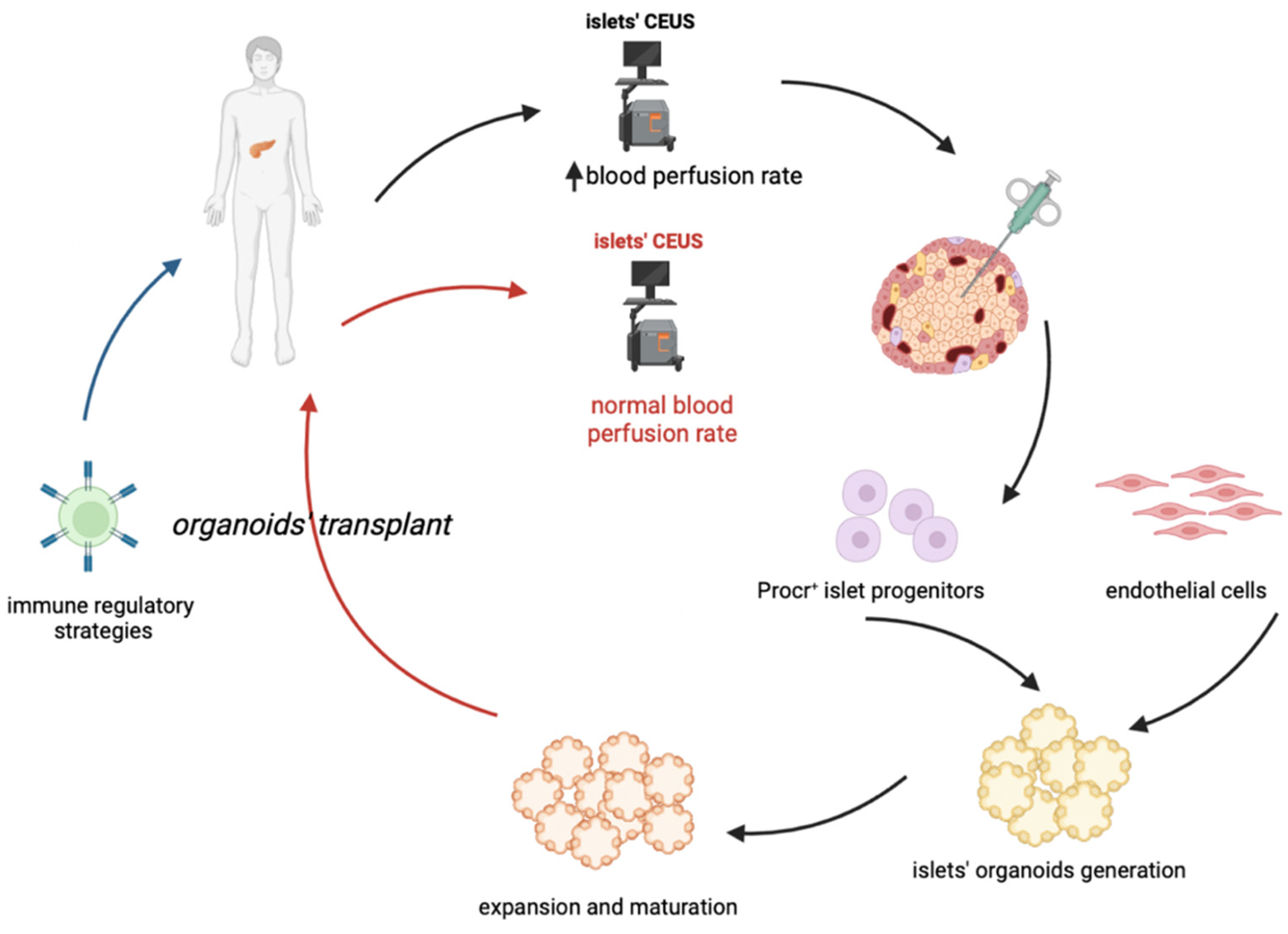
Type 1 diabetes (T1D) is an autoimmune disease with immune cells’ islet infiltration (called “insulitis”), which leads to beta cell loss. Despite being the critical element of T1D occurrence and pathogenesis, insulitis is often present in a limited percentage of islets, also at diagnosis. Therefore, it is needed to define reproducible methods to detect insulitis and beta-cell decline, to allow accurate and early diagnosis and to monitor therapy. This goal is still far due to the morphological aspect of islet microvasculature, which is rather dense and rich, and is considerably rearranged during insulitis. More studies on microvasculature are required to understand if contrast-enhanced ultrasound sonography measurements of pancreatic blood-flow dynamics may provide a clinically deployable predictive marker to predict disease progression and therapeutic reversal in pre-symptomatic T1D patients. It is needed to clarify the relation between insulitis and the dynamics of β cell loss and with coexisting mechanisms of dysfunction, according to clinical stage, as well as the micro vessels’ dynamics and microvasculature reorganization. The ideal cell-based therapy of T1D should start from an early diagnosis allowing a sufficient isolation of specific Procr+ progenitors, followed by the generation and expansion of islet organoids, which could be transplanted coupled to an immune-regulatory therapy which will permit the maintenance of pancreatic islets and an effective and long-lasting insulitis reversal.
- insulitis
- stem cells
- type 1 diabetes
1. Insulitis in Human Type 1 Diabetes
2. Stem Cell Grafting
3. Cell Therapy and the Organoid Perspective
References
- Pugliese, A. Advances in the etiology and mechanisms of type 1 diabetes. Discov. Med. 2014, 18, 141–150.
- Krogvold, L.; Wiberg, A.; Edwin, B.; Buanes, T.; Jahnsen, F.L.; Hanssen, K.F.; Larsson, E.; Korsgren, O.; Skog, O.; Dahl-Jørgensen, K. Insulitis and characterisation of infiltrating T cells in surgical pancreatic tail resections from patients at onset of type 1 diabetes. Diabetologia 2016, 59, 492–501.
- Campbell-Thompson, M.; Fu, A.; Kaddis, J.S.; Wasserfall, C.; Schatz, D.A.; Pugliese, A.; Atkinson, M.A. Insulitis and β-Cell Mass in the Natural History of Type 1 Diabetes. Diabetes 2016, 65, 719–731.
- Krogvold, L.; Skog, O.; Sundström, G.; Edwin, B.; Buanes, T.; Hanssen, K.F.; Ludvigsson, J.; Grabherr, M.; Korsgren, O.; Dahl-Jørgensen, K. Function of Isolated Pancreatic Islets From Patients at Onset of Type 1 Diabetes: Insulin Secretion Can Be Restored After Some Days in a Nondiabetogenic Environment In Vitro. Diabetes 2015, 64, 2506–2512.
- Keenan, H.A.; Sun, J.K.; Levine, J.; Doria, A.; Aiello, L.P.; Eisenbarth, G.; Bonner-Weir, S.; King, G.L. Residual Insulin Production and Pancreatic β-Cell Turnover After 50 Years of Diabetes: Joslin Medalist Study. Diabetes 2010, 59, 2846–2853.
- Coppieters, K.T.; Wiberg, A.; Amirian, N.; Kay, T.W.; von Herrath, M.G. Persistent glucose transporter expression on pancreatic beta cells from longstanding type 1 diabetic individuals. Diabetes Metab. Res. Rev. 2011, 27, 746–754.
- Gianani, R.; Campbell-Thompson, M.; Sarkar, S.A.; Wasserfall, C.; Pugliese, A.; Solis, J.M.; Kent, S.C.; Hering, B.J.; West, E.; Steck, A.; et al. Dimorphic histopathology of long-standing childhood-onset diabetes. Diabetologia 2010, 53, 690–698.
- Morgan, N.G.; Leete, P.; Foulis, A.K.; Richardson, S.J. Islet inflammation in human type 1 diabetes mellitus. IUBMB Life 2014, 66, 723–734.
- Nishimura, A.; Matsumura, K.; Kikuno, S.; Nagasawa, K.; Okubo, M.; Mori, Y.; Kobayashi, T. Slowly Progressive Type 1 Diabetes Mellitus: Current Knowledge And Future Perspectives. Diabetes Metab. Syndr. Obes. 2019, 12, 2461–2477.
- Muralidharan, C.; Linnemann, A.K. β-Cell autophagy in the pathogenesis of type 1 diabetes. Am. J. Physiol. Metab. 2021, 321, E410–E416.
- Wan, X.X.; Zhang, D.Y.; Khan, M.A.; Zheng, S.Y.; Hu, X.M.; Zhang, Q.; Yang, R.H.; Xiong, K. Stem Cell Transplantation in the Treatment of Type 1 Diabetes Mellitus: From Insulin Replacement to Beta-Cell Replacement. Front. Endocrinol. (Lausanne) 2022, 13, 859638.
- Pixley, J.S. Mesenchymal stem cells to treat type 1 diabetes. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165315.
- Cuesta-Gomez, N.; Graham, G.J.; Campbell, J.D.M. Chemokines and their receptors: Predictors of the therapeutic potential of mesenchymal stromal cells. J. Transl. Med. 2021, 19, 156.
- Burke, G.W.; Vendrame, F.; Virdi, S.K.; Ciancio, G.; Chen, L.; Ruiz, P.; Messinger, S.; Reijonen, H.K.; Pugliese, A. Lessons From Pancreas Transplantation in Type 1 Diabetes: Recurrence of Islet Autoimmunity. Curr. Diab. Rep. 2015, 15, 121.
- Chhabra, P.; Brayman, K.L. Stem cell therapy to cure type 1 diabetes: From hype to hope. Stem Cells Transl. Med. 2013, 2, 328–336.
- Wu, S.; Wang, L.; Fang, Y.; Huang, H.; You, X.; Wu, J. Advances in Encapsulation and Delivery Strategies for Islet Transplantation. Adv. Healthc. Mater. 2021, 10, e2100965.
- Yang, S.J.; Singh, A.K.; Drow, T.; Tappen, T.; Honaker, Y.; Barahmand-Pour-Whitman, F.; Linsley, P.S.; Cerosaletti, K.; Mauk, K.; Xiang, Y.; et al. Pancreatic islet-specific engineered Tregs exhibit robust antigen-specific and bystander immune suppression in type 1 diabetes models. Sci. Transl. Med. 2022, 14, eabn1716.
- Marfil-Garza, B.A.; Imes, S.; Verhoeff, K.; Hefler, J.; Lam, A.; Dajani, K.; Anderson, B.; O’Gorman, D.; Kin, T.; Bigam, D.; et al. Pancreatic islet transplantation in type 1 diabetes: 20-year experience from a single-centre cohort in Canada. Lancet Diabetes Endocrinol. 2022, 10, 519–532.
- Loretelli, C.; Assi, E.; Seelam, A.J.; Ben Nasr, M.; Fiorina, P. Cell therapy for type 1 diabetes. Expert Opin. Biol. Ther. 2020, 20, 887–897.
- Hwang, G.; Jeong, H.; Yang, H.K.; Kim, H.S.; Hong, H.; Kim, N.J.; Oh, I.H.; Yim, H.W. Efficacies of Stem Cell Therapies for Functional Improvement of the β Cell in Patients with Diabetes: A Systematic Review of Controlled Clinical Trials. Int. J. Stem Cells 2019, 12, 195–205.
- Cuesta-Gomez, N.; Verhoeff, K.; Jasra, I.T.; Pawlick, R.; Dadheech, N.; Shapiro, A.M.J. Characterization of stem-cell-derived islets during differentiation and after implantation. Cell Rep. 2022, 40, 111238.
- Verhoeff, K.; Cuesta-Gomez, N.; Jasra, I.; Marfil-Garza, B.; Dadheech, N.; Shapiro, A.M.J. Optimizing Generation of Stem Cell-Derived Islet Cells. Stem Cell Rev. Rep. 2022, 18, 2683–2698.
- Dolgin, E. Diabetes cell therapies take evasive action. Nat. Biotechnol. 2022, 40, 291–295.
- From the American Association of Neurological Surgeons (AANS); American Society of Neuroradiology (ASNR); Cardiovascular and Interventional Radiology Society of Europe (CIRSE); Canadian Interventional Radiology Association (CIRA); Congress of Neurological Surgeons (CNS); European Society of Minimally Invasive Neurological Therapy (ESMINT); European Society of Neuroradiology (ESNR); European Stroke Organization (ESO); Society for Cardiovascular Angiography and Interventions (SCAI); Society of Interventional Radiology (SIR); et al. Multisociety Consensus Quality Improvement Revised Consensus Statement for Endovascular Therapy of Acute Ischemic Stroke. Int. J. Stroke 2018, 13, 612–632.
- Chellappan, D.K.; Sivam, N.S.; Teoh, K.X.; Leong, W.P.; Fui, T.Z.; Chooi, K.; Khoo, N.; Yi, F.J.; Chellian, J.; Cheng, L.L.; et al. Gene therapy and type 1 diabetes mellitus. Biomed. Pharmacother. 2018, 108, 1188–1200.
- Wang, D.; Wang, J.; Bai, L.; Pan, H.; Feng, H.; Clevers, H.; Zeng, Y.A. Long-Term Expansion of Pancreatic Islet Organoids from Resident Procr+ Progenitors. Cell 2020, 180, 1198–1211.
