Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Sirius Huang and Version 1 by Vincenzo Ricci.
Shoulder disorders are very common in clinical practice. Among several other pathologies, calcific tendinopathy of the rotator cuff tendons is frequently observed during the ultrasound examination of patients with painful shoulder. The deposition of hydroxyapatite calcium crystals should not be considered as a static process but rather a dynamic pathological process with different/possible patterns of migration.
- rotator cuff
- ultrasonography
- tendon
- calcification
- migration
1. Introduction
Shoulder disorders are commonplace in daily practice and calcific tendinopathy of the rotator cuff tendons can be the main finding of an ultrasound (US) examination [1]. In calcific tendinopathy, deposition of hydroxyapatite is a dynamic pathological occurrence with different patterns of clinical presentation. Identifying these patterns through US can provide additional insight into this condition as well as optimizing its management [2,3][2][3].
The pathogenesis appears to stem from a low amount of oxygen within the tendons, leading to fibrocartilaginous metaplasia, i.e., switch of tenocytes to chondrocytes. The latter cellular line produces a cartilaginous matrix that progressively calcifies [4,5][4][5]. Indeed, the rotator cuff tendons are poorly vascularized and receive nutrients mainly from the overlying synovial bursa [4,5][4][5]. The primum movens of the pathology could be a “metabolic disorder” characterized by reduced passage of nutrients/oxygen from the vascular network located within the peri-bursal fat tissue to the underlying tendons.
The natural history of calcific tendinopathy is characterized by precalcific, calcific and postcalcific stages (Uhthoff cycle). Moreover, the calcific stage is further divided into formative, resting (Figure 1A) and resorptive phases (i.e., maturation process) [4,5][4][5]. Calcifications display distinct patterns of migration during the maturation process during which hydroxyapatite calcium crystals may migrate from tendons to neighboring tissues under mechanical (tension/compression) forces. The fragments can creep through the intrasubstance gaps of the tendon where the layers/laminae are not tightly attached to each other (delaminating zones) (Figure 1B).
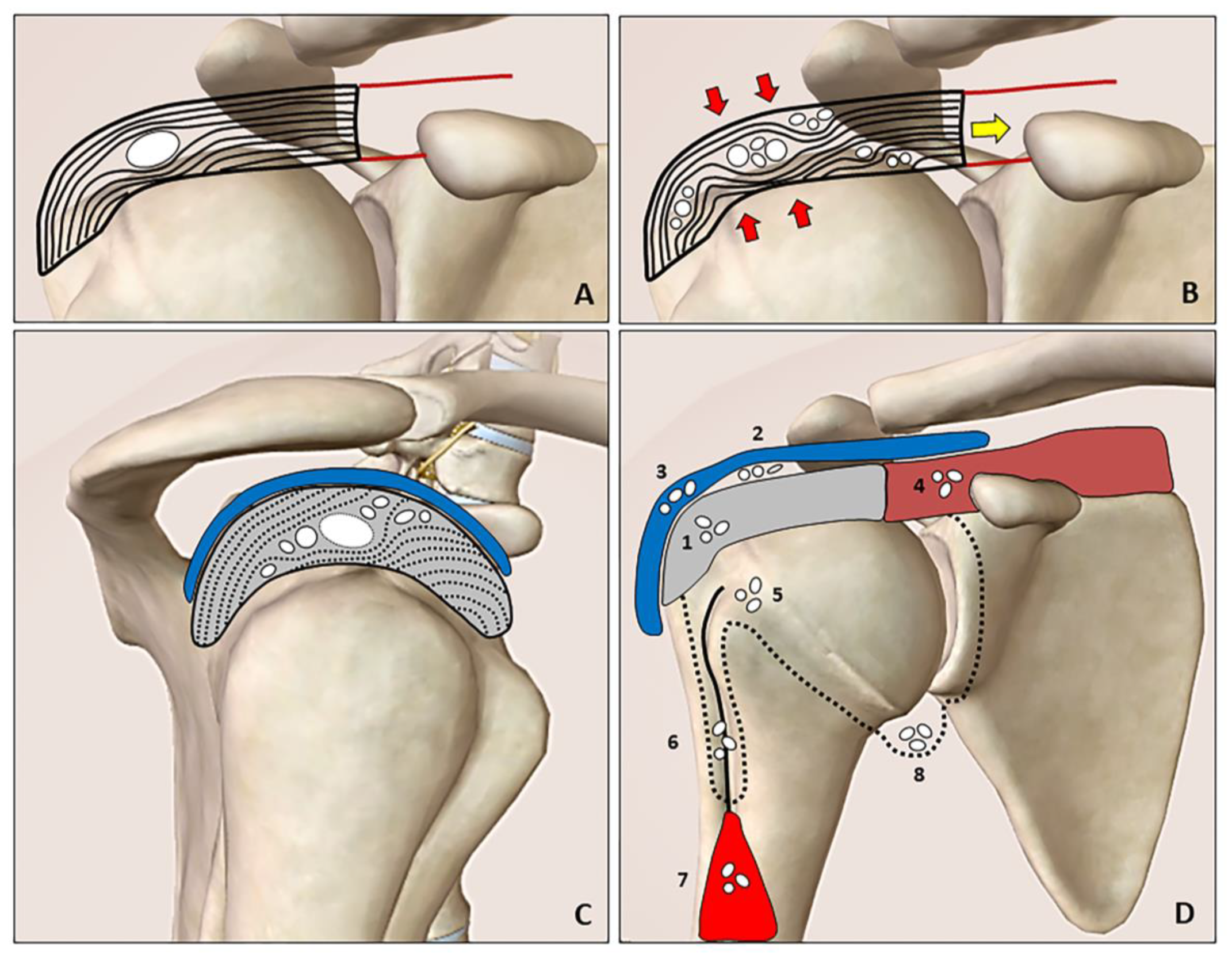
Figure 1. The resting phase is characterized by a hard calcification that shifts the tendon fibers (A). During the resorptive phase (B), the compression (red arrows) and tension (yellow arrow) forces promote slipping of the softly hydrated calcification through the layers/laminae of the tendon (rupture and dispersion of the calcific deposition). The intratendinous bursal-side (cranial direction) and articular side (caudal direction) migration patterns can be clearly identified during the US imaging by “following” the hyperechoic foci of the calcification (white dots) especially in the short axis view (C). Hydroxyapatite calcium crystals (white dots) can develop “migratory patterns”, i.e., moving from the rotator cuff tendons (grey) to several anatomical sites. The spectrum includes the following patterns: intratendinous (1), sub-bursal (2), intrabursal (3), intramuscular (4), intraosseous (5), inside the synovial sheat of the long head of the biceps tendon (i.e., the bicipital recess) (6), between the interfascial planes of the arm (7) and perhaps inside the glenohumeral joint cavity (8) (D). Blue: subacromial bursa, brown: supraspinatus muscle, red: biceps brachii muscle, black line: long head of the biceps tendon, black dotted line: synovium.
Calcium deposition may be extruded from the tendon cranially towards the sub-bursal space and subacromial bursa (i.e., intra-bursal penetration with acute microcrystalline bursitis), or caudally towards bone/synovium of the joint (Figure 1C). The most commonly described patterns are intratendinous, sub-bursal, intrabursal, intramuscular, intraosseous and intraarticular migrations (Figure 1D) [2,3][2][3]. Interfascial migration of softly hydrated calcification slipping from the originating tendon over the superficial fascia of biceps brachii muscle was also described [6]. An intra-articular migration has never been demonstrated by imaging modalities; however, several authors have reported the frequent onset of shoulder stiffness after arthroscopy for calcific tendinopathy—postulating the irritation of the glenohumeral capsule by residual calcium debris as the possible mechanism [7]. Likewise, researchers speculate that chronic micro-leakage of calcium, moving from the rotator cuff tendons inside the synovial recesses of the joint (e.g., axillary recess due to gravity) (Figure 1D), could increase the risk of stiffness through inflammatory involvement of the capsulo-synovial complex (i.e., post-calcific frozen shoulder).
In daily practice, understanding the correlation between the clinical features of the patient and the ultrasonographic pattern of calcific tendinopathy is paramount to plan for an appropriate/substantial rehabilitation program (conservative and interventional alike). In this sense, the next sections will briefly describe the most common clinical-ultrasonographic scenarios of calcific tendinopathy of the shoulder in different phases/stages.
2. Resting Phase
Hard calcification located between the tendon fibers (i.e., displacing them) is associated with focal thickening of the rotator cuff (Figure 2A,B). The possible mechanical impingement to the surrounding bones, ligaments and muscles during motions is referred as the mechanical phase of calcific tendinopathy.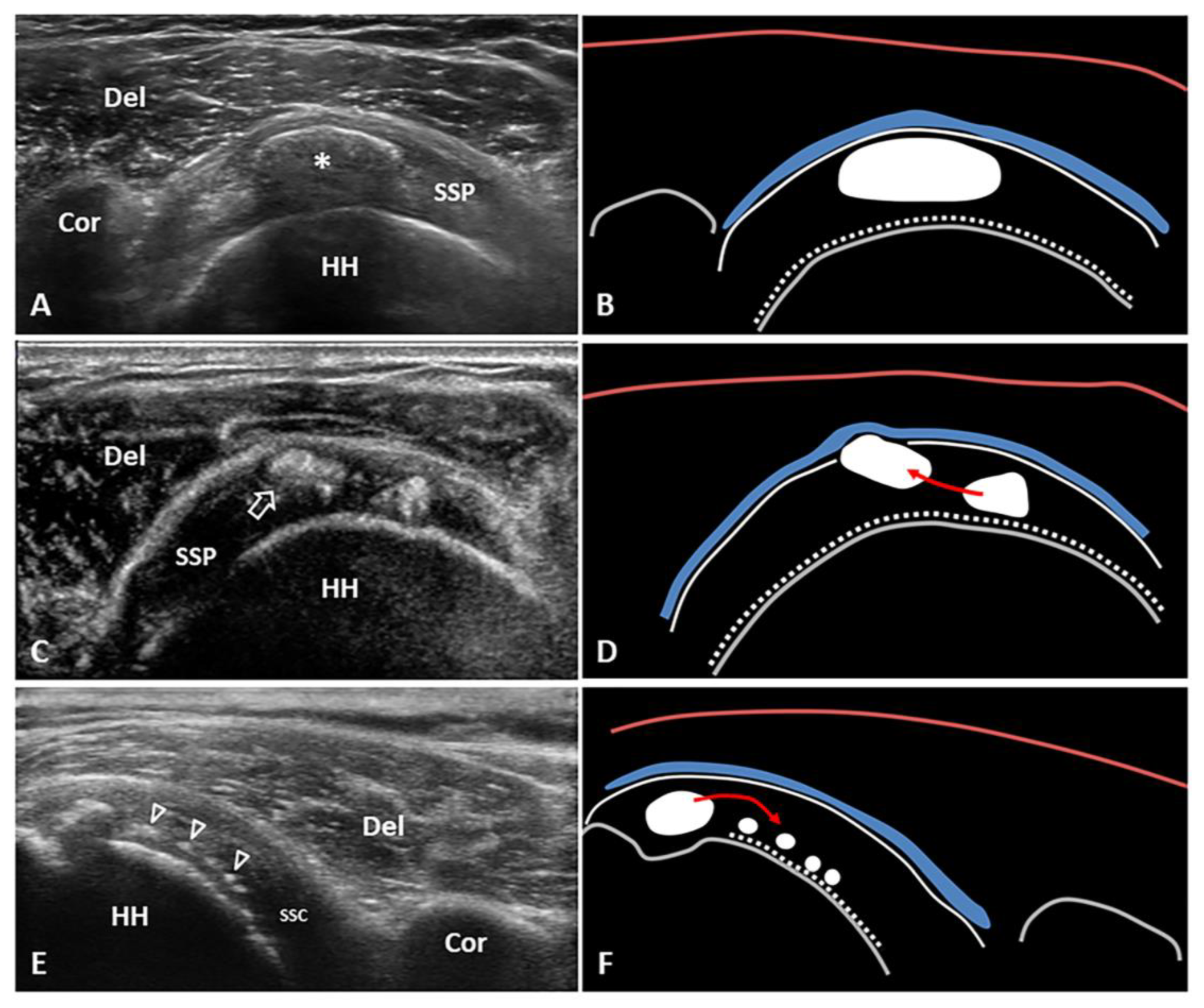
Figure 2. Short-axis view of the supraspinatus (SSP) tendon with elliptical calcification (asterisk) in resting phase—i.e., type 1 Gartner and Heyer (A,B). Short-axis view of the SSP tendon with intratendinous bursal side migration pattern of a softly hydrated fragment (white void arrow) of calcific deposits with sub-bursal space involvement (C,D). Long-axis view of the subscapularis (SSC) tendon with intratendinous articular side migration pattern of multiple hydroxyapatite calcium crystals (white void arrowheads) towards the surface of the humeral head (E,F). The latter might be a “migratory pattern” possibly predisposing to ‘post-calcific’ frozen shoulder. Del: deltoid, Cor: coracoid, HH: humeral head, blue lines: subacromial bursa, white dotted lines: cartilage, white lines: outer surface of the rotator cuff tendons, red lines: superficial fascia of the deltoid muscle, grey lines: bony surface, red arrow: possible direction of migration.
3. Resorptive Phase
During the resorptive phase, intra-tendinous calcific deposition usually breaks down and the sonographic pattern can be highly variable—e.g., fragmented, nodular, cyst-like [18]. Moreover, color/power Doppler signals can be identified surrounding the hydroxyapatite crystals due to the local proliferation of capillaries and thin-walled vascular channels [18,19][18][19]. Of note, the aforementioned intratendinous neovessels allow macrophages and multinucleated giant osteoclast-like cells to reach and degrade the calcific deposits [19]. If the fragments of hydroxyapatite crystals cranially migrate towards the peribursal fat (Figure 2C,D) and/or inside the subacromial-subdeltoid bursa, acute microcrystalline bursitis may develop with a clinical scenario of shoulder hyperalgesia [20]. Intrabursal migration of calcific debris may predominantly involve the lateral recess of the synovial bursa; or, it can present a wide diffusion inside the bursal cavity. The first pattern is usually characterized by a teardrop-shaped bursal effusion located deep in the fibers of the deltoid muscle with pain on the lateral side of the shoulder. The second scenario is commonly that of as an hourglass-shaped bursal effusion with pain spreading throughout the shoulder (Figure 3). Histological studies have shown massive cell infiltration—mainly composed of polymorphonuclear cells and mononuclear phagocytic cells—which occurs within the whole bursal cavity and the synovial lining in response to hydroxyapatite crystal stimulation [21]. The patient typically complains of a pseudoparalytic shoulder with the upper limb adducted to the trunk in order to protect the shoulder and reduce pain [22]. Night rest is severely compromised and the patient is unable to lie on the affected side. In some patients, the sonographic visualization of intrabursal migration of calcific fragments can be challenging; dynamic scanning with gentle active/passive movements of the shoulder can be necessary to promptly identify “bright spots” floating within the bursal cavity [23].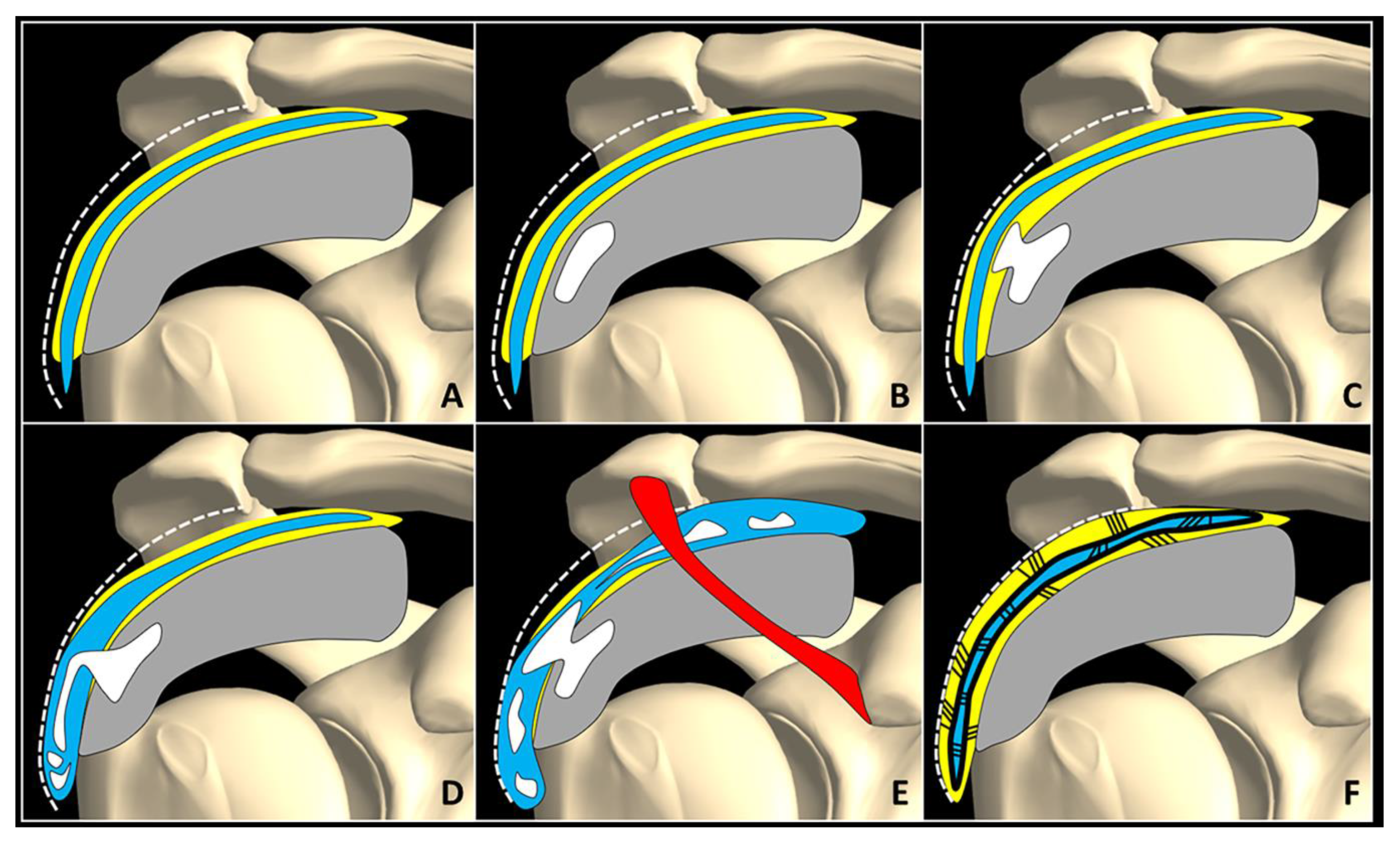
Figure 3. Normally, fat tissue (yellow) is located in between the SASD bursa (blue), rotator cuff tendons (grey), and the subdeltoid fascia (white dotted line) (A). Intra-tendinous calcification (white) (B) may progressively migrate within the peribursal space (C), and perforating the synovial lining, may slip inside the bursal cavity involving its lateral recess (D) or the entire chamber (E). A potential complication of acute microcrystalline bursitis is adhesive bursopathy (F) characterized by thickening of the synovial walls (thick black line) and intra/peri-bursal adhesions (thin black lines). Adhesions reduce the bursal gliding, “gluing” it to the rotator cuff tendons and subdeltoid fascia. Red: coracoacromial ligament.
4. Post-Calcific Stage
Even several months after resolution of the acute phase of calcific tendinopathy (e.g., resorptive phase) the patient may develop shoulder stiffness with or without pain [7,31][7][31]. The most probable cause of this clinical condition is adhesive capsulitis with thickening and fibrosis of the capsular connective tissue in the glenohumeral joint. The pathophysiological link between calcific tendinopathy of the rotator cuff and adhesive capsulitis is not well understood; but chronic micro-leakage of calcium moving from the tendon fibers into the synovial recesses of the joint (Figure 2E,F) could be involved (i.e., chronic chemical synovitis) [32]. Interestingly, some authors have demonstrated that routine arthroscopic glenohumeral exploration performed before the calcification removal is associated with higher risk of post-operative adhesive capsulitis, probably related to the intra-articular diffusion of calcium debris coming from the rotator cuff tendons [33]. As such, the articular penetration of calcium—as a starter of synovial/capsular inflammation—can be considered to be a very likely link between the two shoulder disorders. The prolonged pain-induced hypomobility of the shoulder also seems to play important role in this aspect [7,31][7][31]. The main sonographic findings of adhesive capsulitis described in the literature are thickening of the axillary pouch (i.e., inferior capsular recess of the glenohumeral joint) and coracohumeral ligament in B-mode, and hypervascularization of the rotator cuff interval soft tissues in color/power Doppler mode [32,34][32][34]. Herein, the latter sonographic sign has been arthroscopically confirmed to be related to hyperemic synovial tissue surrounding the proximal segment of the long head of the biceps tendon (LHBT) within the rotator cuff interval [34]. Progressively, synovitis can evolve to synovial hypertrophy by replacement of the fat tissue located inside the rotator cuff interval with fibrous tissue. Hereby, US examination can reveal an irregular hypoechoic coat surrounding the proximal portion of the LHBT in more advanced stages of the disease [35]. Notably, capsular contracture tends to shift the articular effusion towards the bicipital and subcoracoid recesses of the shoulder, where the synovial tissue lacks the capsular coat and is more stretchable [13,36][13][36]. Moreover, dynamic and comparative US assessment—pathological vs. normal shoulder—can be performed to demonstrate the rotational blockade of the humeral head as well as the disappearance of physiological retroflection of the posterior glenohumeral recess under the infraspinatus muscle during external rotation [37]. During the post-calcific stiffness phase of the shoulder, the main purpose of rehabilitation is rapid recovery of the active and passive range of motions. The pertinent literature shows the efficacy of combined treatment with US-guided glenohumeral injection and rehabilitation training to accelerate the improvement in pain and function in case of adhesive capsulitis [38]. Likewise, some authors have also suggested using high-volume injections to mechanically expand the joint space (i.e., hydrodilatation or intra-articular hydraulic distension) to stretch the capsule and “break” the adhesions, i.e., a mechanical effect in addition to the pharmacological effect of the corticosteroid over the chronic glenohumeral synovitis [39]. Of note, concerning US-guided hydrodilatation, the anterior approach through the rotator cuff interval seems to be more effective (than the posterior approach targeting the glenohumeral recess) in reducing pain during shoulder movements [40]. Accordingly, for dilating the anterior capsule of the glenohumeral joint, the needle’s tip can be advanced within the histological interface between the LHBT and the stabilizing pulley (i.e., coracohumeral and superior glenohumeral ligaments) [41] or in the gap between the superior edge of subscapularis tendon and the proximal segment of the LHBT [42]. After the US-guided injection/hydrodilatation, immediate rehabilitation including passive mobilization of the glenohumeral joint in different spatial planes (i.e., angular and translational mobilizations) and the end-of-range capsular stretching of the shoulder is mandatory to progressively improve the active/passive range of motions [43]. As regards active exercises, muscle energy techniques with isometric contraction against an operator’s resistance (in a controlled direction/position) seems to be more effective to improve function and disability—when compared to other types of therapeutic exercises in shoulder adhesive capsulitis [44]. Of note, the capsular tissue is richly innervated; therefore, the reseauthorchers suggest to accurately plan for progressive stretching, to minimize the onset of local/radiating pain along the anterolateral surface of the arm—i.e., C5 and C6 dermatomes. The latter component of pain is mainly related to the innervation of the rotator cuff interval by suprascapular and subscapularis nerves that are derived from the anterior branches of C5–C6 nerve roots [45,46][45][46]. For sure, in case of advanced stiffness of the shoulder, US-guided suprascapular nerve block can also be performed—to facilitate pain-free mobilization of the glenohumeral joint during the functional recovery [47].References
- Özçakar, L.; Çarl, A.B.; Tok, F.; Tekin, L.; Akkaya, N.; Kara, M. The utility of musculoskeletal ultrasound in rehabilitation settings. Am. J. Phys. Med. Rehabil. 2013, 92, 805–817.
- Bianchi, S.; Becciolini, M. Ultrasound appearance of the migration of tendon calcifications. J. Ultrasound Med. 2019, 38, 2493–2506.
- Ricci, V.; Özçakar, L. Life after ultrasound: Are we speaking the same (or a new) language in physical and rehabilitation medicine? J. Rehabil. Med. 2019, 51, 234–235.
- Uhthoff, H.; Sarkar, K. Calcifying tendinitis. Baillieres Clin. Rheumatol. 1989, 3, 567–581.
- Uhthoff, H.K.; Loehr, J.W. Calcific tendinopathy of the rotator cuff: Pathogenesis, diagnosis, and management. J. Am. Acad. Orthop. Surg. 1997, 5, 183–191.
- Cocco, G.; Ricci, V.; Boccatonda, A.; Iannetti, G.; Schiavone, C. Migration of calcium deposit over the biceps brachii muscle, a rare complication of calcific tendinopathy: Ultrasound image and treatment. J. Ultrasound 2018, 21, 351–354.
- Merolla, G.; Bhat, M.G.; Paladini, P.; Porcellini, G. Complications of calcific tendinitis of the shoulder: A concise review. J. Orthop. Traumatol. 2015, 16, 175–183.
- Guillin, R.; Marchand, A.J.; Roux, A.; Niederberger, E.; Duvauferrier, R. Imaging of snapping phenomena. Br. J. Radiol. 2012, 85, 1343–1353.
- Akkaya, N.; Akkaya, S.; Gungor, H.R.; Yaşar, G.; Atalay, N.S.; Sahin, F. Effects of weighted and un-weighted pendulum exercises on ultrasonographic acromiohumeral distance in patients with subacromial impingement syndrome. J. Back Musculoskelet. Rehabil. 2017, 30, 221–228.
- Tate, A.R.; McClure, P.W.; Young, I.A.; Salvatori, R.; Michener, L.A. Comprehensive impairment-based exercise and manual therapy intervention for patients with subacromial impingement syndrome: A case series. J. Orthop. Sports Phys. Ther. 2010, 40, 474–493.
- Mira, R.M.; Molinari Tosatti, L.; Sacco, M.; Scano, A. Detailed characterization of physiological EMG activations and directional tuning of upper-limb and trunk muscles in point-to-point reaching movements. Curr. Res. Physiol. 2021, 4, 60–72.
- Ricci, V.; Galletti, S.; Chang, K.; Özçakar, L. Ultrasound imaging and guidance in the management of adhesive bursopathy of the shoulder: A video demonstration. J. Ultrasound Med. 2020, 39, 633–635.
- Ricci, V.; Mezian, K.; Naňka, O.; Özçakar, L. Assessing/imaging the subcoracoid space: From anatomy to dynamic sonography. J. Ultrasound Med. 2022, 41, 2149–2155.
- Klontzas, M.E.; Vassalou, E.E.; Zibis, A.H.; Karantanas, A.H. The effect of injection volume on long-term outcomes of US-guided subacromial bursa injections. Eur. J. Radiol. 2020, 129, 109113.
- Machida, A.; Sugamoto, K.; Miyamoto, T.; Inui, H.; Watanabe, T.; Yoshikawa, H. Adhesion of the subacromial bursa may cause subacromial impingement in patients with rotator cuff tearsPressure measurements in 18 patients. Acta Orthop. Scand. 2004, 75, 109–113.
- Chou, W.-Y.; Wang, C.-J.; Wu, K.-T.; Yang, Y.-J.; Ko, J.-Y.; Siu, K.-K. Prognostic factors for the outcome of extracorporeal shockwave therapy for calcific tendinitis of the shoulder. Bone Jt. J. 2017, 99-B, 1643–1650.
- Chiou, H.-J.; Chou, Y.-H.; Wu, J.-J.; Huang, T.-F.; Ma, H.-L.; Hsu, C.-C.; Chang, C.-Y. The role of high-resolution ultrasonography in management of calcific tendonitis of the rotator cuff. Ultrasound Med. Biol. 2001, 27, 735–743.
- Chiou, H.-J.; Chou, Y.-H.; Wu, J.-J.; Hsu, C.-C.; Huang, D.-Y.; Chang, C.-Y. Evaluation of calcific tendonitis of the rotator cuff: Role of color Doppler ultrasonography. J. Ultrasound Med. 2002, 21, 289–295.
- Uhthoff, H.K.; Sarkar, K.; Maynard, J.A. Calcifying tendinitis: A new concept of its pathogenesis. Clin. Orthop. Relat. Res. 1976, 118, 164–168.
- Draghi, F.; Scudeller, L.; Draghi, A.G.; Bortolotto, C. Prevalence of subacromial-subdeltoid bursitis in shoulder pain: An ultrasonographic study. J. Ultrasound 2015, 18, 151–158.
- Darrieutort-Laffite, C.; Blanchard, F.; Le Goff, B. Calcific tendonitis of the rotator cuff: From formation to resorption. Jt. Bone Spine 2018, 85, 687–692.
- Greenberg, D.L. Evaluation and treatment of shoulder pain. Med. Clin. N. Am. 2014, 98, 487–504.
- Ricci, V.; Chang, K.-V.; Güvener, O.; Mezian, K.; Kara, M.; Leblebicioğlu, G.; Stecco, C.; Pirri, C.; Ata, A.M.; Dughbaj, M.; et al. EURO-MUSCULUS/USPRM dynamic ultrasound protocols for shoulder. Am. J. Phys. Med. Rehabil. 2021, 101, e29–e36.
- de Witte, P.B.; Kolk, A.; Overes, F.; Nelissen, R.G.; Reijnierse, M. Rotator cuff calcific tendinitis: Ultrasound-guided needling and lavage versus subacromial corticosteroids: Five-year outcomes of a randomized controlled trial. Am. J. Sports Med. 2017, 45, 3305–3314.
- Messina, C.; Sconfienza, L.M. Ultrasound-guided percutaneous irrigation of calcific tendinopathy. Semin. Musculoskelet. Radiol. 2016, 20, 409–413.
- Messina, C.; Banfi, G.; Orlandi, D.; Lacelli, F.; Serafini, G.; Mauri, G.; Secchi, F.; Silvestri, E.; Sconfienza, L.M. Ultrasound-guided interventional procedures around the shoulder. Br. J. Radiol. 2016, 89, 20150372.
- Orlandi, D.; Mauri, G.; Lacelli, F.; Corazza, A.; Messina, C.; Silvestri, E.; Serafini, G.; Sconfienza, L.M. Rotator cuff calcific tendinopathy: Randomized comparison of US-guided percutaneous treatments by using one or two needles. Radiology 2017, 285, 518–527.
- Mitchell, M.J.; Causey, G.; Berthoty, D.P.; Sartoris, D.J.; Resnick, D. Peribursal fat plane of the shoulder: Anatomic study and clinical experience. Radiology 1988, 168, 699–704.
- Järvinen, M.; Józsa, L.; Kannus, P.; Järvinen, T.L.N.; Kvist, M.; Leadbetter, W. Histopathological findings in chronic tendon disorders. Scand. J. Med. Sci. Sports 1997, 7, 86–95.
- Moseley, H.F. The natural history and clinical syndromes produced by calcified deposits in the rotator cuff. Surg. Clin. N. Am. 1963, 43, 1489–1493.
- Merolla, G.; Singh, S.; Paladini, P.; Porcellini, G. Calcific tendinitis of the rotator cuff: State of the art in diagnosis and treatment. J. Orthop. Traumatol. 2016, 17, 7–14.
- Stella, S.M.; Gualtierotti, R.; Ciampi, B.; Trentanni, C.; Sconfienza, L.M.; Del Chiaro, A.; Pacini, P.; Miccoli, M.; Galletti, S. Ultrasound features of adhesive capsulitis. Rheumatol. Ther. 2022, 9, 481–495.
- Ecalle, A.; Julien, C.; Chaouche, S.; Cungi, P.-J.; Anger, F.; Galland, A.; Gravier, R.; Airaudi, S. Is routine gleno-humeral exploration a risk factor for adhesive capsulitis after arthroscopic removal of rotator cuff calcifications? A comparative retrospective study in 340 cases. Orthop. Traumatol. Surg. Res. 2021, 107, 102915.
- Lee, J.C.; Sykes, C.; Saifuddin, A.; Connell, D. Adhesive capsulitis: Sonographic changes in the rotator cuff interval with arthroscopic correlation. Skelet. Radiol. 2005, 34, 522–527.
- Tamborrini, G.; Möller, I.; Bong, D.; Miguel, M.; Marx, C.; Müller, A.M.; Müller-Gerbl, M. The rotator interval—A link between anatomy and ultrasound. Ultrasound Int. Open 2017, 03, E107–E116.
- Carbone, S.; Napoli, A.; Gumina, S. MRI of adhesive capsulitis of the shoulder: Distension of the bursa in the superior subscapularis recess is a suggestive sign of the pathology. Eur. J. Radiol. 2014, 83, 345–348.
- Ricci, V.; Özçakar, L. Looking into the joint when it is frozen: A report on dynamic shoulder ultrasound. J. Back Musculoskelet. Rehabil. 2019, 32, 663–665.
- Fields, B.K.K.; Skalski, M.R.; Patel, D.B.; White, E.A.; Tomasian, A.; Gross, J.S.; Matcuk, G.R. Adhesive capsulitis: Review of imaging findings, pathophysiology, clinical presentation, and treatment options. Skelet. Radiol. 2019, 48, 1171–1184.
- Catapano, M.; Mittal, N.; Adamich, J.; Kumbhare, D.; Sangha, H. Hydrodilatation with corticosteroid for the treatment of adhesive capsulitis: A systematic review. PM&R 2018, 10, 623–635.
- Wang, J.-C.; Tsai, P.-Y.; Hsu, P.-C.; Huang, J.-R.; Wang, K.A.; Chou, C.-L.; Chang, K.-V. Ultrasound-guided hydrodilatation with triamcinolone acetonide for adhesive capsulitis: A randomized controlled trial comparing the posterior glenohumeral recess and the rotator cuff interval approaches. Front. Pharmacol. 2021, 12, 686139.
- Wang, J.-C.; Chang, K.-V.; Wu, W.-T.; Han, D.-S.; Özçakar, L. Ultrasound-guided standard vs dual-target subacromial corticosteroid injections for shoulder impingement syndrome: A randomized controlled trial. Arch. Phys. Med. Rehabil. 2019, 100, 2119–2128.
- Ricci, V.; Chang, K.; Özçakar, L. Ultrasound-guided hydrodilatation of the shoulder capsule at the rotator interval: Technical tips and tricks. Pain Pract. 2020, 20, 948–949.
- Noten, S.; Meeus, M.; Stassijns, G.; Van Glabbeek, F.; Verborgt, O.; Struyf, F. Efficacy of different types of mobilization techniques in patients with primary adhesive capsulitis of the shoulder: A systematic review. Arch. Phys. Med. Rehabil. 2016, 97, 815–825.
- Mertens, M.G.; Meert, L.; Struyf, F.; Schwank, A.; Meeus, M. Exercise therapy is effective for improvement in range of motion, function, and pain in patients with frozen shoulder: A systematic review and meta-analysis. Arch. Phys. Med. Rehabil. 2022, 103, 998–1012.e14.
- Candela, V.; Giannicola, G.; Passaretti, D.; Venditto, T.; Gumina, S. Adhesive capsulitis of the shoulder: Pain intensity and distribution. Musculoskelet. Surg. 2017, 101 (Suppl. S2), 153–158.
- Hsu, P.-C.; Chang, K.-V.; Mezian, K.; Naňka, O.; Wu, W.-T.; Yang, Y.-C.; Meng, S.; Ricci, V.; Özçakar, L. Sonographic pearls for imaging the brachial plexus and its pathologies. Diagnostics 2020, 10, 324.
- Chang, K.-V.; Hung, C.-Y.; Wu, W.-T.; Han, D.-S.; Yang, R.-S.; Lin, C.-P. Comparison of the effectiveness of suprascapular nerve block with physical therapy, placebo, and intra-articular injection in management of chronic shoulder pain: A meta-analysis of randomized controlled trials. Arch. Phys. Med. Rehabil. 2016, 97, 1366–1380.
More
