Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Matteo Nadile and Version 3 by Beatrix Zheng.
Overall, the scientific data show that resveratrol has the ability to target/inhibit certain signaling molecules (EGFR, VEGFR, PKC, JNK, ERK, NF-kB, and STAT3) involved in cervical cancer cell proliferation and survival. Further in vivo experiments and clinical studies are required to better understand the potential of resveratrol against cervical cancer.
- cervical cancer
- resveratrol
- proliferation
- survival
- apoptosis
1. Resveratrol against Cervical Cancer: In Vitro Studies
Using HeLa and SiHa cervical cancer cells, Zoberi et al. found that RSV enhanced the response to ionizing radiation treatment [1][36]. Cell growth and survival were significantly reduced in the presence of RSV compared to irradiated cells without RSV. This radiosensitizing effect was associated with a significant cell cycle arrest at the S phase and a significant inhibition of cyclooxygenase-1 (COX-1) activity [1][36].
Treatment of HeLa cells with RSV resulted in the inhibition of cell migration and invasion [2][37]. Furthermore, RSV treatment significantly reduced the phorbol myristate acetate (PMA)-induced matrix metalloproteinase-9 (MMP-9) mRNA, protein, and activity levels. This inhibition was associated with an inhibition of PMA-induced c-Jun N-terminal kinase (JNK) and protein kinase C (PKCδ)-activation [2][37]. In addition, RSV significantly reduced activator protein-1 (AP1) and nuclear factor-kappa B (NFkB) signaling [2][37].
Kramer et al. found that treatment of HeLa cells with RSV significantly reduced their proliferation and induced cell cycle arrest at the S-phase (observed 24 h after RSV treatment) [3][38]. However, this cell cycle arrest seemed to be reversible as the G2/M-cell population reappeared after 32–48 h of RSV treatment. Overall, this researchtudy shows that RSV has the ability to induce the S-phase arrest in HeLa cells but in a transient and reversible way [3][38].
Tang et al. found that human cervical cancer C-33A and HeLa cells transfected with the HPV-16 oncoproteins E6 and E7 had increased expression of HIF-1a and VEGF [4][39]. The phosphatidylinositol-3-kinase (PI3K) and the ERK signaling cascades were involved in this process, as seen by the increased levels of phosphorylated Akt and ERK. The use of specific inhibitors of these cascades (LY294002 to block PI3K and PD98059 to block ERK) significantly reduced the E6 and E7-induced HIF-1a levels. Importantly, treatment with RSV dose-dependently reduced the E6 and E7-induced HIF-1a and VEGF levels. Treatment with RSV had the same effect on HIF-1a levels as treatment with HIF-1a siRNA. This researchtudy shows that RSV has a potent anti-angiogenic effect and inhibits HPV E6 and E7-driven cervical cancer.
Hsu et al. found that treatment of HeLa, Cx, SiHa, and SKGIIIb cervical cancer cells with RSV (100–200 μΜ) resulted in inhibition of growth and induction of apoptosis, as seen by the increase in cytochrome C and cleaved caspase-3 levels [5][40]. Furthermore, treatment of HeLa and Cx cells with RSV time-dependently increased autophagy, as seen by the increased levels of LC3-II, a marker of autophagy. In addition, treatment of these two cell lines with 100 μΜ RSV resulted in increased cytosolic levels and activity of cathepsin L (cat L). Employment of a small interfering RNA (siRNA) approach to down-regulate cat L abolished the RSV-induced apoptosis while siRNA down regulation of squamous cell carcinoma antigen 1 (SCCA1) promoted RSV-induced apoptosis indicating a role as a negative regulator. Treatment with RSV increased lysosomal membrane permeability (LMP). Inhibition of autophagy with wortmannin or asparagine attenuated the RSV-induced responses (decreased LMP, LC3-II, and apoptosis). Overall, this researchtudy indicates that RSV induces autophagy resulting in a downstream increase in LMP, cytosolic cathepsin L, cytochrome C, and cleaved caspase-3 and apoptosis of cervical cancer cells [5][40].
Treatment of several cervical cancer cells (SKG-I, SKG-II, SKG-IIIa, Nuz & HeLa) with RSV (10, 30 & 100 μΜ) resulted in reduced survival that was associated with a reduction in the anti-autophagy factor ATPase family AAA domain containing 3A (ATAD3A) expression [6][41]. In addition, treatment of these cells with RSV increased autophagy and apoptosis. ATAD3A is increased in HPV-induced cervical cancer, and this researchtudy shows that RSV has the potential to counteract HPV-driven processes.
Kim et al. treated HeLa cells with RSV (10, 30 & 100 μΜ) and found a significant inhibition of phorbol 12-myristate 13-acetate (PMA)-induced cell migration and invasion [7][42]. This effect was associated with a decrease in matrix metalloproteinase-9 (MMP-9) levels and activity, NF-κB, and AP-1. In addition, RSV inhibited NF-κB mediated MMP-9 transcription. Overall, this researchtudy indicates that RSV has the potential to inhibit metastasis of cervical cancer [7][42].
Treatment of HeLa cervical cancer cells with RSV (25 μΜ) resulted in increased apoptosis, as was evident by the increased cleaved caspase-3 and caspase-9 levels, reduced mitochondrial membrane potential, and increased DNA fragmentation. [8][43]. A decrease in the level of HDM2 gene expression was detected after RSV treatment. Overall, this researchtudy shows that RSV has the ability to cause apoptosis in cervical cancer cells through the activation of the caspase cascade and by lowering the expression of HDM2 [8][43].
García-Zepeda et al. treated several cervical cancer cell lines (C33A, HeLa, CaLo, CaSki & SiHa), including HPV 16 and HPV18 positive ones, with RSV (150–250 μM for 48 h) and found a significant reduction in cell proliferation and induction of apoptosis and autophagy [9][44]. In addition, the majority of the cell lines showed an increased expression of p53, while the expression of p65 NFkB was reduced by RSV treatment.
HeLa and SiHa cervical cancer cells treated with RSV (100 µM for 48 h) had reduced growth and increased apoptosis. Western blot analysis revealed decreased phosphorylation of Notch1/2, Hes1, Wnt2/5a, β-catenin, and signal transducer and activator of transcription (STAT3) while the levels of PIAS3 were increased [10][45]. Treatment of the cells with RSV resulted in S-phase cell cycle arrest and induction of apoptosis, effects that were similar to the treatment with AG490, a selective STAT3/JAK3 inhibitor. The use of L-685,458, a Notch inhibitor, or XAV-939, a Wnt inhibitor, did not mimic the RSV-induced effects [10][45]. These data suggest that RSV-induced apoptosis is mediated by the inhibition of STAT3 [10][45].
Similar to these findings [10][45], Li et al. found that treatment of HeLa cervical cancer cells with RSV (10–100 μΜ) resulted in reduced viability, proliferation, and survival that was associated with a decrease in STAT3 phosphorylation and an increase in the expression of the gene associated with retinoid–IFN-induced mortality-19 (GRIM-19). Overexpression of GRIM-19 reduced p-STAT3, cyclin B1, VEGF, and Bcl-2 protein levels while downregulation of GRIM-19, using a siRNA approach, increased phosphorylation/activation of STAT3 and attenuated the effects of RSV. Overall, this researchtudy provides evidence that RSV targets the GRIM-19-STAT3 signaling cascade resulting in anticancer effects [11][46].
Zhang et al. treated three different cervical cancer cell lines (HeLa, SiHa & C33A) with 100 µM of RSV for 48 h and found a decrease in growth, proliferation, and induction of apoptosis. Immunocytochemical (ICC) staining, western blot, and RT-PCR analysis revealed a decrease in p-STAT3 and inhibition of survivin, c-Myc, cyclin D1, and VEGF expression in all three cell lines. Furthermore, basal levels of suppressor of cytokine signaling 3 (SOCS3) and protein inhibitor of activated STAT3 (PIAS3) were low and increased with RSV treatment, as seen by ICC staining and western blot analysis. Nuclear labeling revealed a negative correlation between PIAS3 expression and STAT3 nuclear translocation. These data suggest that RSV is able to modify STAT3 phosphorylation, activation, and nuclear translocation by increasing the expression of PIAS3 resulting in the inhibition of proliferation and survival of cervical cancer cells [12][47].
Ruíz et al. used HeLa cervical cancer cells enriched with cancer stem cells (CSC) positive for CD49f called HeLa Sphere (HeLa SP), which are less sensitive to Etoposide (VP16) [13][48]. Treatment of these cells with RSV and VP16 resulted in a significant decrease in cell viability and an increase in apoptosis that was associated with a reduction in RAD51 protein levels. Downregulation of RAD51, using siRNA, had similar effects as RSV treatment [13][48]. This researchtudy indicates that RSV has the ability to target RAD51 and increase the effectiveness of VP16 treatment in CSC-enriched cervical cancer cells.
Chatterjee et al. found that treatment of HeLa cervical cancer cells with RSV (5–40 µM for 24–48 h) reduced cell viability and cell migration and arrested the cell cycle to the S phase [14][49]. More importantly, RSV downregulated the HPV oncoprotein E6, induced caspase-3 activation, and upregulated p53 protein levels [14][49].
HeLa cervical cancer cells treated with RSV showed decreased proliferation (IC50: 21.76 µM), increased cell cycle arrest at the G1/S phase, and increased apoptosis. Western blot analysis revealed a significant increase in p53 protein levels with RSV treatment [15][50]. These effects of RSV were also seen in HeLa cells lacking the aryl hydrocarbon receptor (AHR), indicating that the RSV-induced effects are AHR-independent.
Exposure of HeLa cervical cancer cells to RSV reduced viability inhibited proliferation, and induced apoptosis in a dose- and time-dependent manner [16][51]. Treatment with RSV increased caspase-3 and -9 cleavages and increased the expression of the pro-apoptotic protein Bax whereas the levels of the antiapoptotic proteins Bcl-2 and Bcl-XL were decreased. Lastly, p53 expression levels were increased, and Cyclin B1 expression levels were decreased [16][51]. Overall, this researchtudy shows that RSV has a strong inhibitory effect on HeLa cells.
Treatment of HeLa cervical cancer cells with RSV reduced viability and proliferation was correlated with the downregulation of phospholipid scramblase 1 (PLSCR1) [17][52].
SiHa cervical cancer cells treated with RSV (100 µM for 24 h) showed reduced expression of survivin, an anti-apoptotic protein, which is often over-expressed in cervical cancer cells [18][53]. Knocking down of survivin using siRNA resulted in effects similar to RSV treatment. Furthermore, when SiHa cells were treated with RSV in combination with tumor necrosis factor-related apoptosis-inducing ligand (TRAIL), there was a notable decrease in cell viability, cell cycle arrest at the G2/M phase, increased apoptosis, and upregulation of E-cadherin. These data suggest that RSV, in combination with TRAIL, has a synergistic effect on inducing apoptosis in SiHa cancer cells [18][53].
Assad et al., treated HeLa cells with RSV and found reduced viability and cytotoxicity similar to that seen when cells were treated with the mTOR inhibitors Temsirolimus and Everolimus used in cancer treatment [19][54]. Furthermore, treatment with RSV, similar to treatment with Temsirolimus and Everolimus, enhanced the cytotoxic effect of radiation treatment (2Gy) [19][54]. This researchtudy indicates radio-sensitizing properties of RSV.
Treatment of HeLa and SiHa, human cervical cancer cells with RSV, resulted in reduced proliferation, reduced wound healing, migration, and invasion, indicating a reduction in metastasis [20][56]. Treatment with RSV (40 µM for 24 h) resulted in a significant increase in E-cadherin and a reduction in N-cadherin and vimentin, suggesting inhibition of epithelial to mesenchymal transition (EMT). Further supporting the decrease in the invasion, western blot analysis revealed a decrease in MMP-3 and -13 protein levels with RSV treatment [20][56]. In addition, treatment with RSV reduced the phosphorylation/activation of STAT3 protein [20][56].
HeLa cells treated with RSV showed reduced proliferation and increased apoptosis, as revealed by both flow cytometry/annexin measurements and the increased levels of the pro-apoptotic protein BIM [21][55]. Western blot analysis revealed reduced phosphorylated ERK and FOXO3a levels with RSV treatment [21][55].
Pani et al. found that treatment of HeLa cells with RSV resulted in reduced viability (MTT assay) that was associated with a decrease in intracellular glucose, suggesting a decrease in glucose uptake by the cancer cells [22][57]. Similarly, lactate levels were shown to be decreased, whereas pyruvate levels were increased, and the NADH/NAD+ ratio was decreased with RSV treatment suggesting RSV has effects that combat the Warburg Effect [22][57].
Treatment of HeLa and Ca Ski cells with RSV inhibited proliferation, induced cell cycle arrest, and promoted apoptosis. Flow cytometry results revealed an increased proportion of cells in the G1 phase of the cell cycle, while there was a significant decrease in the proportion of cells in the S phase with RSV treatment. The mRNA levels of p16, p21, and p27 were increased, whereas protein levels of CDK4, E2F1 and p-pRb1 were decreased. The mRNA and protein levels of BCL-2 were decreased, while mRNA and protein levels of BAX were increased with RSV treatment. In addition, immunocytochemistry and western blot analysis revealed a significant decrease in E6, E7, and p-pRb1 and an increase in p53 [23][59].
HT-3 cervical cancer cells treated with RSV had reduced viability and proliferation and increased apoptosis [24][60]. Maximum induction of apoptosis was seen at a concentration of 1.25 µM RSV. Unfortunately, this researchtudy did not examine the mechanisms involved in mediating these effects of RSV.
Treatment with HeLa cells with RSV resulted in reduced viability [25][61] and increased the expression (mRNA levels measured) of the sodium/lithium/calcium exchanger (NCLX). NCLX is a mitochondria membrane protein moving calcium out of mitochondria (into cytoplasm) in exchange for sodium or lithium. The increased expression of NCLX seen with RSV treatment correlated with increased cytosolic calcium levels. These data suggest that the reduced cell viability seen with RSV is due to calcium-induced cytotoxicity. The effect of RSV on cell viability was similar to that seen with the downregulation of NCLX by the siRNA approach [25][61]. Downregulation of NCLX leads to the overloading of the mitochondria with calcium resulting in cytotoxicity. The combination of RSV and NCLX siRNA resulted in a greater reduction in cell viability compared to each treatment alone. Overall, this researchtudy indicates that disruption of intracellular calcium homeostasis leads to cytotoxicity, and a combination of RSV and NCLX siRNA treatment approach should be further explored as an anticancer treatment.
When subclones of W12 cells (derived from cervical precancerous lesions containing episomal and integrated HPV16 DNA) were treated with RSV, their proliferation was significantly reduced. The IC50 RSV values in 20,861, 20,822, 201,402, 20,862, and 20,850 subclones of cells were in the range of 10–53.7 µM. [26][58].
Treatment of HeLa cells with RSV resulted in reduced survival and cell cycle arrest at the S-phase [27][62]. Immunofluorescence imaging revealed a significant downregulation of epidermal growth factor receptor (EGFR) in HeLa cells treated with RSV [27][62].
Although the in vitro studies presented above and in Table 1 utilized RSV concentrations in the range of 0.16 to 262 µM, the majority of the studies found strong anticancer effects with 50–100 µM RSV. Unfortunately, none of the studies summarized here utilized non-cancerous human cervical epithelial cells to examine the effects of RSV on cells representing normal/healthy cells. ThWe researchers performed a further search of the literature and found no studies examining the effects of RSV on healthy cervical epithelial cells. However, in the past, the researchers we found a significant inhibition of survival of PC3 and 22RV1 prostate cancer cells by RSV treatment, while PNT1A cells representing normal prostate epithelial cells were not affected [28][63].
Table 1. Effects of Resveratrol against Cervical Cancer: in vitro studies.
| Cell | Resveratrol Concentration/Duration | Effect | Reference |
|---|---|---|---|
| HeLa, SiHa cervical cancer cells | 10, 25 µM 1–8 days |
Increased effects of IR ↓cell growth ↓cell survival ↑cell cycle arrest (S phase) ↓COX-1 activity |
[1] |
| HeLa cells | 50 & 75 µM 24 h |
↓PMA effects ↓MMP-9 mRNA, protein & activity ↓JNK ↓PKC δ ↓AP-1 ↓NFkB |
[2] |
| HeLa cervical cancer cells | 5, 25 & 50 µM 24–48 h |
↓cell growth Accumulation in the S phase of the cell cycle |
[3] |
| C-33A and HeLa Expressing HPV E6, E7 |
25, 50 &100 µM 16 h |
↓HIF-1a ↓VEGF |
[4] |
| hHeLa, Cx, SiHa and SKGIIIb cervical cancer cells |
100–400 µM 24–72 h |
↓cell growth ↑autophagy ↑apoptosis ↑LC3-II ↑LMP ↑Cat L ↑Cytochrome C ↑caspase-3 |
[5] |
2. Resveratrol Analogs against Cervical Cancer: In Vitro
RSV is a molecule with low bioavailability, and new analogs are being developed with the goal of increasing bioavailability as well as effectiveness while decreasing potential toxicity to healthy/normal tissues.
Kim et al. examined the effects of nine synthetic analogs of RSV (styryl quinazoline derivatives) and found that (E)-8-acetoxy2-[2-(3,4-diacetoxyphenyl) ethenyl]-quinazoline (8-ADEQ) was the most potent in inhibiting the proliferation of HeLa cells (Table 2) Further examination of the mechanism involved revealed induction of cell cycle arrest at the G2/M phase that was associated with increased cyclin B1 expression, cyclin-dependent protein kinase 1 (Cdk1), cell division cycle 25C (Cdc25C) and p53 phosphorylation, checkpoint kinases 1 (Chk1) and Chk2 activation as well as activation of the ataxia telangiectasia mutated (ATM)/ataxia telangiectasia-Rad3-related (ATR) kinases. Inhibition of the ATM/ATR pathway using the inhibitor caffeine, or a siRNA approach attenuated the 8-ADEQ-induced G2/M cell cycle arrest. These data indicate that the RSV analog 8-ADEQ activates the ATM/ATR-p53 pathway leading to the inhibition of cervical cancer cell proliferation [29][64].
Table 2. Effects of resveratrol analogs against cervical cancer: in vitro evidence.
| Cell | Analog Name | Resveratrol Concentration/Duration | Effect | Reference | ||||
|---|---|---|---|---|---|---|---|---|
| HeLa | 8-ADEQ | 8 µM 25 h |
↓proliferation ↑Cell cycle arrest at G2/M phase ↑cyclin B1 levels ↑Cdk1, Cdc25C phosphorylation ↑Chk1, Chk2 activation ↑ATM/ATR activation |
[29] | ||||
| HeLa | Pterostilbene | 0–400 µM 24–48 h |
↑Cell morphology ↓Cell growth ↑DNA fragmentation ↓Proliferation ↑Apoptosis ↓p-mTOR ↓p-PI3K ↓p-Akt |
[30] | ||||
| HeLa | N-(4-methoxyphenyl)-3,5-dimethoxybenamide (MPDB) | 35 µM 15 h |
↓Cell growth ↓Survival ↑Cell cycle arrest at G2/M phase ↓Proliferation ↑DNA fragmentation ↑Apoptosis ↑Cdc2 ↑Cdc25c ↑Chk1/2 ↑p53 ↓Bcl-xL ↑Fas ↑Caspases-3/-8/-9 ↑Cleaved PARP |
[31] | ||||
| TC1 | Pterostilbene | 20, 30 µM 48 h |
↓Cell viability ↑Cytotoxicity ↑Apoptosis ↓E6 |
[32] | ||||
| HeLa | Pterostilbene | 20 µM 24–48 h |
↓Cell growth ↓Survival ↓Metastasis ↑Cell cycle arrest at S and G2/M phase ↑p21/53 protein levels ↓Cyclin E1/B1 ↓Bcl-2 protein levels ↓Bcl-xL protein levels ↑Cleaved caspases-3/-9 ↓MMPs-2/-9 |
[33] | SKG-I SKG-II SKG-IIIa Nuz HeLa Cervical cancer cells |
10, 30 & 100 μΜ | ↑autophagy ↑apoptosis ↓drug resistance ↓ATAD3A ↑abrasion of the mitochondrial outer membrane ↑autophagosomes |
[6] |
| HeLa cervical cancer cells | 10, 30 & 100 μΜ 24 h |
↓invasion ↓metastasis ↓MMP9 levels-activity ↓NF κΒ ↓AP-1 |
[7] | |||||
| HeLa cervical cancer cells | 25 μΜ 24, 48 & 72 h |
↓cell proliferation ↑apoptosis ↑caspase-9 ↑caspase-3 ↓mitochondrial membrane potential -JC-1 in monomeric form ↓HDM2 |
[8] |
|||||
| C33A (with mutated p53) HeLa(HPV18positive) CaLo(HPV18positive) CaSki(HPV16positive) SiHa(HPV16 positive) |
150–250 µM 48 h |
↓proliferation ↑apoptosis ↓mitochondrial membrane potential ↑mitochondrial and lysosomal permeability ↑p53 levels ↓p65 NF κB levels |
[9] | |||||
| HeLa SiHa cervical cancer cells |
100 μΜ 12–48 h |
↑S-phase cell cycle arrest ↑apoptosis ↓p-STAT3 ↓Notch1/2 ↓Hes1 ↓Wnt2/5a ↓β-catenin ↑PIAS3 |
[10] | |||||
| HeLa cervical cancer cells | 10 & 100 μΜ 24 h |
↓cell viability ↓cell proliferation ↓cell survival ↑GRIM-19 ↓p-STAT3 ↓cyclin B1 ↓VEGF ↓Bcl-2 |
[11] | |||||
| HeLa SiHa C33A cervical cancer cells |
100 μΜ 12, 24, 36 & 48 h |
↓cell growth ↓proliferation ↑apoptosis ↓p-STAT3 ↓survivin ↓c-Myc ↓cyclin D1 ↓VEGF ↑SOCS3 ↑PIAS3 |
[12] | |||||
| cancer stem cells (CSC) from HeLa cultures (HeLa SP) | 137 μM 48–72 h |
↓cell viability ↑apoptosis ↓RAD51 |
[13] | |||||
| HeLa cervical cancer cells | 5–40 µM 24–48 h |
↓cell viability ↓cell migration ↑cell cycle arrest (S phase) ↓viral oncogene E6 ↑p53 levels |
[14] | |||||
| HeLa | 10–80 µM 12–36 h |
↓cell proliferation ↑cell cycle arrest at G1/S phase ↑p53 levels ↑apoptosis |
[15] | |||||
| HeLa | 10–40 µM 24–48 h |
↓cell viability ↓cell proliferation ↑apoptosis ↑caspase-3 ↑caspase-9 ↑Bax ↓Bcl-2 ↓Bcl-XL ↑p53 ↓Cyclin B1 |
[16] | |||||
| HeLa | 0–100 µM 24–96 h |
↓Cell growth ↓Cell viability ↓Proliferation ↓Phospholipid scramblase 1 |
[17] | |||||
| SiHa | 100 µM 24 h |
↓Cell viability ↑Cell cycle arrest in G2/M ↑Apoptosis ↓Survivin mRNA levels ↓Survivin protein levels ↑E-cadherin |
[18] | |||||
| HeLa | 2.5–150 µM 24–48 h |
↓Cell viability ↑Cytotoxicity ↑necrosis |
[19] | |||||
| HeLa | 0–80 µM 48 h |
↓Proliferation ↑Apoptosis ↓p-FOXO3a ↑FOXO3a ↑Bim ↓p-ERK |
[21] | |||||
| HeLa SiHa |
0–40 µM 24 h |
↓Proliferation ↓Wound healing ↓Migration/invasion ↓Metastasis ↑E-cadherin ↓N-cadherin ↓vimentin ↓MMP-3/13 protein levels ↓STAT3 protein levels |
[20] | |||||
| HeLa | 20 µM 24 h |
↓Cell viability ↑Cytotoxicity ↓Glucose uptake ↓NADH/NAD+ ratio ↓Lactate ↑Pyruvate |
[22] | |||||
| W12 | 0–100 µM | ↓Proliferation | [26] | |||||
| HeLa Ca Ski |
5–40 µM 24 h |
↓Proliferation ↑Cell cycle arrest in S phase ↑Apoptosis ↑p16/21/27 ↓CDK4 ↓E2F1 ↓p-pRb1 ↓Bcl-2 mRNA & protein levels ↓Bcl-xL mRNA levels ↑Bax protein levels ↑E6/7 ↑p53 |
[23] | |||||
| HT-3 | 0.16–1.25 µM 0–48 h |
↓Cell viability ↓Cell growth ↓Proliferation ↑Apoptosis |
[24] | |||||
| HeLa | 262.87 µM 24 h |
↓Cell viability ↑Apoptosis ↑mRNA caspases-3/-8/-9 levels ↑NCLX |
[25] | |||||
| HeLa | 20 µM 24 h |
↑Cell cycle arrest in S phase ↓Colony formation ↓EGFR |
[27] |
Overall, the in vitro studies described in the above section provide evidence that RSV has the potential to inhibit proliferation and survival and induce apoptosis of cervical cancer cells (Table 1). Evidence regarding the mechanisms involved in these anticancer effects of RSV reveals inhibition of EGFR, ERK, JNK, PKC, STAT3, and NF-kB. The levels of the tumor suppressor p53 and the pro-apoptotic cleaved caspases-3, -8, and -9 and PARP were increased, while the levels of the anti-apoptotic Bcl-2 and Bcl-XL were reduced. Furthermore, RSV increased LC3-II levels indicating induction of autophagy, while reduced MMP 3, 9, and 13 levels reveal a potential to reduce metastasis and invasion (Figure 12).
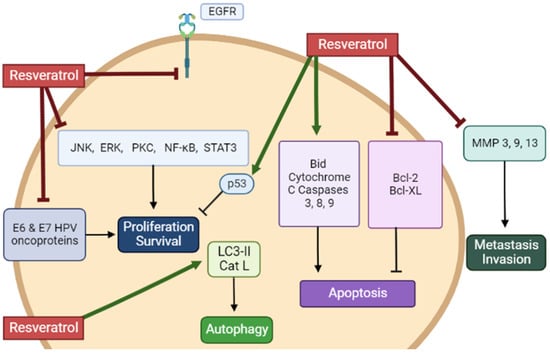
Figure 12. Summary of the effects of resveratrol in cervical cancer cells in vitro. RSV reduced proliferation and survival and induced apoptosis of cervical cancer cells. The figure, created using BioRender.com, is based on the data of the studies [1][2][3][4][5][6][7][8][9][10][11][12][13][14][15][16][17][18][19][20][21][22][23][
HeLa cells treated with the RSV analog pterostilbene had altered cell morphology, reduced proliferation, increased DNA fragmentation, and induced apoptosis, as indicated by the reduced mitochondrial membrane potential. Western blot analysis revealed a dose-dependent decrease in phosphorylation (activation) of mTOR and PI3K/Akt, which are implicated in cell proliferation and survival [30][65] (Table 2).
HeLa cells treated with N-(4-methoxyphenyl)-3,5-dimethoxybenamide (MPDB), an RSV analog, showed reduced proliferation and survival and cell cycle arrest at G2/M phase while p53 phosphorylation levels were elevated [31][66]. An increase in phosphorylation of Cdc2, Cdc25c, and Chk1/2 was seen. Treatment with MPDB reduced total and phosphorylated levels of the antiapoptotic protein Bcl-xL, increased cleavage of caspases-3, -8, and -9 and PARP, and increased phosphorylation of the Fas death receptor. The use of the caspase inhibitor, z-VAD-fmk (50 µM), abolished the MPDB-induced DNA fragmentation [31][66]. These data indicate that MPDB induced apoptosis by altering both the intrinsic and extrinsic pathways of apoptosis.
Both RSV and pterostilbene showed a significant decrease in TC1 cell viability. However, pterostilbene was found to be more potent, with an IC50 value of 15.61 µM compared to the parent RSV compound, with an IC50 value of 34.46 µM. Immunocytochemical analysis revealed a significant downregulation of E6 oncoprotein with both RSV and pterostilbene treatment of TC1 cells [32][67].
3. Resveratrol against Cervical Cancer: In Vivo Animal Studies
BALB/C nude mice were subcutaneously injected with HeLa cells and were treated with RSV (10 mg/kg of body weight) daily for 28 days. RSV treatment resulted in a significant reduction in tumor weight [17][52]. Unfortunately, no other measurements or tumor analysis was performed in this researchtudy.
Female C57BL/6 mice were subcutaneously injected with TC-1 cells (expressing the oncogenes: HPV16-E6, -E7, and H-Ras) in the nape. After tumors were well established (15 to 20 days following cancer cell injection), tumors were sectioned into four quadrants, and each quadrant was treated with RSV (10 μL of 1 mM stock solution, a total of 40 μL injected intralesionally) for 5 consecutive days. Although the concentration of RSV within the tumor was not measured, it was expected to be above the lethal dose of 80 µM. This treatment resulted in significantly smaller tumor size and volume compared to animals treated with vehicle control (PBS). Immunohistochemistry of the tumor tissue revealed a significant reduction in E6 oncoprotein levels, vascular endothelial growth factor (VEGF), and proliferating cellular nuclear antigen (PCNA) of 79%, 60.5%, and 75.5%, respectively. Taken together, these data indicate that direct treatment of tumors with RSV resulted in significant tumor shrinkage that was due to inhibition of proliferation and cell cycle arrest associated with a reduction in E6 protein levels [32][67].
Athymic BALB/C nude mice were divided into an RSV pre-treatment and treatment group [20][56]. The pretreated group was administered intragastrically with 30 mg/kg of RSV 3 times per week for two weeks before being subcutaneously injected with HeLa cells (5 × 106 cells), then treated with RSV for an additional 3 weeks. The treated group was administered intragastrically with RSV (30 mg/kg, 3 times/week) 10 days after HeLa cell injection for a total of 5 weeks. The data from the RSV pre-treatment and treatment groups illustrated a significant decrease in tumor size, weight, and volume. Western blot analysis of the tumor samples showed a significant reduction in STAT3 phosphorylation, b-catenin, vimentin, MMP-3, and MMP-13, levels. IHC also revealed it decreased in p-STAT3, N-cadherin, and vimentin and increased E-cadherin [20][56]. Unfortunately, with this experimental design of the researchtudy, it is not clear what is the effect of the RSV pretreatment alone. The researchers should have included another group of animals that received cervical cancer cells pretreated with RSV and not treated with RSV for the rest of the researchstudy.
Antitumor effects were also witnessed in another researchtudy conducted by Hao et al. BALB/C female nude mice subcutaneously injected with 2 × 106 HeLa cells and 1 week later, were orally treated with 30 mg/kg RSV, three times per week, for 3 weeks. This treatment resulted in decreased tumor volume and weight compared to the control. mRNA and protein levels of both E6 and E7 were found to be reduced, while protein levels (measured by western blotting and immunostaining) of tumor suppressors p53 and Rb1 were significantly increased in tumor tissues [34][69].
Nude Balb/C female mice subcutaneously injected with HeLa cells were intragastrically administered 15 mg/kg of RSV three times a week for 5 consecutive weeks. With this treatment course, tumor weight and volume decreased significantly. Western blot analysis of the tumor tissue revealed a decrease in E6 and E7, and p-pRb1 with an increase in the tumor suppressor p53 [23][59].
Although the above-described in vivo animal studies show that administration of RSV in mice xenografted with cervical cancer cells resulted in a significant reduction in tumor volume and weight compared to non-treated mice (Figure 23), unfortunately, none of the studies measured blood RSV levels. The absorption of RSV through the gastrointestinal tract is low, and this results in low bioavailability [35][70]. In addition, RSV is rapidly metabolized and eliminated by systemic circulation, further contributing to its low bioavailability [35][70]. Intragastric administration of RSV (472 mg) in pigs, followed by an examination of RSV and its metabolites, showed dihydro-resveratrol (DHR) and RSV-3-O-glucuronide as the main metabolites [36][71]. A recent study has found that oral (in the diet) administration of RSV in mice for 4 weeks (human equivalent dose of 4.6 mg/kg/day) resulted in the detection by HPLC-MS/MS of 11 RSV metabolites in tissues (bile, kidney, liver) and plasma that included dihydro-resveratrol (DHR), lunularin (LUN), and sulfate and glucuronide conjugates of RSV [37][72]. In plasma DHR, LUN, and RSV conjugates of 2–7 µM were found. Importantly, the evidence indicates that DHR and LUN are gut bacteria-derived metabolites of RSV, as these metabolites were not detected in antibiotic-treated mice. Furthermore, DHR and LUN, at the concentrations found in mouse tissues, had stronger anti-inflammatory and anti-cancer effects than RSV. This study provides evidence of the important role of gut microbiota on RSV metabolism. Importantly, the gut microbiota-generated RSV metabolites appear to significantly contribute to the biological effects of RSV in vivo.
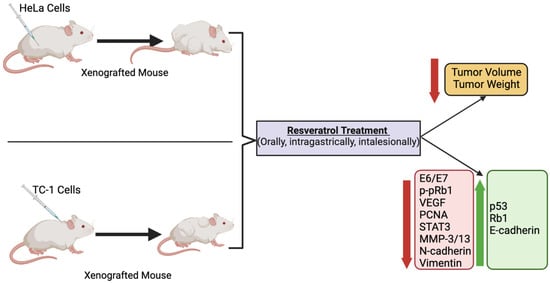
Figure 23. Summary of the effects of resveratrol in cervical cancer in animals. Treatment of animals (mice) xenografted with cervical cancer cells with RSV resulted in a significant reduction in tumor volume and weight compared to non-treated mice. The figure, created using BioRender.com, is based on the data of the studies [17][20][23][32][34][52,56,59,67,69].
