Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Catherine Yang and Version 1 by Agnishwar Girigoswami.
Silica nanoparticles (SNPs) are available in many forms, including core-shell silica nanoparticles, nonporous SNPs, hollow mesoporous silica nanoparticles (HMSN), and mesoporous silica nanoparticles (MSN). The advancements in nanotechnology have quickly developed a new subject with vast applications of nanostructured materials in medicine and pharmaceuticals. The enormous surface-to-volume ratio, ease of surface modification, outstanding biocompatibility, and, in the case of mesoporous nanoparticles, the tunable pore size make the SNPs a promising candidate for nano-based medical applications.
- silica nanoparticles
- synthesis
- therapy
- diagnosis
- nanomedicine
1. Drug Delivery System
Due to the advancement of nanotechnology, materials generated at the nanoscale level have received growing attention in disciplines such as drug delivery [3,51,52,53][1][2][3][4]. Because of its unique qualities, such as a vast surface-to-volume ratio, controlled particle size, pore volume, and high biocompatibility, porous silica nanoparticles (NPs) have been studied extensively among all known nanomaterials [54,55,56,57][5][6][7][8]. Mesoporous silica nanoparticles (MSNs) are good prospects for drug delivery and biological applications, with pore sizes ranging from 2 nm to 50 nm. MSNs are synthesized in the presence of a supramolecular assembled surfactant that functions as a targeting moiety [25][9]. MSN-based biological applications have shown to be highly promising (Figure 31). The advantages of MSNs include: (i) pore channels with a vast surface area and pore volume have a lot of potential for drug adsorption and loading; (ii) compared to other nanostructures, the mesoporous silica has a large drug-loading capacity and release kinetics because of its porous nature and tunable pore size; (iii) the therapeutic effectiveness of drugs is improved, and toxicity is reduced when delivered via a surface that can be easily adjusted for the regulation and targeted drug administration [58,59][10][11]. In vivo biosafety studies for cytotoxicity, biodegradation, biodistribution, and excretion have given positive results. (iv) Drug delivery and bioimaging can be performed simultaneously when magnetic and/or luminous substances are used; (v) bioactive materials with good surface characteristics and porosity have proven to be good options for bone regeneration [60][12]. The number of studies on mesoporous silica materials has risen considerably as a result of these distinct properties. Additional research on MSNs, which are covalently attached to dipalmitoyl molecules with the support of phosphorylated lipids, were effectively produced into a controlled release mechanism. Through a chemically induced disulfide reduction, the system may release fluorescein moieties from the porous structure of MSNs coated with the lipid bilayer (LB-MSNs) [61][13]. According to the system, LB-MSNs can be employed to create a controlled release of drug delivery system.
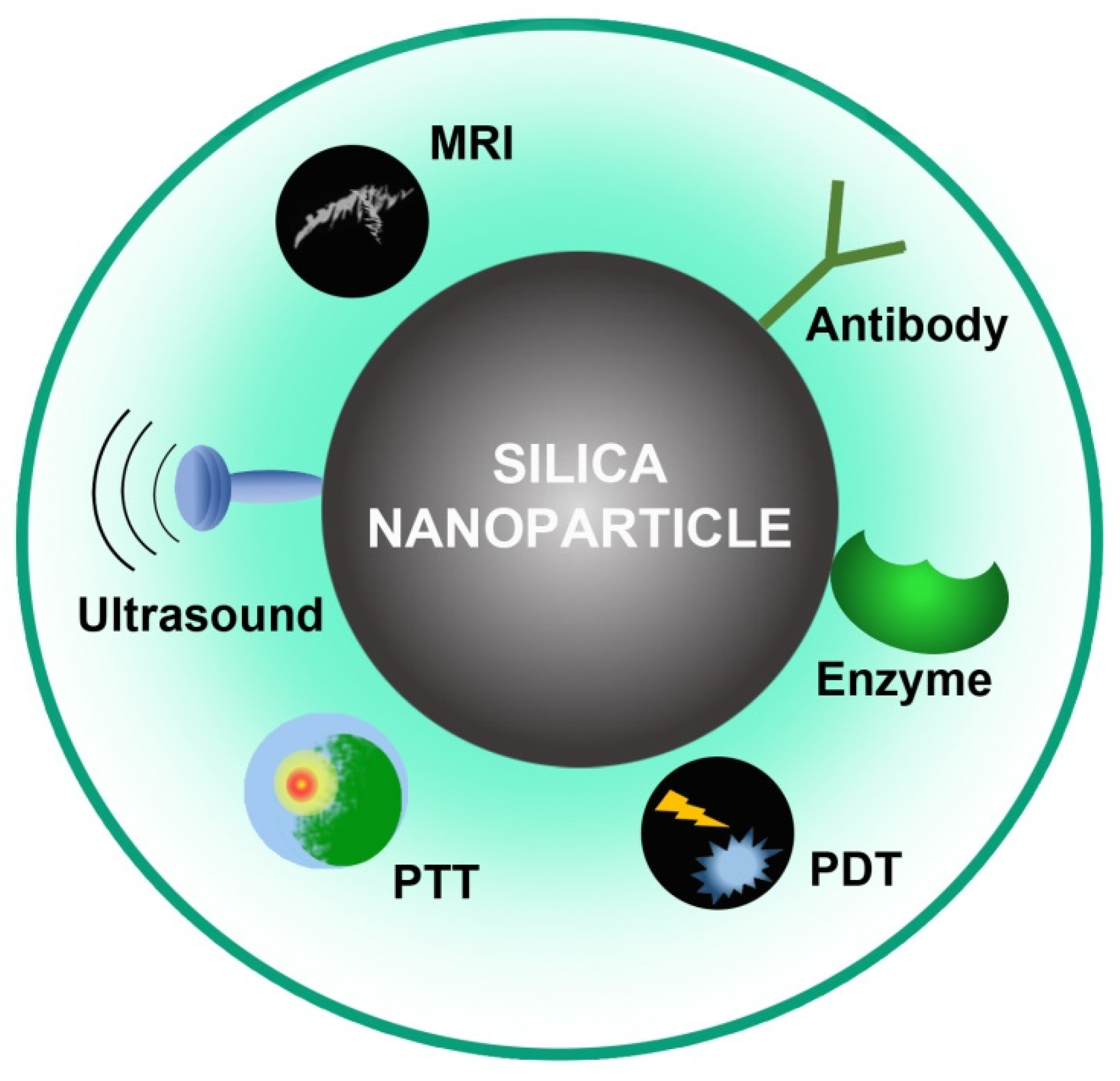
Figure 31.
Biomedical applications of SNPs.
2. Photodynamic Therapy
PDT is an emerging noninvasive therapeutic strategy based on the activation of a photosensitizer (PS) with the help of light in a specific wavelength. In the process of illumination, the excited PS transfers its energy to the molecular oxygen, leading to the generation of reactive oxygen species (ROS). The dioxygen, otherwise called singlet oxygen produced, can efficiently oxidize the main cellular macromolecules that result in vascular closure and tumor cell death [52][3]. PDT does not pose harmful risks to the biological system, as in the case of ionizing radiation therapy that causes damage to the surrounding normal cells.
It is clear that, compared with traditional treatment modalities, PDT has its advantages due to its limited invasiveness and negligible cumulative toxicity. Therefore, PDT aims to boost the quality of life of cancer patients. PDT is highly successful in treating lung, skin, head, and neck cancers [62][14]. PDT has not yet been accepted as a first-line cancer treatment process due to the lack of perfect PS and difficulties experienced during the PS formulation. Nanostructured micelles, liposomes, polymers, proteins, or metals are currently available for pharmaceutical purposes in various forms. PS-loaded silica nanoparticles are shown to be potential singlet oxygen-producing platforms for increasing therapeutic-possessing photoactive activity and improves selectivity and solubility [63,64,65][15][16][17]. The photosensitizers can be either tagged or encapsulated onto silica nanoparticles in their inner or outer surfaces. As a result, loading the photosensitizer into the nanomaterial provides outstanding photostability while restraining oxygen species diffusion.
The surface functionalized silica nanoparticles exhibit a greater therapeutic profile in photodynamic therapy against cancer. The porphyrin dye used in PDT suffers from low solubility and selectivity. SNPs are widely used to overcome the issues faced by the PS. The anticancer effect of core-shell nanoparticles where the core was made up of silica and coated with xylan bearing 5-(4-hydroxyphenyl)-10,15,20-triphenylporphyrin (TPPOH) dye was investigated. The prepared hybrid particle was then characterized to assess the pharmacokinetic profile. The in vitro analysis showed better anticancer activity in colorectal cancer compared to the dye alone. The photodynamic activity of mesoporous silica nanoparticle coated with two different photosensitizers, namely, indocyanine Green and Chlorin e6, against prostate cancer was also reported. The formulations have enhanced cancer killing capacity compared to the nude dye. The dual combination of dyes exhibits better therapeutic efficiency when nanoformulated using SNPs.
3. Photothermal Therapy
Photothermal therapy (PTT) utilizes a photothermal agent (PA) to convert heat from light energy. It is a noninvasive therapeutic modality with a minimum side effect and is widely used for the treatment of drug-resistant tumors. Mesoporous silica nanoparticles are the most efficient drug delivery agent used for the codelivery of PA and chemotherapeutics in combination therapy [66][18]. Wang et al. developed NIR-triggered photothermal nanoparticles incorporating indocyanine green into amino-group-modified silica nanoparticles [67][19]. They have successfully demonstrated that the synthesized photothermal nanoparticles have the potential for enhanced cancer cell killings. Wang et al. formulated multifunctional Janus nanoparticles combining gold triangle and mesoporous silica nanoparticles as drug delivery vehicles to deliver hypoxia-active prodrugs for topical PTT [68][20]. The folate functionalization on the pegylated Janus structure helps in targeting cancer cells. Therefore, MSN is useful in cancer therapy and a widely used system for theranostics.
4. Imaging and Monitoring
Recent studies have explored the usage of SNPs in imaging modalities and found that contrast agents encapsulated in SNPs produce high-resolution images compared to conventional imaging techniques [69][21]. These investigations revealed that SNPs could deliver contrast agents with high specificity and sensitivity for targeting and better imaging contrast. In addition, SNPs have a controlled release in the body because they have a substantial delay. In biomedical imaging, tumor imaging is an essential factor. Other findings indicate that intravenous administrations of core-shell nanoparticles (silica core and metallic shells) result in an improvement in computed tomography (CT) and magnetic resonance imaging (MRI). By using MSNs as a multimodal strategy, researchers successfully detected tumor blood capillaries in live rat model organisms according to near-infrared (NIR) imaging and positron emission tomography (PET) and other techniques [70][22]. The results showed that this combination of devices improves optical and PET imaging resolutions. Furthermore, fluorescently tagged mesoporous silica nanoparticles were utilized as an endoscopic contrast medium to diagnose abnormal cells of the gut. The researchers developed a new type of Magnus nano-bullet (Mn-DTPA-F-MSNs), which consists of a head with magnetic property (Fe3O4-NPs) and mesoporous silica (SiO2) body [71][23]. Researchers redesigned the mesoporous SiO2 before loading it with Mn2+. Both in vitro and in vivo studies demonstrated that the Magnus nano-bullet accelerated the T1-weighted MR images. The magnus nano-bullets showed enhancement in the detection of GSH, a biomarker responsible for redox responsive T1 MRI. A macrophage-driven PET imaging tracker was engineered using aza-dibenzocyclooctyne-tethered PEGylated MSNs (DBCO-MSNs) combining short-lived 18F labeled radiotracer. The in vivo cancer cell tracking and imaging were performed by the PET imaging protocol with the movement of macrophage cells into the tumor site by the bio-orthogonal SPAAC reactions to form 18F-labeled aza-dibenzocycloocta-triazolic MSNs inside RAW 264.7 cells [12][24]. In response, the macrophage was more capable of moving towards the tumor effectively. Finally, the tissue radioactive dispersion measurements were consistent with the PET imaging data. A novel cup-shaped SNP, which was effective for ultrasonic imaging, was also discovered.
Using an emulsification slicing process, researchers developed exosomes-like silica nanoparticles (ELS), or cup-shaped drops were constructed [72][25]. Researchers discovered that ELS was more effective at enhancing particle echogenicity and cell tagging when compared to SNPs shaped like cup-shaped drops. The study demonstrated that stem cells can be used in ultrasound imaging in the future and that SNPs can improve the echogenicity of stem cells in vitro and in vivo [73][26]. Furthermore, they described a biodegradable ultrasound-capable material. These SNPs did not witness cytotoxic effects at the 250 µg/mL concentration necessary for tagging and then were removed from cells within three weeks [73][26]. SNP-based imaging techniques have both advantages and disadvantages. Simple optical imaging with a modest imaging resolution is limited, whereas high-quality CT imaging may contaminate the body with radiation. Researchers show that multimodal imaging is capable of enhancing individual imaging methods. Long-term imaging of sentinel lymph nodes draining tumor utilizing mesoporous nanosilica-based triple-modal probes was demonstrated in a study [74][27]. Due to the excellent stability and resilience of nanoprobes, imaging results from various modalities are coherent and comparable. Furthermore, biocompatibility is a crucial aspect of the use of SNPs in nanomedicine and imaging techniques. Nanomaterials tagged with multimodal imaging nanoprobes were successfully identified using optical imaging, as well as MRI, and also provide more outstanding biocompatibility [74][27].
5. Protein Recognition and Isolation
The versatile properties of silica set it apart from other materials. The ease of synthesis, high surface area, low cost, and surface functionalization are the major reasons for their biomedical applications. Other than drug delivery and diagnostic applications, they can be used to recognize and separate proteins from the sample. Researchers are keen on the application of silica, since the toxicity inside the biological system is negligible. In order to afford simple and quick separation of proteins, iron oxide nanoparticles (IONP) can be coated with silica [75][28]. The magnetic property of IONP is used to achieve the isolation process. The silica nanoparticle allows surface imprinting, which is an efficient strategy to obtain superior adsorption affinity as the initial step for separation. Typically, the nanostructures are fabricated employing iron oxide nanoparticles as a core, which is then laminated with silica to accomplish quick biomolecule separation. The core responds to external magnetic fields, whereas the shell serves as a spot for protein entrapment, providing biocompatibility and stability [76][29]. Core-shell nanoparticles of this sort are implemented in bioseparation, biosensors, immunoassay analysis, nucleic acid detection, and other applications. Mesoporous silica has a higher porous architecture, which offers greater adsorption of the adsorbates. The surface charge of silica nanoparticles is a significant issue. Pure mesoporous silica is said to have a neutral charge. This neutral structure of silica may be susceptible to leaching risks. As a result, chemical modification with tagging of the organic group is used to achieve stability [77][30].
6. Nucleic Acid Detection and Purification
The nucleic acid detection technique is performed in wide areas, including clinical diagnosis, food technology, and environmental safety monitoring [78][31]. Since nucleic acid is a fundamental component for storing and transmitting genetic information, scientists are focusing their efforts on constructing a facile method for detecting and purifying it in preparation for future research. Three main factors that facilitate DNA adsorption are hydrogen bond formation, electrostatic repulsion, and dehydration. Currently, existing approaches have many implications [79][32]. The main concern with the conventional approach is that they require pipetting procedures, which apparently reduce sensitivity. To achieve the desired sensitivity, additional treatment, such as amplification, is required. However, the amplification stage frequently produces aerosol, which is detrimental to the environment. The technique is quite complicated and necessitates a high level of expertise. Researchers must exercise extra precautions when working with extremely contagious viruses. In order to upgrade the method, silica-coated magnetic nanoparticles-driven separation was established [80,81,82][33][34][35]. Several investigations suggest that integrating magnetic nanoparticles (MNPs) as a core with a silica shell could be very effective. Such modified core-shell-structured silica-coated MNPs, were used to extract nucleic acid and amplify them. Therefore, silica shells can be employed to perform simultaneous, automated, and precise nucleic acid sequence detection.
7. Gene Therapy
Nanotechnology-based gene therapy is now emerging as a possible method of delivering genes to treat a wide variety of genetic complications, including cancer. Nanoparticles are expected to be vital vehicles that could carry genetic molecules into cells. Highly porous nanostructures exhibit high loading volume for incorporation of target moiety. Among all the other nanoparticles, the most preferred carrier vehicle is mesoporous silica nanoparticles (MSNs), since they possess a large surface area and high pore volume [83][36]. Silica is considered a nonviral vector that could effectively deliver genetic molecules to the target site. To achieve targeted release, receptor-mediated endocytosis predominates to deliver the cargo or payloads to the diseased site. Different bioconjugation and chemical modification can further upregulate the function of silica inside the biological system. Polymers are generally utilized for surface modification to improve performance while avoiding cytotoxicity [84][37]. In vivo evaluation of chemical modification of silica with sodium chloride or sodium iodide has recently proven to be a promising method for gene transfer without causing any pathological alterations in the cells [85][38]. Since the DNA is negatively charged, the carrier molecule has to be prepared with a positive surface charge. However, the bonding strength should be weak between the carrier molecules and the targeting moiety as the gene involved in breakage and release is weak [86][39]. Strong adhesion might lead to release in undesired sites, or may undergo digestion with no release. Hence, the particle with payload has to be tailored to achieve satisfactory results. The control over the morphology of nanomaterial during fabrication serves the purpose [87][40].
8. Vaccine Delivery
Vaccines were at the frontline to maintain human wellbeing and have been a cost-effective solution to prevent serious infections. Concerns rising regarding conventional viral systems are their immunogenicity and toxicity, which are the primary reasons for gaining research interest for designing novel vaccination methods. The present focus of research seems to be on the development and implementation of effective carriers for the delivery of the genetic payload to the target tissue [88][41]. Three major things to be considered during the formulation of vaccines are effective adjuvant, appropriate antigen, and route of administration. The antigen is used to activate the immunological response, whereas the adjuvant is used to support the antigen to elicit the immune response. Vaccination can be performed either by introducing a protein antigen or by using RNA/DNA that encodes an antigenic protein. Developing a vehicle that can carry the genetic material to the site free of toxicity was a major hurdle for researchers. MSNs are being used in vaccinations that serve as both a carrier and an adjuvant (Table 21). MSNs as a carrier can transport RNA/DNA to the target tissue, promoting protein antigen synthesis, and acts as an adjuvant to induce both cell-mediated and humoral immune responses [89][42]. Several in vitro studies have shown that the vaccines can be delivered based on the acid–base triggered system. Since lymph nodes are the major site for vaccine targets, they need to undergo lymphatic drainage to be taken up by dendritic cells for further action. The surface modification with negative groups makes them a complete lymph-nodes-targeted vehicle. The current pandemic situation due to COVID-19 has evidenced the importance of vaccines. Oligonucleotides-based vaccines are the primary and only therapeutic option available for now. Researchers working on COVID-19 vaccinations are now concentrating on the development of vaccines with nanocarrier for RNA/DNA delivery that encodes specific spike protein [90][43]. The stability, biocompatibility, and release kinetic profile of MSNs makes them a potent tool for antigen carrier and adjuvant.
Table 21.
Summary of relevant theranostic applications of silica nanoparticles.
. In mice in vivo models, it was also found that the 50 nm SNPs also seemed to promote osteoblast proliferation while inhibiting osteoclast differentiation, thereby enhancing bone density as well as biochemical indicators of bone formation. SNPs play a key role in bone regeneration. Researchers discovered that magnetic nanoparticle (Fe3O4)-coated mesoporous silica nanoparticles (M-MSNs) could stimulate bone regeneration in rodents during distraction osteogenesis (DO) in mice [106][59]. A system was constructed using MSCs encapsulated with polymer to stimulate osteogenic differentiation and bone regeneration. SOST siRNA suppressed the osteoporosis-subjecting gene SOST, which lowers osteoblastic development, resulting in increased bone formation. A team of researchers synthesized bioactive glass nanoparticles with monodispersed strontium using improved Stöber’s method. SNPs with diameter 90 ± 10 nm were formed prior to incorporation of calcium and strontium [107][60]. MC3T3-E1 cells from mice were labeled with antibodies against colla1, osteocalcin (OSC), and osteopontin (OSP) for immunohistochemistry to determine the protein expression in response to nanoparticles after three weeks of growth. Furthermore, 14 percent Sr-BGNPs also notably demonstrate a high expression of markers, OSC and OSP, which is representative for osteogenic differentiation compared to zero percent Sr-BGNPs. These results indicate that the Sr-BGNPs serve as an effective agent for bone regeneration [107][60]. All the biomedical applications are summarized in Table 32.
Table 32.
Summary of important silica nanoparticles (SNPs) in theranostics.
| Types of SNPs/Synthetic Routes | Surface Chemistry/Modifications | Biomedical Applications | Ref. | |||||
|---|---|---|---|---|---|---|---|---|
| Fractal SNP/surfactant-free Stöber method | Fractal silica nanoparticles | Sharp edge and rough surface with enhanced adhesion towards enzyme | [18][61] | |||||
| Solid SNP/Stöber method | Amine group/ polyethylenimine | 44 | ] | |||||
| Mimicking virus surface topology and surface roughness enhances the binding of biomolecules | [ | 53 | ][4] | 3. | Mesoporous Silica Nanoparticles | Carboxylic acids | Tuberculosis | [92][45] |
| MSN/co-condensation | - | Ruthenium polypyridyl delivery for cancer treatment | [54][5] | 4. | Silica nanoparticles | Mincle agonists | tuberculosis | [93][46] |
| MSN | Azobenzene-modified | Lubrication enhancement and drug release for osteoarthritis | 5. | Dendrimer-like mesoporous silica nanoparticles | Foot-and-mouth disease VLPs (virus-like particles) | Foot-and-mouth disease | [94][47] | |
| 6. | Rambutan-Like Mesoporous Silica Nanoparticles | DNA vaccine with PEI coating | Chronic infections and cancers. | [95][48] | ||||
| 7. | Extra-large pore MSNs (XL-MSNs) | Cancer antigen with Amine modification | Malignancy | [96][49] | ||||
| 8. | Mesoporous SBA-16 and silanized SBA-16 (APTES-SBA-16) nanoparticles | (3-Aminopropyl) triethoxysilane (APTES) | Paracoccidioidomycosis | [97][50] | ||||
| 9. | MCM-41 type silica nanoparticles | Polymer and amine | Oral protein-based vaccine | [98][51] | ||||
| 10. | Spherical MSNs | Recombinant EspA loaded | Enterohemorrhagic Escherichia coli | [99][52] |
9. Other Applications
Besides the applications listed above, SNPs are also essential to prevent bacterial infections, speed up wound healing, and promote bone regeneration [100,101][53][54]. To enhance the benefits of antibacterial infections, nanocarrier-encapsulated antibiotics have been used to enhance the efficiency of antibacterial treatment with the ability to penetrate bacterial walls [12][24]. SNP characteristics, such as huge surface-to-volume ratio, pore size, and controlled loading, and drug release of antimicrobial agents are remarkable in this regard. When antibacterial peptides such as LL-37, non-steroidal anti-inflammatory medications, and antibiotics using a combination of levofloxacin-loaded MSNs and polycationic dendrimers are added to the mix, the antibiotics are effective at combating Gram-negative bacterial biofilm. The results of the study show Gram-negative E. coli and Gram-positive Staphylococcus aureus do not grow in the presence of SNPs loaded with captured bacteria [12][24]. Another study examined Gram-negative bacteria that were driven by targeted QS into virulence. The unit of study was to test the bacterial communication or quorum quenching (QQ) capacity of metals and metal oxide nanoparticles. Miller created bifunctional SNPs with the quenching molecule cyclodextrin [12,102][24][55]. These results suggest that using functionalized cyclodextrin SNPs, remain in the bacterial microenvironment and inhibit extracellular bacterial communication proteins from seeping out, resulting in antibiotic activity. The study investigated that SNPs were shown to influence migration and invasion of human skin fibroblasts in a local wound healing model (CCD-25SK). The silicic acid is generated by the fibroblasts when the silica nanoparticles become dissolved after absorption in the cells and stimulates wound healing [103,104][56][57]. According to the study, researchers found the biocomposite treatment shortened wound epithelialization time in Wistar rats compared with the control group. Furthermore, the composite has antibacterial properties, good drug availability, and a high absorption rate, which could make SNP biocomposite a potential wound healing material in the future [105][58]. Moreover, silica at the nanoscale has a crucial role in bone regeneration.
The findings also reveal that SNPs are an essential component of bone grafts in this study. SNPs were found to inhibit osteoclasts and activate osteoblasts in vitro. In mice, SNPs improved bone mineral density significantly [60][12]
| [ | |||
| 55 | |||
| ] | |||
| [ | |||
| 6 | ] | ||
| MSN | Carboxyl-functionalized | Controlled delivery of NSAIDs | [56][7] |
| Virus like hollow MSN/self-consuming perovskite | Electrostatic adsorption of doxorubicin | Combination of chemotherapy and immunotherapy | [57][8] |
| MSN/co-condensation | Covalently bound dipalmitoyl | Controlled release of fluorescein | [61][13] |
| Core-shell hybrid SNPs/sol-gel process following modified Stöber method | Silica core with xylan linked 5-(4-hydroxyphenyl)-10,15,20-triphenylporphyrin (TPPOH) | PDT against colorectal cancer cells | [63][15] |
| Hollow-MSN/Stöber method | Hyaluronic acid | Cancer chemo-PDT | [64][16] |
| MSN/ modified Stöber method | haloBODIPYs, PEG and FA | Targeted cancer PDT | [65][17] |
| Silica nanospheres/sol-gel method | Indocyanine green to amino modified surface | PTT against drug resistant tumors | [67][19] |
| Janus-structured gold triangle-MSN/ sol-gel method | Amino functionalization and attachment of tirapazamine (TPZ) | Extrinsic radiosensitization, local PTT and hypoxia-specific chemotherapy | [68][20] |
| FA-Gd-Tb@SiO2/covalent conjugation | Covalent conjugation of luminescent Tb3+ to Si-O-Si framework followed by attachment of Gd and FA (folic acid) | Targeted cellular time-gated luminescence (TGL) and cancer cell MR imaging | [70][22] |
| Janus magnus nano-bullets (Mn-DTPA-F-MSNs)/sol-gel process | Mn-DTPA-functionalized Fe3O4-MSNs | GSH (glutathione) responsive T1/T2 MRI | [71][23] |
| Exosomes-like cup-shaped SNP/emulsion template method | - | Ultra sound contrast for stem cell imaging and drug delivery | [72][25] |
| Theranostic MSN | Amino-silane conjugation of fluorescein followed by Gd-DOTA | Ultra sound and MR imaging of stem cells and slow-release reservoir of insulin-like growth factor (IGF) | [73][26] |
| MSN/sol-gel method | Salicylic acid and ketoconazole | Antifungal and wound healing | [103][56] |
| SNP | Carbon quantum dots/silica nanoparticles/silk fibroin nanocomposites | Wound repair | [104][57] |
| Rod-like silica nanoparticles/one pot method in presence of PVP | Type I collagen-SiO2@Fe3O4 | Cell guidance and drug delivery | [106][59] |
References
- Sharmiladevi, P.; Girigoswami, K.; Haribabu, V.; Girigoswami, A. Nano-enabled theranostics for cancer. Mater. Adv. 2021, 2, 2876–2891.
- Deepika, R.; Girigoswami, K.; Murugesan, R.; Girigoswami, A. Influence of divalent cation on morphology and drug delivery efficiency of mixed polymer nanoparticles. Curr. Drug Del. 2018, 15, 652–657.
- Vimaladevi, M.; Divya, K.C.; Girigoswami, A. Liposomal nanoformulations of rhodamine for targeted photodynamic inactivation of multidrug resistant gram negative bacteria in sewage treatment plant. J. Photochem. Photobiol. B Biol. 2016, 162, 146–152.
- Niu, Y.; Yu, M.; Hartono, S.B.; Yang, J.; Xu, H.; Zhang, H.; Zhang, J.; Zou, J.; Dexter, A.; Gu, W. Nanoparticles mimicking viral surface topography for enhanced cellular delivery. Adv. Mater. 2013, 25, 6233–6237.
- Harun, S.N.; Ahmad, H.; Lim, H.N.; Chia, S.L.; Gill, M.R. Synthesis and optimization of mesoporous silica nanoparticles for ruthenium polypyridyl drug delivery. Pharmaceutics 2021, 13, 150.
- Zhao, W.; Wang, H.; Wang, H.; Han, Y.; Zheng, Z.; Liu, X.; Feng, B.; Zhang, H. Light-responsive dual-functional biodegradable mesoporous silica nanoparticles with drug delivery and lubrication enhancement for the treatment of osteoarthritis. Nanoscale 2021, 13, 6394–6399.
- Gou, K.; Wang, Y.; Guo, X.; Wang, Y.; Bian, Y.; Zhao, H.; Guo, Y.; Pang, Y.; Xie, L.; Li, S. Carboxyl-functionalized mesoporous silica nanoparticles for the controlled delivery of poorly water-soluble non-steroidal anti-inflammatory drugs. Acta Biomater. 2021, 134, 576–592.
- Xu, D.; Song, X.; Zhou, J.; Ouyang, X.; Li, J.; Deng, D. Virus-like hollow mesoporous silica nanoparticles for cancer combination therapy. Colloids Surf. B Biointerfaces 2021, 197, 111452.
- Rosenholm, J.M.; Sahlgren, C.; Lindén, M. Towards multifunctional, targeted drug delivery systems using mesoporous silica nanoparticles—Opportunities & challenges. Nanoscale 2010, 2, 1870–1883.
- Wang, Y.; Zhao, Q.; Han, N.; Bai, L.; Li, J.; Liu, J.; Che, E.; Hu, L.; Zhang, Q.; Jiang, T. Mesoporous silica nanoparticles in drug delivery and biomedical applications. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 313–327.
- Firmansyah, A.; Nugrahani, I.; Wirasutisna, K.; Ibrahim, S. Formation of boron-silica based mesoporous and studies of its adsorption ability for curcuminoids. Biointerface Res. Appl. Chem. 2020, 10, 7977–7981.
- Shadjou, N.; Hasanzadeh, M. Bone tissue engineering using silica-based mesoporous nanobiomaterials: Recent progress. Mater. Sci. Eng. C 2015, 55, 401–409.
- Roggers, R.A.; Lin, V.S.-Y.; Trewyn, B.G. Chemically reducible lipid bilayer coated mesoporous silica nanoparticles demonstrating controlled release and HeLa and normal mouse liver cell biocompatibility and cellular internalization. Mol. Pharm. 2012, 9, 2770–2777.
- Dolmans, D.E.; Fukumura, D.; Jain, R.K. Photodynamic therapy for cancer. Nat. Rev. Cancer 2003, 3, 380–387.
- Bouramtane, S.; Bretin, L.; Pinon, A.; Leger, D.; Liagre, B.; Richard, L.; Brégier, F.; Sol, V.; Chaleix, V. Porphyrin-xylan-coated silica nanoparticles for anticancer photodynamic therapy. Carbohydr. Polym. 2019, 213, 168–175.
- Zhou, Y.; Chang, C.; Liu, Z.; Zhao, Q.; Xu, Q.; Li, C.; Chen, Y.; Zhang, Y.; Lu, B. Hyaluronic acid-functionalized hollow mesoporous silica nanoparticles as pH-sensitive nanocarriers for cancer chemo-photodynamic therapy. Langmuir 2021, 37, 2619–2628.
- Prieto-Montero, R.; Prieto-Castañeda, A.; Katsumiti, A.; Cajaraville, M.P.; Agarrabeitia, A.R.; Ortiz, M.J.; Martínez-Martínez, V. Functionalization of Photosensitized Silica Nanoparticles for Advanced Photodynamic Therapy of Cancer. Int. J. Mol. Sci. 2021, 22, 6618.
- Gao, Y.; Gao, D.; Shen, J.; Wang, Q. A review of mesoporous silica nanoparticle delivery systems in chemo-based combination cancer therapies. Front. Chem. 2020, 8, 1086.
- Wang, Y.; Niu, C.; Fan, S.; Li, Y.; Li, X.; Dai, Y.; Shi, J.; Wang, X. Indocyanine Green Loaded Modified Mesoporous Silica Nanoparticles as an Effective Photothermal Nanoplatform. Int. J. Mol. Sci. 2020, 21, 4789.
- Wang, Z.; Chang, Z.-M.; Shao, D.; Zhang, F.; Chen, F.; Li, L.; Ge, M.-F.; Hu, R.; Zheng, X.; Wang, Y. Janus gold triangle-mesoporous silica nanoplatforms for hypoxia-activated radio-chemo-photothermal therapy of liver cancer. ACS Appl. Mater. Interfaces 2019, 11, 34755–34765.
- Cha, B.G.; Kim, J. Functional mesoporous silica nanoparticles for bio-imaging applications. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnology 2019, 11, e1515.
- Song, B.; Liu, Q.; Ma, H.; Tang, Z.; Liu, C.; Zou, J.; Tan, M.; Yuan, J. Tumor-targetable magnetoluminescent silica nanoparticles for bimodal time-gated luminescence/magnetic resonance imaging of cancer cells in vitro and in vivo. Talanta 2020, 220, 121378.
- Khatik, R.; Wang, Z.; Li, F.; Zhi, D.; Kiran, S.; Dwivedi, P.; Xu, R.X.; Liang, G.; Qiu, B.; Yang, Q. “Magnus nano-bullets” as T1/T2 based dual-modal for in vitro and in vivo MRI visualization. Nanomed. Nanotechnol. Biol. Med. 2019, 15, 264–273.
- Li, Z.; Mu, Y.; Peng, C.; Lavin, M.F.; Shao, H.; Du, Z. Understanding the mechanisms of silica nanoparticles for nanomedicine. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnology 2021, 13, e1658.
- Chen, F.; Ma, M.; Wang, J.; Wang, F.; Chern, S.-X.; Zhao, E.R.; Jhunjhunwala, A.; Darmadi, S.; Chen, H.; Jokerst, J.V. Exosome-like silica nanoparticles: A novel ultrasound contrast agent for stem cell imaging. Nanoscale 2017, 9, 402–411.
- Kempen, P.J.; Greasley, S.; Parker, K.A.; Campbell, J.L.; Chang, H.-Y.; Jones, J.R.; Sinclair, R.; Gambhir, S.S.; Jokerst, J.V. Theranostic mesoporous silica nanoparticles biodegrade after pro-survival drug delivery and ultrasound/magnetic resonance imaging of stem cells. Theranostics 2015, 5, 631.
- Huang, X.; Zhang, F.; Lee, S.; Swierczewska, M.; Kiesewetter, D.O.; Lang, L.; Zhang, G.; Zhu, L.; Gao, H.; Choi, H.S. Long-term multimodal imaging of tumor draining sentinel lymph nodes using mesoporous silica-based nanoprobes. Biomaterials 2012, 33, 4370–4378.
- Lee, H.; Shin, T.-H.; Cheon, J.; Weissleder, R. Recent developments in magnetic diagnostic systems. Chem. Rev. 2015, 115, 10690–10724.
- Pham, X.-H.; Hahm, E.; Kim, H.-M.; Son, B.S.; Jo, A.; An, J.; Tran Thi, T.A.; Nguyen, D.Q.; Jun, B.-H. Silica-coated magnetic iron oxide nanoparticles grafted onto graphene oxide for protein isolation. Nanomaterials 2020, 10, 117.
- Mostafaei, M.; Hosseini, S.N.; Khatami, M.; Javidanbardan, A.; Sepahy, A.A.; Asadi, E. Isolation of recombinant Hepatitis B surface antigen with antibody-conjugated superparamagnetic Fe3O4/SiO2 core-shell nanoparticles. Protein Expr. Purif. 2018, 145, 1–6.
- Choi, J.R.; Hu, J.; Tang, R.; Gong, Y.; Feng, S.; Ren, H.; Wen, T.; Li, X.; Abas, W.A.B.W.; Pingguan-Murphy, B. An integrated paper-based sample-to-answer biosensor for nucleic acid testing at the point of care. Lab. Chip 2016, 16, 611–621.
- Melzak, K.A.; Sherwood, C.S.; Turner, R.F.; Haynes, C.A. Driving forces for DNA adsorption to silica in perchlorate solutions. J. Colloid Interface Sci. 1996, 181, 635–644.
- Min, J.H.; Woo, M.-K.; Yoon, H.Y.; Jang, J.W.; Wu, J.H.; Lim, C.-S.; Kim, Y.K. Isolation of DNA using magnetic nanoparticles coated with dimercaptosuccinic acid. Anal. Biochem. 2014, 447, 114–118.
- Ma, C.; Li, C.; He, N.; Wang, F.; Ma, N.; Zhang, L.; Lu, Z.; Ali, Z.; Xi, Z.; Li, X. Preparation and characterization of monodisperse core–shell Fe3O4@ SiO2 microspheres and its application for magnetic separation of nucleic acids from E. coli BL21. J. Biomed. Nanotechnol. 2012, 8, 1000–1005.
- Yue, H.; Shin, J.M.; Tegafaw, T.; Han, H.S.; Chae, K.-S.; Chang, Y.; Lee, G.H. Magnetic separation of nucleic acids from various biological samples using silica-coated iron oxide nanobeads. J. Nanopart. Res. 2020, 22, 1–12.
- Zhou, Y.; Quan, G.; Wu, Q.; Zhang, X.; Niu, B.; Wu, B.; Huang, Y.; Pan, X.; Wu, C. Mesoporous silica nanoparticles for drug and gene delivery. Acta Pharm. Sin. B 2018, 8, 165–177.
- Bharali, D.J.; Klejbor, I.; Stachowiak, E.K.; Dutta, P.; Roy, I.; Kaur, N.; Bergey, E.J.; Prasad, P.N.; Stachowiak, M.K. Organically modified silica nanoparticles: A nonviral vector for in vivo gene delivery and expression in the brain. Proc. Natl. Acad. Sci. USA 2005, 102, 11539–11544.
- Xia, T.; Kovochich, M.; Liong, M.; Meng, H.; Kabehie, S.; George, S.; Zink, J.I.; Nel, A.E. Polyethyleneimine coating enhances the cellular uptake of mesoporous silica nanoparticles and allows safe delivery of siRNA and DNA constructs. ACS Nano 2009, 3, 3273–3286.
- Roy, I.; Ohulchanskyy, T.Y.; Bharali, D.J.; Pudavar, H.E.; Mistretta, R.A.; Kaur, N.; Prasad, P.N. Optical tracking of organically modified silica nanoparticles as DNA carriers: A nonviral, nanomedicine approach for gene delivery. Proc. Natl. Acad. Sci. USA 2005, 102, 279–284.
- Hartono, S.B.; Phuoc, N.T.; Yu, M.; Jia, Z.; Monteiro, M.J.; Qiao, S.; Yu, C. Functionalized large pore mesoporous silica nanoparticles for gene delivery featuring controlled release and co-delivery. J. Mater. Chem. B 2014, 2, 718–726.
- Mody, K.T.; Popat, A.; Mahony, D.; Cavallaro, A.S.; Yu, C.; Mitter, N. Mesoporous silica nanoparticles as antigen carriers and adjuvants for vaccine delivery. Nanoscale 2013, 5, 5167–5179.
- Gordon, S.; Teichmann, E.; Young, K.; Finnie, K.; Rades, T.; Hook, S. In vitro and in vivo investigation of thermosensitive chitosan hydrogels containing silica nanoparticles for vaccine delivery. Eur. J. Pharm. Sci. 2010, 41, 360–368.
- Qiao, L.; Chen, M.; Li, S.; Hu, J.; Gong, C.; Zhang, Z.; Cao, X. A peptide-based subunit candidate vaccine against SARS-CoV-2 delivered by biodegradable mesoporous silica nanoparticles induced high humoral and cellular immunity in mice. Biomater. Sci. 2021, 9, 7287–7296.
- Lee, J.Y.; Kim, M.K.; Nguyen, T.L.; Kim, J. Hollow mesoporous silica nanoparticles with extra-large mesopores for enhanced cancer vaccine. ACS Appl. Mater. Interfaces 2020, 12, 34658–34666.
- Montalvo-Quirós, S.; Vallet-Regí, M.; Palacios, A.; Anguita, J.; Prados-Rosales, R.C.; González, B.; Luque-Garcia, J.L. Mesoporous Silica Nanoparticles as a Potential Platform for Vaccine Development against Tuberculosis. Pharmaceutics 2020, 12, 1218.
- Abdelwahab, W.M.; Riffey, A.; Buhl, C.; Johnson, C.; Ryter, K.; Evans, J.T.; Burkhart, D.J. Co-adsorption of synthetic Mincle agonists and antigen to silica nanoparticles for enhanced vaccine activity: A formulation approach to co-delivery. Int. J. Pharm. 2021, 593, 120119.
- Liu, Z.; Ru, J.; Sun, S.; Teng, Z.; Dong, H.; Song, P.; Yang, Y.; Guo, H. Uniform dendrimer-like mesoporous silica nanoparticles as a nano-adjuvant for foot-and-mouth disease virus-like particle vaccine. J. Mater. Chem. B 2019, 7, 3446–3454.
- Song, H.; Yang, Y.; Tang, J.; Gu, Z.; Wang, Y.; Zhang, M.; Yu, C. DNA vaccine mediated by rambutan-like mesoporous silica nanoparticles. Adv. Ther. 2020, 3, 1900154.
- Cha, B.G.; Jeong, J.H.; Kim, J. Extra-large pore mesoporous silica nanoparticles enabling co-delivery of high amounts of protein antigen and toll-like receptor 9 agonist for enhanced cancer vaccine efficacy. ACS Cent. Sci. 2018, 4, 484–492.
- Soares, D.C.F.; Soares, L.M.; de Goes, A.M.; Melo, E.M.; de Barros, A.L.B.; Bicalho, T.C.A.S.; Leao, N.M.; Tebaldi, M.L. Mesoporous SBA-16 silica nanoparticles as a potential vaccine adjuvant against Paracoccidioides brasiliensis. Microporous Mesoporous Mater. 2020, 291, 109676.
- Amin, M.K.; Boateng, J.S. Surface modification of mobile composition of matter (MCM)-41 type silica nanoparticles for potential oral mucosa vaccine delivery. J. Pharm. Sci. 2020, 109, 2271–2283.
- Hajizade, A.; Salmanian, A.H.; Amani, J.; Ebrahimi, F.; Arpanaei, A. EspA-loaded mesoporous silica nanoparticles can efficiently protect animal model against enterohaemorrhagic E. coli O157: H7. Artif. Cells Nanomed. Biotechnol. 2018, 46, S1067–S1075.
- Injorhor, P.; Ruksakulpiwat, Y.; Ruksakulpiwat, C. Effect of shrimp shell chitosan loading on antimicrobial, absorption and morphological properties of natural rubber composites reinforced with silica-chitosan hybrid filler. Biointerface Res. Appl. Chem 2020, 10, 5656–5659.
- Alghuthaymi, M. Magnetic-silica nanoshell for extraction of fungal genomic DNA from Rhizopus oryzae. Biointerface Res. Appl. Chem. 2020, 10, 4972–4976.
- Miller, K.P. Bacterial Communication and Its Role As a Target for Nanoparticlebased Antimicrobial Therapy. Doctoral Dissertation, University of South Carolina, Columbia, SC, USA, 2015.
- Masood, A.; Maheen, S.; Khan, H.U.; Shafqat, S.S.; Irshad, M.; Aslam, I.; Rasul, A.; Bashir, S.; Zafar, M.N. Pharmaco-Technical Evaluation of Statistically Formulated and Optimized Dual Drug-Loaded Silica Nanoparticles for Improved Antifungal Efficacy and Wound Healing. ACS Omega 2021, 6, 8210–8225.
- Abolghasemzade, S.; Pourmadadi, M.; Rashedi, H.; Yazdian, F.; Kianbakht, S.; Navaei-Nigjeh, M. PVA based nanofiber containing CQDs modified with silica NPs and silk fibroin accelerates wound healing in a rat model. J. Mater. Chem. B 2021, 9, 658–676.
- Quignard, S.; Coradin, T.; Powell, J.J.; Jugdaohsingh, R. Silica nanoparticles as sources of silicic acid favoring wound healing in vitro. Colloids Surf. B Biointerfaces 2017, 155, 530–537.
- Shi, Y.; Li, Y.; Coradin, T. Magnetically-oriented type I collagen-SiO2@ Fe3O4 rods composite hydrogels tuning skin cell growth. Colloids Surf. B Biointerfaces 2020, 185, 110597.
- Naruphontjirakul, P.; Porter, A.E.; Jones, J.R. In vitro osteogenesis by intracellular uptake of strontium containing bioactive glass nanoparticles. Acta Biomater. 2018, 66, 67–80.
- Fu, J.; Jiao, J.; Song, H.; Gu, Z.; Liu, Y.; Geng, J.; Jack, K.S.; Du, A.; Tang, J.; Yu, C. Fractal-in-a-sphere: Confined self-assembly of fractal silica nanoparticles. Chem. Mater. 2019, 32, 341–347.
More
