You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 2 by Amina Yu and Version 1 by Arrigo Cicero.
Bioactive peptides (BPs) are a heterogeneous class of molecules found in a wide range of plant and animal sources. The first bioactive peptide was identified circa 1900 by Mellander, who isolated BPs from casein and demonstrated its ability to improve bone calcification in rachitic children. BPs can be defined as peptides between 2 and 20 amino acids able to modulate physiological functions. In general, BP consist of an inactive precursor molecule that becomes active after release of the active site by enzymatic or chemical hydrolysis in the gastrointestinal tract, thus allowing BP to be absorbed through specific peptide transporters. Thus, BP can be classified into exogenous and endogenous molecules, obtained via gastrointestinal digestion or artificially, respectively.
- bioactive peptides
- nutraceuticals
- food supplements
- functional foods
1. New Sources
1.1. From Production to Commercialisation
Bioactive P eptides (BPs) can be obtained using several methods, which generally include the extraction and the isolation of proteins from a food source, followed by the isolation of a specific protein isolate that undergoes in vitro or in vivo enzymatic hydrolysis. Different enzymes can be used to generate short-chain peptides. Once the protein hydrolysate is obtained, peptides can be separated and purified, and their bioactivities assayed. Subsequently, the peptide fraction of interest is analyzed to determine the amino acid sequence. Once the peptide sequence is known, BP can be synthetized and studied to determine bioavailability and bioactivity before proceeding with clinical trials (Figure 1) [38][1]. As a recent application, that shows how the in silico methodologies coupled to in vitro tests may be useful for the identification of potentially bioactive peptides, this study methods has been applied to the evaluation of the overall nutritional value of the Parma ham [39][2].
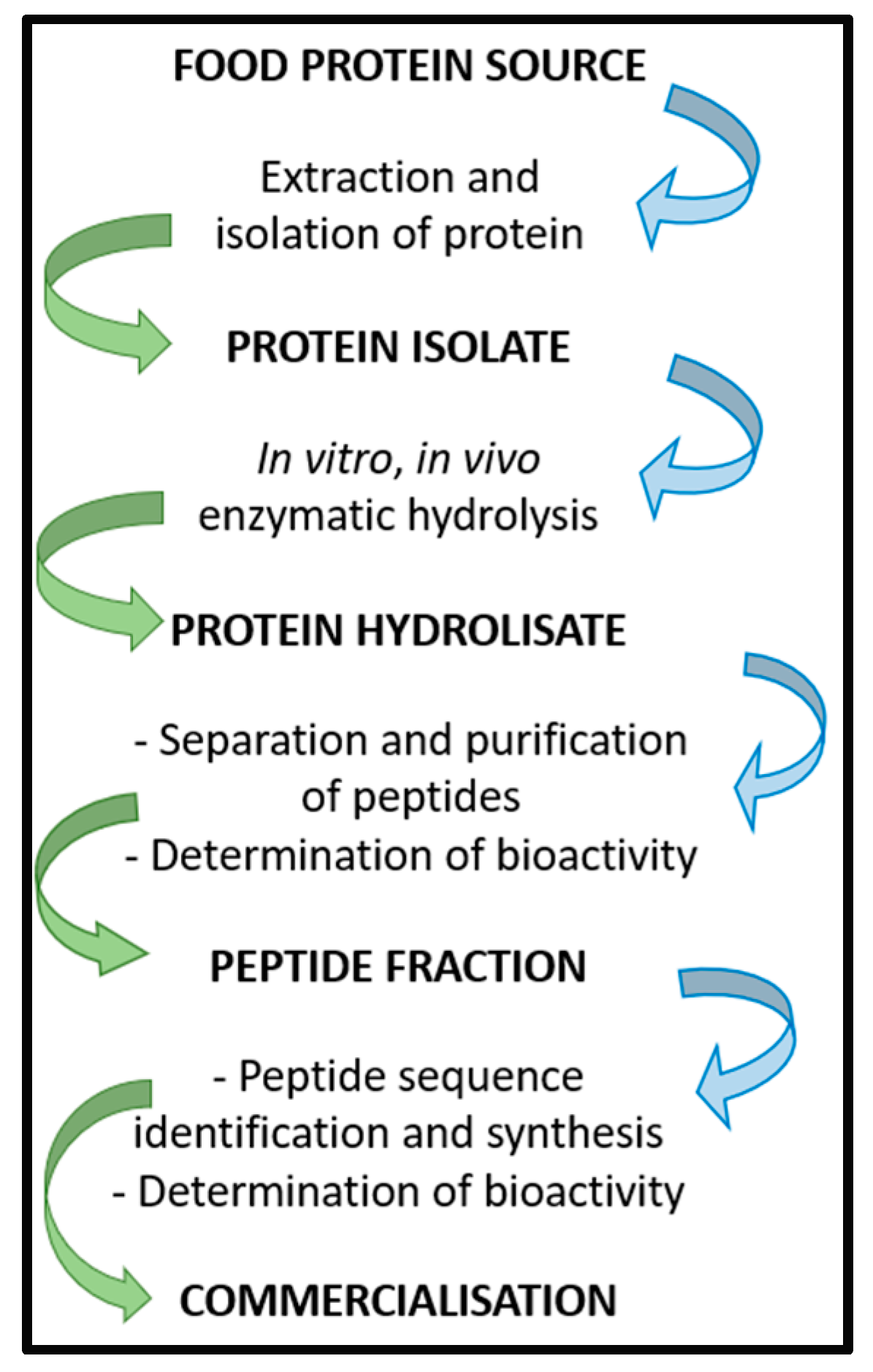
Figure 1.
Scheme of BP isolation, preparation, and commercialization.
Enzymatic hydrolysis, microbial fermentation, and chemical hydrolysis are the three main methods of BP production. Chemical hydrolysis consists of the formation of peptide hydrolysates using an acid or base at high temperatures resulting in the peptide cleavage. However, though this technique yields robust hydrolysis, it is nonspecific, poorly reproducible, and leads to amino acid denaturation [40][3]. Enzymatic digestion produces peptides from soy, corn, potato, peanut, milk, whey, egg, and meat proteins with target functionalities including antioxidant, anti-inflammatory, anti-hypertensive, anti-diabetic, anti-microbial, and anti-cancer activities. The efficacy of protein hydrolysates depends on several factors such as the protein substrate pre-treatment, type of proteases used, and the hydrolysis conditions applied [41][4]. Microbial fermentation produces BP from a variety of sources with a variety of bioactivities. BP produced by microbial fermentation can differ in type, amount and activity depending on the cultures used [42][5]. Recently, the fermentation of milk proteins with specific microbial strains (e.g., Lactobacillus bulgaricus, Lactobacillus helveticus MB2-1, Lactobacillus plantarum B1-6 and 70810) has produced anti-hypertensive and lipid-lowering peptides [43][6].
BP with antihypertensive, antioxidant, and antidiabetic activities account for the majority of peptides in the Biopep database, but antimicrobial, antiproliferative, and lipid-lowering activities have also been described [44][7]. The main limiting factor in the generation of BP is not at the level of raw materials, but the technological processes and the difficulty in efficiently generating a specific BP sequence without altering its functionalities or bioaccessibility [44][7]. New separation and purification techniques (e.g., ultrafiltration and/or advanced chromatography techniques) have been developed to overcome these problems [45][8].
1.2. Stability and Bioavailability
Once a peptide is ingested, it faces physicochemical environments that can negatively influence its stability and bioactivity. The bioactivities of BP are highly variable and depend on the degree of hydrolysis during the isolation processes, the gastrointestinal environment, BP size, and peptide hydrophobicity [46][9]. In addition, the food matrix could interact negatively or positively with the chemical structure of BP, modifying both the stability and bioavailability [40][3]. BP can undergo chemical hydrolysis in the stomach where either acidic conditions or gastric enzymes interact and hydrolyze proteins and peptides. Additionally, pancreatic enzymes, proteases from the microbiota, and the drastic change in pH (from ~2 of the stomach to 7 of the large intestine) can influence the hydrolysis of BP in the gastrointestinal (GI) tract [47][10]. BP can also be degraded at the brush border, in the cytosol of enterocytes, or even within lysosomes and other cellular organelles [48][11]. Thus, in vitro studies using simulated gastrointestinal digestion systems are needed to investigate the stability and bioaccessibility of many peptides from food proteins [49][12]. However, observations from in vitro studies might not be reflective of in vivo conditions, especially for BP with low bioavailability, which is strongly influenced by the absorption and susceptibility to physiological enzymes that breakdown the peptides into inactive fragments [50][13]. For example, the peptides MAP1 and MAP2 derived from milk proteins demonstrated in vitro ACE inhibitory activity, but only MAP1 has an acceptable stability and can reach the desired cellular sites of action and act as an anti-hypertensive peptide in vivo [51][14].
2. Applications
2.1. Anti-Inflammatory Activity
Bioactive peptides from plant and animal sources have known anti-inflammatory activities (Figure 32). However, the mechanisms of action are mostly unknown and only a few of these peptides have been investigated with both in vitro and in vivo studies [64][15]. A study by Majumder et al. suggests that BP are anti-inflammatory because they cause transcriptional downregulation of inflammatory kinases such as NF-kB and MAPK. It is unknown whether BP act directly on cell membranes or by engaging cell receptors, but it is likely that both mechanisms play a role depending on the specific peptide [65][16]. Other suggested mechanisms of action include the inhibition of the pro-inflammatory JNK-MAPK pathway, reducing the formation of atherosclerotic plaques (obtained with IPP and VPP peptides) [66][17] and the modulation of the expression of intestinal chemokines and cytokines (obtained with beans, milk, and soy peptides) [67][18]. Several BP act through different pathways. Lunasin inhibits IL-6, IL-1β PGE2 production, the expression of COX-2 and inducible NOS, as well as the activation of NF-kB via the Akt-mediated route [68][19]. The polypeptide DMPIQAFLLYQEPVLGPVR derived from β-casein and a tripeptide derived from ovotransferrin from egg albumin, inhibit the NF-kB pathway, reducing the transcription of vascular cell adhesion molecule 1 (VCAM1) and intracellular adhesion molecule 1 (ICAM-1) [24,69][20][21]. The ɣ-glutamyl cysteine peptide extracted from beans inhibits JNK and IkB phosphorylation, while valyl-prolyl-tyrosine (VPY) reduces IL-8 and TNF-α secretion [64][15]. An interesting anti-inflammatory peptide has been extracted from milk fermented with Lactobacillus plantarum strains resulting in a BP with effects comparable to sodium diclofenac in preclinical studies [68][19]. BP derived from milk reduce postprandial inflammation in obese people as shown by reduced plasma monocyte chemoattractant protein-1 (MCP-1) and chemokine ligand 5 (CCL5) concentrations [70][22].
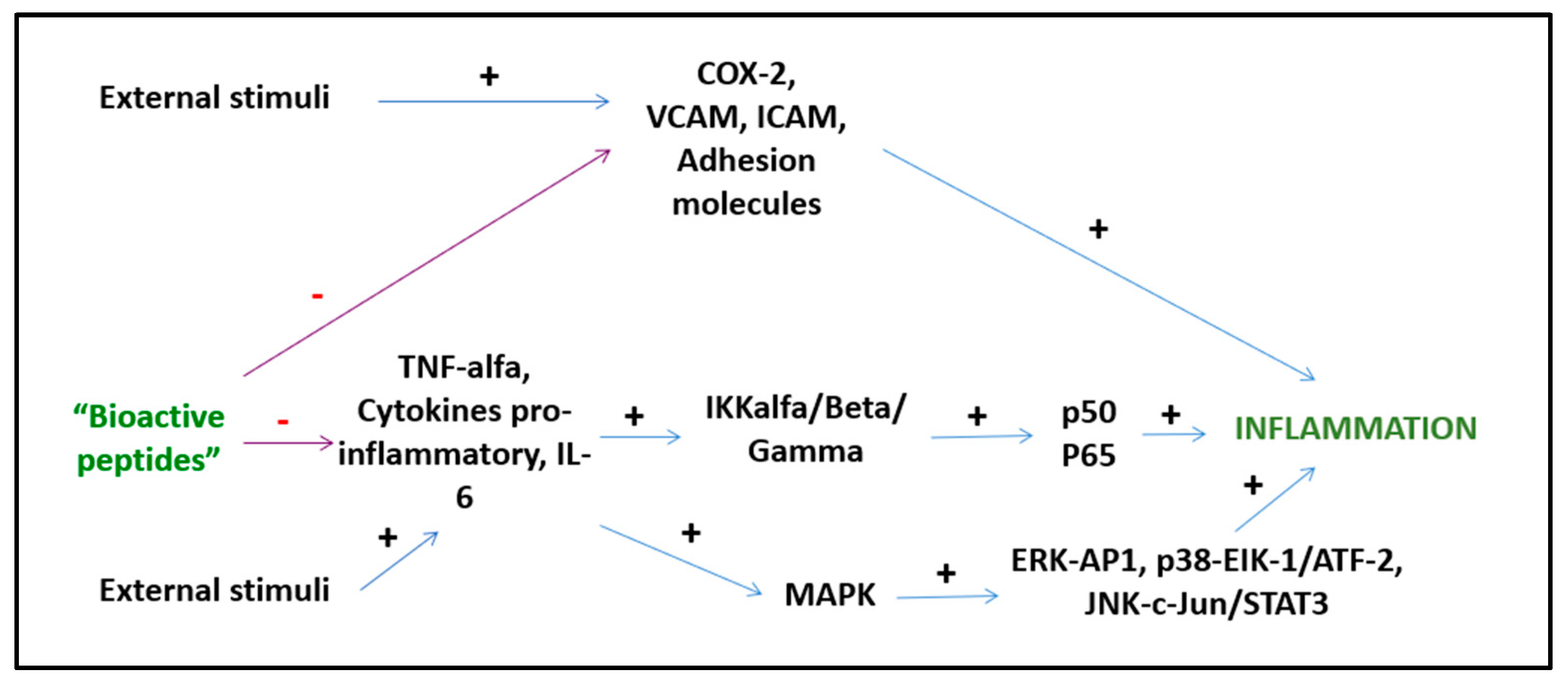
Figure 32.
Anti-inflammatory activities of BPs.
Although preliminary data suggest an interesting anti-inflammatory activity of BP related to the modulation of transcription factors and the inhibition of the expression of pro-inflammatory cytokines and chemokines, data on humans are still lacking and long-term RCTs are urgent to investigate and consolidate these results.
2.2. Anti-Hypertensive Activity
Hypertension is one of the most important cardiovascular risk factors and data indicate that the lifetime risk of developing hypertension is a staggering 90%. The estimated global burden of hypertension will increase to 1.56 billion afflicted individuals by 2025 [70][22]. Hypertension accounts for about 7.6 million premature deaths and 92 million DAILYs (disability-adjusted life-years: 1 DAILY = 1 lost year of healthy life) [71][23]. In thHereis contextn, the European guidelines for hypertension include the nutraceutical approach for both pre-hypertensive subjects with borderline blood pressure values and hypertensive patients in combination with conventional treatments [72,73][24][25].
Numerous bioactive peptides from different sources (milk, fish, plants, and meat) have demonstrated anti-hypertensive activities. They act through several pathways, such as inhibiting the renin-angiotensin system, increasing the activity of certain vasodilating agents (nitric oxide), or reducing the activity of the sympathetic nervous system [74][26]. The most common target of BP lowering molecules is the renin-angiotensin system, with the specific inhibition of renin or angiotensin converting enzyme (ACE) resulting in reduced levels of angiotensin I and angiotensin II [75][27]. One of the richest sources of anti-hypertensive peptides is milk. Milk is particularly rich in tripeptides (valyl-prolyl-proline (VPP) and isoleucyl-prolyl-proline (IPP)) and polypeptides (e.g., FFVAPFPEVFGK and YLGYLEQLLR) [24][20]. Numerous RCTs have investigated the effects of milk BPs on blood pressure. In a meta-analysis of 18 RCTs, the lactotripeptides (LTP) IPP and VPP (dosages from 5 to 100 mg/day) reduced systolic blood pressure −3.73 mmHg (95% CI: −6.70, −1.76) and diastolic blood pressure −1.97 mmHg (95%CI: −3.85, −0.64) [76][28]. Interestingly, these effects were more evident in Asian people, suggesting a possible genetic/population-dependent effect. LTP also improves arterial stiffness, measured as pulse wave velocity, in mildly hypertensive subjects [77][29].
The enzymatic or pepsin hydrolysis of whey proteins generates several BP with anti-hypertensive activities. The decapeptide DRVYIHPFHL, octapeptide DRVYIHPF and heptapeptide RVYIHPF inhibit the renin-angiotensin-aldosterone (RAS) system [19][30]. Casein proteins and BP from cow’s milk whey demonstrate significant blood pressure lowering activities in pre-hypertensive and hypertensive subjects [78][31]. The enzymatic hydrolysis of whey proteins also produces the ACE-inhibitor peptides α-lactalbumin and β-lactoglobulin, and lactorphins, which lower the blood pressure by normalizing the endothelial function [79][32].
Other anti-hypertensive tripeptides (LKP, IKP, LRP) are extracted from fish (e.g., bonito, tuna, sardine). These BP increase the endothelial NO levels and aorta vasodilatation in rats [80][33]. The peptides MVGSAPGVL and LGPLGHQ (from Okamejei kenojei), and AHIII (from Styela clava) exhibit anti-hypertensive activity in pre-clinical studies.
BP extracted from numerous plant species (e.g., soy, barley, oak, pea) appear to reduce blood pressure through several mechanisms; however, it is not always possible to discriminate between the effects of plant proteins and other plant components that could contribute to the antihypertensive effects [81][34].
Therefore, BP derived from both plant and animal sources can moderately reduce blood pressure in humans. However, long-term RCTs, especially in normotensive and pre-hypertensive patients, are needed.
2.3. Lipid-Lowering Activity
Elevated plasma concentrations of total cholesterol (TC) and LDL cholesterol (LDL-C) and, under certain conditions, low concentrations of HDL cholesterol (HDL-C) are among the main modifiable risk factors for cardiovascular diseases [82][35]. An examination of data from over 18,000 individuals aged >20 years who participated in national health and nutrition surveys in the United States from 1999 to 2006 demonstrated that the unadjusted prevalence of hypercholesterolemia varies from 53.2% to 56.1%. Indeed, a recent report from the American Heart Association (AHA) has confirmed that in the United States, only 75.7% of children and 46.6% of adults have targeted TC levels (TC < 170 mg/dL for children and <200 mg/dL for adults, in subjects not treated pharmacologically) [83][36]. These percentages are comparable with other western countries [84,85][37][38].
BP from soy, lupine, and milk proteins are in the lipid-lowering nutraceuticals class. A meta-analysis of 35 RCTs investigated the effects of soy protein (B-conglycinin globulin) on cholesterolaemia. The results demonstrated the reduction in LDL-C of 3% (−4.83 mg/L; 95% CI: −7.34, −2.31), in TC of 2% (−5.33 mg/L; 95% CI: −8.35, −2.30) and in triacylglycerol of 4% (−4.92 mg/L; 95% CI: −7.79, −2.04). The period of treatment was from 4 weeks to 1 year and the lipid-lowering effects was greater in moderately hypercholesterolaemic patients (−7.47 mg/L; 95% CI: −11.79, −3.16) compared with healthy people (−2.96 mg/L; 95%CI: −5.28, −0.65) [86][39]. BP likely improve the lipid profile by acting as hydroxymethylglutaril-CoA (HMG-CoA) reductase inhibitors, up-regulators of LDL receptors, regulators of the sterol regulatory element-binding protein 2 (SREBP2) pathway, or by stimulating the faecal excretion of bile salts [87][40]. The hydrolysate extract of Mucuna pruriens and BP from cowpea reduced LDL-C and TC by affecting micelle formation and exogenous cholesterol absorption [88,89][41][42]. The lunasin peptide extracted from soy has demonstrated lipid-lowering activities in animal models [90][43].
Therefore, several lipid-lowering peptides can improve the lipid profiles of mildly dyslipidaemic patients. However, evaluation of both pharmacodynamic and pharmacokinetic profiles and the long-term effective dosages in humans are necessary.
2.4. Anti-Cancer Activity
One of the most important areas of research for bioactive peptides concerns their role as anti-cancer agents. BP from plants, milk, egg, and marine organisms possess cytotoxic activity against numerous cancer cell lines. These BP have the advantages of low-toxicity, and high tissue penetration, cell diffusion, and permeability [91][44]. BP can use different mechanisms to inhibit cell migration, affect gene transcription/cell proliferation, inhibit tumor angiogenesis, or alter cancer cell tubulin structures [92][45].
The BP lunasin has anticancer activity when tested against breast, skin, colon, prostate, leukaemia, and lymphoma cell lines [93][46]. Lunasin suppresses the transformation of cells by chemical carcinogens (in mouse fibroblast, and human breast cancer MCF-7), induces cell cycle arrest in G2/M phase and apoptosis through the activation of caspase-3 (in L1210 leukaemic and human colon adenocarcinoma cells) and inhibits the metastasis of human colon cancer cells [94][47]. In vivo studies, especially in mice models, demonstrated the reduction of lymphoma volume and liver metastasis of colon cancer by lunasin [95][48].
The peptide Glutammyl-Glycyl-Argininyl-Prolyl-Arginine from rice, at the dose of 600–700 μg/mL, inhibits the growth of colon cancer cells (Caco-2 and human colorectal adenocarcinoma cell line, HCT-116) by 84%, breast cancer cells (MCF-7, MDA-MB-231) by 80%, and liver cancer cells by 84% (HepG-2) [96][49].
Other rich sources of anticancer BP are the legume seeds that contain protease inhibitors, such as the Bowman-Birk inhibitor that has an inhibitory effect against prostate, breast, and colon cancers in vitro [97][50]. The Bowman-Birk inhibitor has Food and Drug Administration (FDA) approval, especially for people with oral leukoplakia or benign prostatic hyperplasia [98][51]. Plant-derived lectins (from tepary bean and mistletoe) have cytotoxic effects on the cervical carcinoma cell line C33-A and human colon carcinoma cell line, the Sw480 [99][52].
Among the BP of animal origin, milk lactoferrin inhibits the growth of breast cancer (MDA-MB-231) and nasopharyngeal carcinoma cells in vitro and in vivo by arresting the cell cycle at the G1/S transition, or suppressing Akt signaling, respectively [100][53]. It also induces cell apoptosis, modulates gene expression and reduces angiogenesis [101][54]. Hydrolysates of the egg proteins lysozyme and ovomucin inhibit tumor cell proliferation, improving the effectiveness of chemotherapy (colorectal cancer; B16 melanoma) [102][55]. Many marine peptides (e.g., from tuna, sponges, squid) show antiproliferative activity against different cancer types in vitro, but these results have not been confirmed in vivo [103][56].
Thus, several BP demonstrate potential as anti-cancer peptides, with demonstrated cytotoxic and anti-tumoural activity in vitro and in animal models. However, controlled human clinical trials are still needed to evaluate the true therapeutic efficacy of this class of compounds.
2.5. Immunomodulatory Activity
BP can also modulate immune responses. Studies in vitro and in vivo demonstrated that αS1-casein and β-casein stimulated phagocytes, B lymphocyte IgG production, and T cell proliferation [24][20]. Peptides from fish have immunomodulatory activities, especially enhancing macrophage and natural killer cells activity, and lymphocyte proliferation (e.g., BP from Chum Salmon or from Atlantic cod) [104][57]. BP can also regulate immunity by modulating the gut-associated immunity affecting phagocytic cell activity and IgA-secreting cells in the small intestine lamina propria [105][58]. Studies in mice demonstrated that oyster hydrolysates can enhance lymphocyte proliferation, natural killer cell activity, and macrophage phagocytosis [106][59]. Similar results were observed with tryptic hydrolysates of soybean and rice proteins [107][60].
Thus, BP exhibit immunomodulatory effects on both innate and adaptive immunity. RCTs are clearly needed to determine the efficacy and the safety profile of these molecules.
2.6. Other Biological Activities
BP from wheat gliadin, soy proteins, egg-yolk proteins, porcine myofibrillar proteins, pea, and aquatic by-product proteins are well known to possess antioxidant properties and to protect against the oxidative stress typical of major chronic diseases [108,109][61][62].
BP antioxidant activity can be a result of the metal ion chelation that inhibits enzymatic and non-enzymatic peroxidation of lipids and essential fatty acids. BP can also reduce reactive oxygen species by acting as free radical scavengers. Carnosine and anserine, two abundant BP in meats, reduce or prevent oxidative stress-related diseases [28][63]. Lunasin protects human Caco-2 cells in vitro from oxidative stress caused by treatment with hydrogen peroxide or tert-butylhydroperoxide by scavenging peroxyl and superoxide radicals [110][64]. BP isolated from oyster, shrimp, squid, and bluemussel have demonstrated antioxidant properties in vitro and in animal models [111][65].
The peptides α-lactorphin and β-lactorphin (opioid peptides) exert analgesic activity on the opiate receptor agonist. In in vitro studies, α-lactalbumin and β-lactoglobulin BP have demonstrated analgesic activity at micromolar concentrations [112][66]. Analgesic peptides have also been isolated from rice albumin, gluten, and spinach [113][67].
The BP lactoferrin (fragment 17–41) and peptides derived from bovine meat (GFHI, DFHING, FHG, GLSDGEWQ) are being studied in vitro and in vivo for their antimicrobial effects. BP have demonstrated a large spectrum of action against viruses, bacteria, protozoa, and fungi. For example, the fragment 17–41 of lactoferrin, also known as lactoferricin, has bactericidal activity due to its ability to alter bacterial membrane permeability by interacting with the lipid A portion of bacterial lipopolysaccharides [114][68]. Preliminary data indicate that the BP are well tolerated, do not induce pathogen resistance, and demonstrate activity against both Gram-positive and Gram-negative bacteria [115][69].
References
- Sun, X.; Acquah, C.; Aluko, R.E.; Udenigwe, C.C. Considering food matrix and gastrointestinal effects in enhancing bioactive peptide absorption and bioavailability. J. Funct. Foods 2020, 64, 103680.
- Dellafiora, L.; Paolella, S.; Dall’Asta, C.; Dossena, A.; Cozzini, P.; Galaverna, G. Hybrid in Silico/in Vitro Approach for the Identification of Angiotensin I Converting Enzyme Inhibitory Peptides from Parma Dry-Cured Ham. J. Agric. Food Chem. 2015, 63, 6366–6375.
- Akbarian, M.; Khani, A.; Eghbalpour, S.; Uversky, V.N. Bioactive Peptides: Synthesis, Sources, Applications, and Proposed Mechanisms of Action. Int. J. Mol. Sci. 2022, 23, 1445.
- Zarei, M.; Ebrahimpour, A.; Abdul Hamid, A.; Anwar, F.; Saari, N. Production of defatted palm kernel cake protein hydrolysate as a valuable source of natural antioxidants. Int. J. Mol. Sci. 2012, 13, 8097–8111.
- Hayes, M.; Ross, R.P.; Fitzgerald, G.F.; Stanton, C. Putting microbes to work: Dairy fermentation, cell factories and bioactive peptides. Part I: Overview. Biotechnol. J. 2007, 2, 426–434.
- Rui, X.; Wen, D.; Li, W.; Chen, X.; Jiang, M.; Dong, M. Enrichment of ACE inhibitory peptides in navy bean (Phaseolus vulgaris) using lactic acid bacteria. Food Funct. 2015, 6, 622–629.
- Bechaux, J.; Gatellier, P.; Le Page, J.F.; Drillet, Y.; Sante-Lhoutellier, V. A comprehensive review of bioactive peptides obtained from animal byproducts and their applications. Food Funct. 2019, 10, 6244–6266.
- Piovesana, S.; Capriotti, A.L.; Cavaliere, C.; La Barbera, G.; Montone, C.M.; Zenezini Chiozzi, R.; Laganà, A. Recent trends and analytical challenges in plant bioactive peptide separation, identification and validation. Anal. Bioanal. Chem. 2018, 410, 3425–3444.
- Toldrá, F.; Gallego, M.; Reig, M.; Aristoy, M. Bioactive peptides generated in the processing of dry-cured ham. Food Chem. 2020, 321, 126689.
- Amigo, L.; Hernández-Ledesma, B. Current Evidence on the Bioavailability of Food Bioactive Peptides. Molecules 2020, 25, 4479.
- Renukuntla, J.; Vadlapudi, A.D.; Patel, A.; Boddu, S.H.; Mitra, A.K. Approaches for enhancing oral bioavailability of peptides and proteins. Int. J. Pharm. 2013, 447, 75–93.
- Li, T.; Shi, C.; Zhou, C.; Sun, X.; Ang, Y.; Dong, X.; Huang, M.; Zhou, G. Purification and characterization of novel antioxidant peptides from duck breast protein hydrolysates. LWT Food Sci. Technol. 2020, 125, 109215.
- Fitzgerald, R.J.; Murray, B.A.; Walsh, D.J. Hypotensive peptides from milk proteins. J. Nutr. 2004, 134, 980S–988S.
- Boelsma, E.; Kloek, J. IPP-rich milk protein hydrolysate lowers blood pressure in subjects with stage 1 hypertension, a randomized controlled trial. Nutr. J. 2010, 9, 52.
- Malinowski, J.; Klempt, M.; Clawin-Rädecker, I.; Lorenzen, P.C.; Meisel, H. Identification of a NFκB inhibitory peptide from tryptic β-casein hydrolysate. Food Chem. 2014, 165, 129–133.
- Aihara, K.; Ishii, H.; Yoshida, M. Casein-derived tripeptide, Val-Pro-Pro (VPP), modulates monocyte adhesion to vascular endothelium. J. Atheroscler. Thromb. 2009, 16, 594–603.
- Majumder, K.; Mine, Y.; Wu, J. The potential of food proteinderived anti-inflammatory peptides against various chronic inflammatory diseases. J. Sci. Food Agric. 2016, 96, 2303–2311.
- Majumder, K.; Chakrabarti, S.; Morton, J.S.; Panahi, S.; Kaufman, S.; Davidge, S.T.; Wu, J. Egg-derived tri-peptide IRW exerts antihypertensive effects in spontaneously hypertensive rats. PLoS ONE 2013, 8, e82829.
- Aguilar-Toalá, J.E.; Santiago-Lòpez, L.; Peres, C.M.; Peres, C.; Garcia, H.S.; Vallejo-Cordoba, B.; Gonzàlez-Còrdova, A.F.; Hernàndez-Mendoza. Assessment of multifunctional activity of bioactive peptides derived from fermented milk by specific Lactobacillus plantarum strains. J. Dairy Sci. 2017, 100, 65–75.
- Cicero, A.F.G.; Fogacci, F.; Colletti, A. Potential role of bioactive peptides in prevention and treatment of chronic diseases: A narrative review. Br. J. Pharmacol. 2016, 174, 1378–1394.
- Holmer-Jensen, J.; Karhu, T.; Mortensen, L.S.; Pedersen, S.B.; Herzig, K.H.; Hermansen, K. Differential effects of dietary protein sources on postprandial low-grade inflammation after a single high fat meal in obese non-diabetic subjects. Nutr. J. 2011, 10, 115.
- McInnes, G.T. Lowering blood pressure for cardiovascular risk reduction. J. Hypertens. Suppl. 2005, 23, S3–S8.
- Lawes, C.M.; Vanders, H.S.; Rodgers, A. Global burden of bloodpressure related disease. Lancet 2008, 371, 1513–1518.
- Borghi, C.; Cicero, A.F. Nutraceuticals with clinically detectable blood pressure lowering effect: A review of available randomized clinical trials and their meta-analyses. Br. J. Clin. Pharmacol. 2017, 83, 163–171.
- Cicero, A.F.; Colletti, A. Nutraceuticals and blood pressure control: Results from clinical trials and meta-analyses. High Blood Press. Cardiovasc. Prev. 2015, 22, 203–213.
- Aluko, R.E. Antihypertensive peptides from food proteins. Annu. Rev. Food Sci. Technol. 2015, 6, 235–262.
- Cicero, A.F.; Aubin, F.; Azais-Braesco, V.; Borghi, C. Do the lactotripeptides isoleucine–proline–proline and valine–proline–proline reduce systolic blood pressure in European subjects? A meta-analysis of randomized controlled trials. Am. J. Hypertens. 2013, 26, 442–449.
- Cicero, A.F.; Gerocarni, B.; Laghi, L.; Borghi, C. Blood pressure lowering effect of lactotripeptides assumed as functional foods: A meta-analysis of current available clinical trials. J. Hum. Hypertens. 2011, 25, 425–436.
- Yadav, J.S.; Yan, S.; Pilli, S.; Kumar, L.; Tyagi, R.D. Surampalli RY Cheese whey: A potential resource to transform into bioprotein, functional/nutritional proteins and bioactive peptides. Biotechnol. Adv. 2015, 33, 756–774.
- Cicero, A.F.; Colletti, A.; Rosticci, M.; Cagnati, M.; Urso, R.; Giovannini, M.; Borghi, C.; D’Addato, S. Effect of Lactotripeptides (Isoleucine-Proline-Proline/Valine-Proline-Proline) on Blood Pressure and Arterial Stiffness Changes in Subjects with Suboptimal Blood Pressure Control and Metabolic Syndrome: A Double-Blind, Randomized, Crossover Clinical Trial. Metab. Syndr. Relat. Disord. 2016, 14, 161–166.
- Nongonierma, A.B.; FitzGerald, R.J. Bioactive properties of milk proteins in humans: A review. Peptides 2015, 73, 20–34.
- Dong, J.Y.; Szeto, I.M.Y.; Makinen, K.; Gao, Q.; Wang, J.; Li-Qin, Q.; Zhao, Y. Effect of probiotic fermented milk on blood pressure: A meta-analysis of randomised controlled trials. Br. J. Nutr. 2013, 110, 1188–1194.
- Cheung, R.C.; Ng, T.B.; Wong, J.H. Marine peptides: Bioactivities and applications. Mar. Drugs 2015, 13, 4006–4043.
- Nirupama, G.; Mohammad, B.; Hossain, D.K.R.; Nigel, P.B. A review of extraction and analysis of bioactives in oat and barley and scope for use of novel food processing technologies. Molecules 2015, 20, 10884–10909.
- Ford, E.S.; Li, C.; Pearson, W.S.; Zhao, G.; Mokdad, A.H. Trends in hypercholesterolemia, treatment and control among United States adults. Int. J. Cardiol. 2010, 140, 226–235.
- Writing Group Members; Mozaffarian, D.; Benjamin, E.J.; Go, A.S.; Arnett, D.K.; Blaha, M.J.; Cushman, M.; Das, S.R.; de Ferranti, S.; Després, J.P.; et al. Heart disease and stroke statistics—2016 update: A report from the American Heart Association. Circulation 2016, 133, e38–e360.
- Baigent, C.; Keech, A.; Kearney, P.M.; Blackwell, L.; Buck, G.; Pollicino, C.; Kirby, A.; Sourjina, T.; Peto, R.; Collins, R.; et al. Efficacy and safety of cholesterol-lowering treatment: Prospective metaanalysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 2005, 366, 1267–1278.
- Zdrojewski, T.; Solnica, B.; Cybulska, B.; Bandosz, P.; Rutkowski, M.; Stokwiszewski, J.; Gaciong, Z.; Banach, M.; Wojtyniak, B.; Pencina, M.; et al. Prevalence of lipid abnormalities in Poland. The NATPOL 2011 survey. Kardiol. Pol. 2016, 74, 213–223.
- Tokede, O.A.; Onabanjo, T.A.; Yansane, A.; Gaziano, J.M.; Djoussé, L. Soya products and serum lipids: A meta-analysis of randomised controlled trials. Br. J. Nutr. 2015, 114, 831–843.
- Lammi, C.; Zanoni, C.; Scigliuolo, G.M.; D’Amato, A.; Arnoldi, A. Lupin peptides lower low-density lipoprotein (LDL) cholesterol through an up-regulation of the LDL receptor/sterol regulatory element binding protein 2 (SREBP2) pathway at HepG2 cell line. J. Agric. Food Chem. 2014, 62, 7151–7159.
- Marques, M.R.; Soares Freitas, R.A.; Corrêa Carlos, A.C.; Siguemoto, É.S.; Fontanari, G.G.; Arêas, J.A. Peptides from cowpea present antioxidant activity, inhibit cholesterol synthesis and its solubilisation into micelles. Food Chem. 2014, 168, 288–293.
- Herrera Chalé, F.; Ruiz Ruiz, J.C.; Betancur Ancona, D.; Acevedo Fernández, J.J.; Segura Campos, M.R. The hypolipidemic effect and antithrombotic activity of Mucuna pruriens protein hydrolysates. Food Funct. 2016, 7, 434–444.
- Lule, V.K.; Garg, S.; Pophaly, S.D.; Hitesh; Tomar, S.K. Potential health benefits of lunasin: A multifaceted soy-derived bioactive peptide. J. Food. Sci. 2015, 80, R485–R494.
- Otvos, L., Jr. Peptide-based drug design: Here and now. Methods Mol. Biol. 2008, 494, 1–8.
- Tyagi, A.; Tuknait, A.; Anand, P.; Gupta, S.; Sharma, M.; Mathur, D.; Joshi, A.; Singh, S.; Gautam, A.; Raghava, G.P. CancerPPD: A database of anticancer peptides and proteins. Nucl. Acids. Res. 2015, 43, D837–D843.
- Hernández-Ledesma, B.; Hsieh, C.C.; de Lumen, B.O. Chemopreventive properties of peptide lunasin: A review. Protein Pept. Lett. 2013, 20, 424–432.
- Dia, V.P.; de Mejia, E.G. Lunasin potentiates the effect of oxaliplatin preventing outgrowth of colon cancer metastasis, binds to α5β1 integrin and suppresses FAK/ERK/NF-κB signaling. Cancer Lett. 2011, 313, 167–180.
- Chang, H.C.; Lewis, D.; Tung, C.Y.; Han, L.; Henriquez, S.M.P.; Voiles, L.; Lupov, I.P.; Pelloso, D.; Sinn, A.L.; Pollok, K.E.; et al. Soypeptide lunasin in cytokine immunotherapy for lymphoma. Cancer Immunol. Immunother. 2014, 63, 283–295.
- Kannan, A.; Hettiarachchy, N.S.; Lay, J.O.; Liyanage, R. Human cancer cell proliferation inhibition by a pentapeptide isolated and characterized from ricebran. Peptides 2010, 31, 1629–1634.
- Park, J.H.; Jeong, H.J.; de Lumen, B.O. Contents and bioactivities of lunasin, bowman-birk inhibitor, and isoflavones in soybean seed. J. Agric. Food Chem. 2005, 53, 7686–7690.
- Malkowicz, S.B.; McKenna, W.G.; Vaughn, D.J.; Wan, X.S.; Propert, K.J.; Rockwell, K.; Marks, S.H.; Wein, A.J.; Kennedy, A.R. Effects of Bowman-Birk inhibitorconcentrate (BBIC) in patients with benign prostatic hyperplasia. Prostate 2001, 48, 16–28.
- Valadez-Vega, C.; Alvarez-Manilla, G.; Riverón-Negrete, L.; GarcíaCarrancá, A.; Morales-González, J.A.; Zuñiga-Pérez, C.; Zuñiga-Pérez, C.; Madrigal-Santillán, E.; Esquivel-Soto, J.; Esquivel-Chirino, C.; et al. Detection of cytotoxic activity of lectin on human colon adenocarcinoma (Sw480) and epithelial cervical carcinoma (C33-A). Molecules 2011, 16, 2107–2118.
- Deng, M.; Zhang, W.; Tang, H.; Ye, Q.; Liao, Q.; Zhou, Y.; Wu, M.; Xiong, W.; Zheng, Y.; Guo, X.; et al. Lactotransferrin acts as a tumor suppressor in nasopharyngeal carcinoma by repressing AKT through multiple mechanisms. Oncogene 2013, 32, 4273–4283.
- Varadhachary, A.; Wolf, J.S.; Petrak, K.; O’Malley, B.W., Jr.; Spadaro, M.; Curcio, C.; Forni, G.; Pericle, F. Oral lactoferrin inhibits growth of established tumors and potentiates conventional chemotherapy. Int. J. Cancer 2004, 111, 398–403.
- Azuma, N.; Suda, H.; Iwasaki, H.; Yamagata, N.; Saeki, T.; Kanamoto, R.; Iwami, K. Antitumorigenic effects of several food proteins in a rat model with colon cancer and their reverse correlation with plasma bile acid concentration. J. Nutr. Sci. Vitaminol. 2000, 46, 91–96.
- Ledesma-Martínez, E.; Aguíñiga-Sánchez, I.; Weiss-Steider, B.; Rivera-Martínez, A.R.; Santiago-Osorio, E. Casein and Peptides Derived from Casein as Antileukaemic Agents. J. Oncol. 2019, 2019, 8150967.
- Yang, Y.; Zhang, Z.; Pei, X.; Han, H.; Wang, J.; Wang, L.; Long, Z.; Shen, X.; Li, Y. Immunomodulatory effects of marine oligopeptide preparation from chum salmon (Oncorhynchus keta) in mice. Food Chem. 2009, 113, 464–470.
- Duarte, J.; Vinderola, G.; Ritz, B.; Perdigon, G.; Matar, C. Immunomodulating capacity of commercial fish protein hydrolysate for diet supplementation. Immunobiology 2006, 211, 341–350.
- Wang, Y.K.; He, H.L.; Wang, G.F.; Wu, H.; Zhou, B.C.; Chen, X.L.; Zhang, Y.Z. Oyster (Crassostrea gigas) hydrolysates produced on a plant scale have antitumor activity and immunostimulating effects in BALB/c mice. Mar. Drugs 2010, 8, 255–268.
- Kitts, D.D.; Weiler, K. Bioactive proteins and peptides from food sources. Applications of bioprocesses used in isolation and recovery. Curr. Pharm. Des. 2003, 9, 1309–1323.
- Wang, J.; Zhao, M.; Zhao, Q.; Jiang, Y. Antioxidant properties of papain hydrolysates of wheat gluten in different oxidation systems. Food Chem. 2007, 101, 1658–1663.
- Hipkiss, A.R.; Brownson, C. A possible new role for the anti-ageing peptide carnosine. Cell. Mol. Life Sci. 2000, 7, 747–753.
- Malaguti, M.; Dinelli, G.; Leoncini, E.; Bregola, V.; Bosi, S.; Cicero, A.; Hrelia, S. Bioactive peptides in cereals and legumes: Agronomical, biochemical and clinical aspects. Int. J. Mol. Sci. 2014, 15, 21120–21135.
- Garcia-Nebot, M.J.; Recio, I.; Hernandez-Ledesma, B. Antioxidant activity and protective effects of peptide lunasin against oxidative stress in intestinal Caco-2 cells. Food. Chem. Toxicol. 2014, 65, 155–161.
- Harada, K.; Maeda, T.; Hasegawa, Y.; Tokunaga, T.; Tamura, Y.; Koizumi, T. Antioxidant activity of fish sauces including puffer (Lagocephalus wheeleri) fish sauce measured by the oxygen radical absorbance capacity method. Mol. Med. Rep. 2010, 3, 663–668.
- Pihlanto-Leppälä, A. Bioactive peptides derived from bovine whey proteins: Opioid and ace-inhibitory peptides. Trends Food Sci. Technol. 2010, 11, 347–356.
- Teschemacher, H. Opioid receptor ligands derived from food proteins. Curr. Pharm. Des. 2003, 9, 1331–1344.
- Orsi, N. The antimicrobial activity of lactoferrin: Current status and perspectives. Biometals 2004, 17, 189–196.
- Jang, A.; Jo, C.; Kang, K.S.; Lee, M. Antimicrobial and human cancer cell cytotoxic effect of synthetic angiotensin-converting enzyme (ACE) inhibitory peptides. Food Chem. 2008, 107, 327–336.
More
