1. Insulitis in Human Type 1 Diabetes
As highlighted before, insulitis in human T
ype 1
diabetes (T1D)D pancreas can be detected in a low percentage of cases and up to 30%
[1]. The variability depends on age and disease duration. Insulitis is predominant in younger patients and when they are tested near diagnosis
[2][16]. The frequency of insulitis may display limited inverse correlation with diabetes duration and has no correlation with age, it predominantly affects insulin-positive islets and has been also observed in patients with residual beta cells, also years after diagnosis
[3][14].
The results obtained analysing serial biopsies up to now highlight the chronicity of this process, detectable for years after diagnosis, either in children or young adults.
Moreover, insulitis does not affect all the islets at the same time, confirming that this is a long evolving process.
Of relevance, there was no correlation of beta cell mass with insulitis, disease duration and age of onset. Inflammation and beta cell dysfunction may be an important pathogenic mechanism at the time of initial disease manifestation and contribute to cause the symptoms of severe hyperglycaemia
[4][32].
All those findings suggest that beta cell destruction is quite heterogeneous and is not likely to be completed until several years after diagnosis. Studies have reported persistence of insulin-positive beta cells even decades after diagnosis
[5][33] and that glucose transporters continue to be expressed
[6][34]. In some patients with long disease duration, beta cells express the survivin molecule, possibly a factor involved in the persistence of the beta cell phenotype
[7][35].
Low levels of beta cell apoptosis have been noted in the pancreas of patients with long disease duration, implying the existence of some beta cell turnover
[5][33].
The above observations are usually present in the context of chronic signs of islet inflammation, including insulitis and increased expression of H
uman L
eucocyte Antigen (HLA) A class I molecules
[8][36].
Therefore, this kind of disease onset does not help either an early diagnosis or therapeutic design. On the other hand, it would also be important to prevent the disease in some cases when familiarity is present.
Recent data have highlighted that a Slowly Progressive type 1 insulin-dependent Diabetes Mellitus (SPIDDM), sometimes indicated to as a Latent Autoimmune Diabetes in Adults (LADA), is a heterogeneous disease often confused with type 1 and type 2 diabetes. In the pancreas of patients with SPIDDM, which includes a T-cell-mediated insulitis, the following features can be detected: pseudo-atrophic islets, absence of beta cells, and the lack of amylin (i.e., islet amyloid polypeptide) deposition into the islet cells (a pathologic marker of type 2 diabetes). Being therefore this a T1D syndrome with a slow evolution, the authors claim for prevention strategies for patients with SPIDDM. The proposed strategies aim at the preservation of beta-cell function and include the early administration of low doses of insulin or of dipeptidyl peptidase-4 (DPP-4) inhibitors
[9][37].
Recently, beta cell autophagy has been hypothesized to be involved in T1D pathogenesis
[10][38]. This is in line with the knowledge that T1D is a multifactorial autoimmune disease involving multiple environmental and genetic factors, but still without a clear aetiology. Early signalling defects within the beta cells may promote a change in the local immune milieu leading to autoimmunity. Therefore, some studies have been focused on intrinsic beta-cell mechanisms that aid in the restoration of cellular homeostasis under environmental conditions that cause dysfunction. One of these intrinsic mechanisms to promote homeostasis is autophagy (linked with beta-cell dysfunction in type 2 diabetes).
2. Stem Cell Grafting
Insulitis and the related micro vessel alterations remain a critical element of T1D pathology, having a great relevance to design more effective targeting of pathogenic mechanisms.
It is well known that combination therapies promoting immune regulation and addressing beta cell dysfunction should be more effective in treating this chronic disease, but studies should be much more focused on the phenotypic features of infiltrating cells in the insulitis lesion, also in relation to other abnormalities, as well as gene expression profile and epigenetic regulation of the beta cells, which could be a major contributor to their dysfunction and death.
More recently the use of stem cell grafting has been proposed. Clinical good-manufacturing practice (cGMP)-grade stem cell products have been used in human clinical trials, showing that stem cell transplantation has beneficial effects on T1D, with no obvious adverse reactions. To better achieve remission of T1D by stem cell transplantation, innovative approaches such as mesenchymal stem cells (MSCs), human embryonic stem cells (hESCs), and bone marrow hematopoietic stem cells (BM-HSCs) to restore the immunotolerance and preserve the islet beta-cell function of T1D have been investigated
[11][63], but the results are, up to now, discouraging.
In fact, studies lacked a good base of pre-clinical investigations attempting endocrine pancreas recovery using stem cell transplantation. The focus was on the use of hematopoietic and mesenchymal stem cells (MSCs)
[12][64]. However, it should be considered that MSCs could exert an anti-inflammatory role, thanks to the secretion of chemokines and cytokines capable of immunomodulation and T cell inhibition, with the potential of improving strategies of engraftment of donor islets
[13][65].
Authors tried to use all types of stem cells with partial, incomplete, or largely negative results, mainly because the goal to overcome the disease is far from being obtained. Stem cells can only replace for a given time the lost cells, but they will inevitably convert to new targets of the immune attack. Sometimes they can have more time or better endure the insults, but the true resistant ones are far from being obtained.
Therefore, more effective therapeutic approaches are needed
[14][66].
To overcome these issues, combinatorial strategies have been proposed for a curative treatment of T1D
[15][67]. Combining safe and effective stem cell strategies with reliable existing therapies such as islet transplantation, as well as the immunosuppressive and immunomodulatory drug regimens and/or novel bioengineering techniques and/or gene therapies, would ensure an optimistic scenario for successful translation of stem cell therapy in the cure of T1D.
EIn our opinion, every strategy including stem cells of every origin albeit differentiated into insulin-containing cells or whole islets for a mere grafting doom to failure, as the history of the previous studies teaches. This since the grafted cells or islets will be inevitably de novo attacked, unless other strategies are undertaking, for instance by encapsulation
[16][68] or gene editing approaches that protect them from the immune attack
[17][69]. Indeed, encouraging data come from the 20-year outcomes of a cohort study employing islet transplantation
[18][70].
3. Cell Therapy and the Organoid Perspective
Cell therapy, instead of a mere stem cell grafting, provides an opportunity to prevent or reverse T1D
[19][71].
In fact, the clinical trial results of stem cell therapies for T1D are largely dissatisfactory
[20][72], and many questions and technical hurdles still need to be solved. The major points that researchers should overcome include: (i) how to generate more mature functional beta cells in vitro from hPSCs; (ii) how to improve the differentiation efficiency of IPCs from hPSCs; (iii) how to protect implanted IPCs from autoimmune attack; (iv) how to generate enough desired cell types for clinical transplantation and (v) how to establish thorough insulin independence
[21][22][73,74].
However, they seem to not take into consideration that it is needed to solve the main problem at the basis of the disease. Also, in this case it seems that effectively diabetes stem cell therapy takes evasive actions
[23][75].
Therefore, the application of cell-based therapy for T1D albeit represents the most advanced approach for curing it, must take into consideration also the main variables.
Firstly, protective encapsulating devices and gene-editing technologies could obviate the need for antirejection drugs in stem-cell-derived therapies for diabetes.
Then, adoptive transfer of autologous cells having enhanced immunomodulatory properties could suppress autoimmunity and preserve beta-cells. Such therapies have been made possible by a combination of genome-editing techniques and transplantation of tolerogenic cells. In-vitro modified autologous hematopoietic stem cells and tolerogenic dendritic cells may protect endogenous and newly generated beta-cells without hampering immune surveillance for infectious agents and malignant cellular transformation.
The technological advances will allow to test the proposed new strategies directly in human cell models, as the gold standard in biomedical research fields. Omics approaches to characterize the molecular signatures of the better cell populations, should be given priority to accelerate bioengineering strategies. This will help to decipher the still mysterious treasure of human islet beta-cell regeneration, as well as it will prevent the recurrent drawbacks in the later stages of clinical testing or the overlooking of potentially effective treatments that fail in mouse model testing.
However, methods to generate cells that meet quality and safety standards for clinical applications have been recently addressed
[22][74], despite they are still far from application in clinical setting.
On the other hand, a vast number of studies that have been reported on gene therapy for the management of T1D have been conducted in animal models and in preclinical studies. Some recent reports from phase I/II clinical trials showed the feasibility of such treatments in humans, although with a small
n [24][76]. Currently, there are several gene level interventions that are being investigated, notably overexpression of genes and proteins, transplantation of cells that express the genes against T1D, stem-cells mediated gene therapy, genetic vaccination, immunological precursor cell-mediated gene therapy and vectors. The way to better address these issues has been indicated
[25][77].
Anyway, cell therapy is a much more reliable perspective and studies must be performed to this aim.
The latest research interest for either deeper knowledge or possible therapeutic advances are pancreatic islet organoids.
It is well known how hard it is to produce functional beta cells in vitro. Recently
[26][78], a previously unidentified protein C receptor positive (Procr+) cell population has been identified in adult mouse pancreas through single-cell RNA sequencing (scRNA-seq). The cells are present inside the islets, do not express differentiation markers, and show epithelial-to-mesenchymal transition characteristics. By means of genetic lineage tracing, it has been demonstrated that Procr+ islet cells undergo clonal expansion and generate all four endocrine islet cell types during adult homeostasis. Sorted Procr+ cells, representing ∼1% of islet cells, have been demonstrated to be capable to form islet-like organoids when cultured at clonal density. In addition, exponential expansion can be maintained over long periods by serial passaging, while differentiation can be induced at any time point in culture. Interestingly, beta cells dominate in differentiated islet organoids, while α, δ, and PP cells occur at much lower frequencies. The organoids are glucose-responsive and insulin-secreting. Of great scientific significance, upon transplantation in diabetic mice, these organoids have been found to reverse the disease. Therefore, the adult mouse pancreatic islet contains a population of Procr+ endocrine progenitors that can be the more effective cells to be used for replacement also in T1D
[26][78]. This approach, if confirmed in humans, could represent an interesting source of human islets.
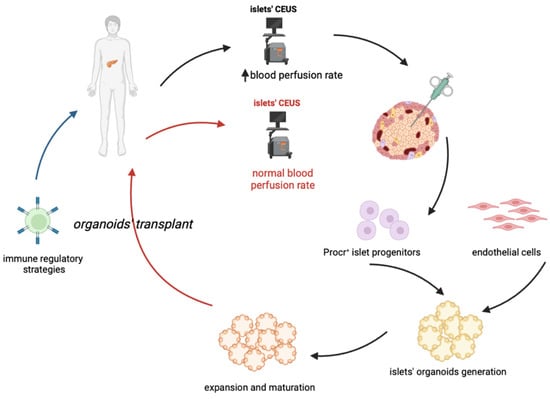
In conclusion, one of the proposed approaches for cell-based therapy of T1D in the future (Figure 1) could combine an early diagnosis (possibly thanks to CEUS) with a sufficient isolation of Procr+ progenitors, followed by the generation and expansion of islet organoids, which could be transplanted coupled to an immune-regulatory therapy which will permit the maintenance of pancreatic islets and an effective and long-lasting insulitis reversal. A key issue to resolve would be the maximization of progenitors’ isolation and organoids expansion, such that only a few biopsies would ensure an optimal outcome.
Figure 1. Proposed cell-based treatment of type 1 diabetes. The proposed approach should combine an early diagnosis of T1D, through CEUS (Contrast-Enhanced Ultrasonography) with the proper quantity of Procr+cell progenitors, combined with endothelial cells, derived from IPSCs. This in order to generate and expand islet organoids, that can be then grafted in patients undergoing an immune-regolatory therapy. The latter is required to guarantee a long-term maintenance of the transplanted pancreatic islets.