You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 2 by Catherine Yang and Version 1 by Samir Kumar.
Glancing angle deposition (GLAD) is a technique for the fabrication of sculpted micro- and nanostructures under the conditions of oblique vapor flux incident and limited adatom diffusion. GLAD-based nanostructures are emerging platforms with broad sensing applications due to their high sensitivity, enhanced optical and catalytic properties, periodicity, and controlled morphology. GLAD-fabricated nanochips and substrates for chemical and biosensing applications are replacing conventionally used nanomaterials due to their broad scope, ease of fabrication, controlled growth parameters, and hence, sensing abilities.
- Glancing angle depositon
- Biosensors
- Sculptured thin films
- SERS
- Thin films
- SPR
1. SPR-Based Detection
Surface plasmon resonance is a powerful analytical method for detecting the high sensitivity of biomolecules and is based on plasmonic materials’ optical properties. The SPR method has shown great promise with large sensitivity for biomolecular applications [106][1], especially for real-time biomolecule interaction, protein action, antigen, and nucleic acid detection [107,108,109][2][3][4]. SPR-based sensors are useful for label-free detection because of the very high sensitivity of the surface plasmon polaritons (SPP) to changes in the local environment in the measurement of both refractive indices and dielectric constants [110,111][5][6]. SPR-based biosensors possess the advantage of high versatility and can be tailored for the detection of a vast number of analytes since this type of detection does not demand any special characteristic of the analyte, such as fluorescence properties or absorption or scattering bands. Additionally, SPR-based bio-detection does not require analytes or biomolecules to possess fluorescent or radioactive labels.
The primary sensing phenomenon in SPR is related to propagating surface plasmon polaritons. A surface plasmon polariton (SPP) is an electromagnetic wave propagating at the metal–dielectric boundary. The electromagnetic field of an SPP confined at the metal–dielectric interface decreases exponentially in both metals and dielectrics [112][7]. The dielectric constant of the propagating surface plasmon wave is highly sensitive to changes in the local refractive index of the dielectric. This property is the underlying principle of SPR biosensors. Metal-binding molecules or the recognition element attached to the surface of the metal capture the analyte, resulting in a change in the local refractive index at the metal surface. This change in the local refractive index creates a change in the propagation constant, which can be easily measured using various methods112. Figure 61 shows the underlying mechanism followed during SPR sensing using the Kretschmann configuration.
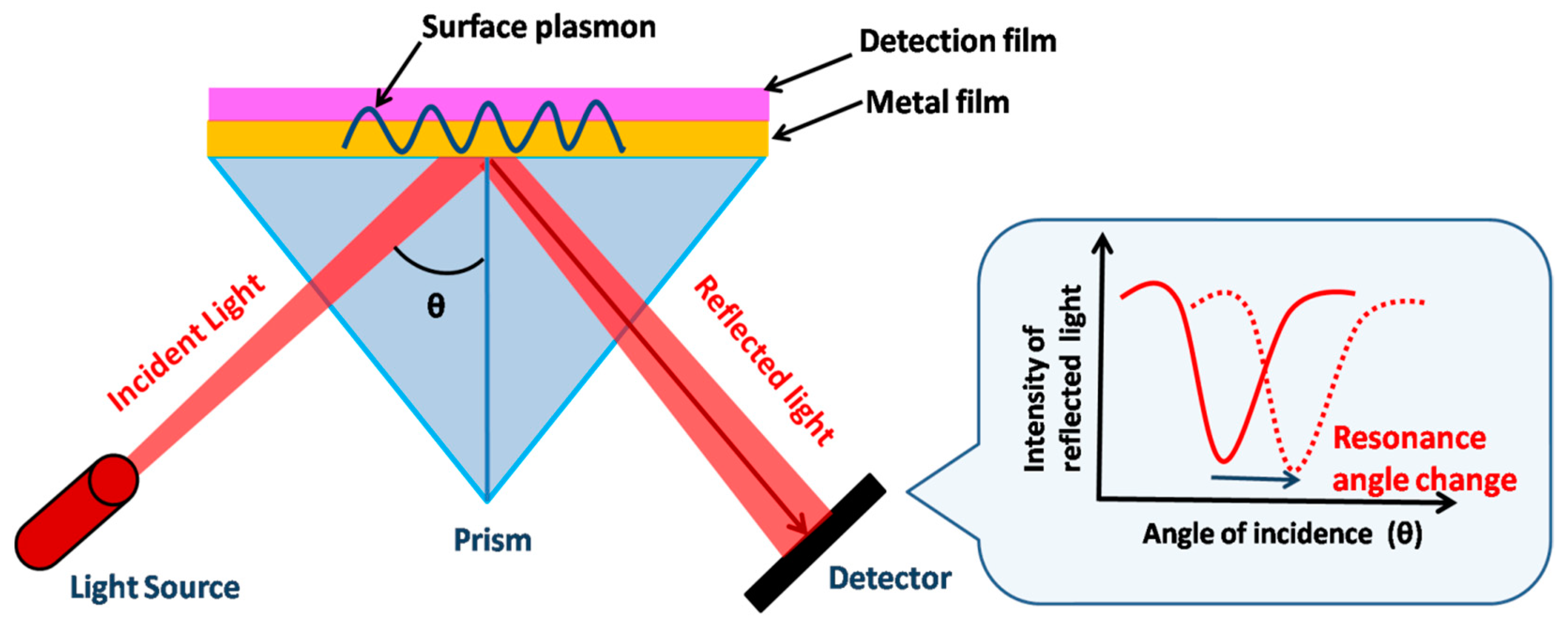
Figure 61. Schematic of SPR-based detection of biomolecules using Kretschmann configuration. The dip in the resonance peak shifts due to the change in the refractive index.
Recently, new strategies, such as the generation of frictional charges using triboelectric surfaces, have been employed to detect and generate localized surface plasmon resonance (LSPR) wavelength shifts in large-area Au nanostructured surfaces [113][8]. These LSPs with frictional charges, using current methods such as spectroscopic and triboelectric measurements, have shown a complex interplay of bioconjugation and bio-complex detection. These budding strategies, combined with GLAD-fabricated nanostructures, can be employed for enhanced biosensing detection and applications. GLAD-fabricated metallic thin films facilitate the development of SPR- and LSPR-based biosensors, possessing the advantage of a considerable increment in sensitivity. These nano-sculptured thin films have been theoretically and experimentally demonstrated to possess high SPR sensitivity [114,115][9][10]. Additionally, their periodicity, gap, etc., can be tailored in a desired way, hence the binding of bioanalytes and sensitivity enhancement [115][10].
2. SERS-Based Biosensing
Surface-enhanced Raman scattering (SERS) is the enormous amplification of Raman signals from molecules by several orders of magnitude when adsorbed on metal colloidal nanoparticles or a rough metal surface, discovered in 1974 by Fleischmann et al. [132,133][11][12]. The detection of analyte molecules, even at a single-molecule level, can be realized by employing the SERS technique, which expands its practical applications [134][13]. SERS has found applications in various fields, including the detection of trace chemicals [135][14] such as dye molecules, food additives, [136][15] and pesticides, [77][16] and in bioanalysis, medical diagnosis, [36][17] the detection of biomolecules, [137][18] cancer diagnosis, [138][19] in vivo molecular probing in live cells, [139][20] and explosives detection [140][21]. SERS substrates are the nanostructured platform that supports plasmon resonance and amplifies Raman signals [141,142,143][22][23][24] and are broadly classified as random morphology or ordered/periodic SERS substrates. Random morphology SERS substrates (which include roughened electrodes, metallic silver and gold colloids, and metal-island film on a planar substrate) are inhomogeneous and are not highly reproducible [144][25]. Periodic arrays of metallic nanostructures (using nanolithography and other physical vapor deposition techniques) can overcome this issue by providing uniform and controlled morphology of SERS substrates [145][26].
SERS has become a powerful chemical and biological sensing technique due to its excellent sensitivity and specificity. Uniform and highly reproducible SERS substrates with batch-to-batch reproducibility that have a SERS signal variation of less than 15% can be fabricated using the GLAD technique [146][27]. Various pathogen species or even strains can be differentiated using SERS multivariate statistical analysis. Therefore, extrinsic detection using Raman labels is more widely explored.
3. Fluorescence-Based Biosensing
Fluorescence is a short-lived kind of luminescence’, created as a result of electromagnetic excitation caused when light energy is absorbed in a short wavelength and then emits light at a longer wavelength [156][28]. Fluorophores or fluorochromes are molecules that show fluorescence. Fluorophores emit energy in the form of light radiation or sometimes dissipate in the form of heat. The basic concept of biosensing using a fluorophore involves the coupling of target recognition with some change in the fluorescence of the reporter molecule. Different types of fluorescence biosensors can be proposed based on the nature of the sensing and binding elements [157][29]. The change in the intensity of fluorophores attached to the recognition element is susceptible to the local environment and is monitored directly. This change in the fluorescence intensity may be due to a shift in fluorophore–biomolecule interactions [158][30] or fluorophore–target molecule interactions, for example, the interaction of single-stranded DNA protein binding [159][31]. The primary advantages of fluorescence-based biosensors are their simplicity, ability to detect smaller ligands, availability of many donors and acceptors, convenient transducing optical signals, and suitability for short distances. Metallic nanostructures are known to enhance the fluorescence of fluorophores [160,161,162][32][33][34]. The fluorophores interact with the plasmonic field at the metal surface, increasing the local field and enhancing fluorescence intensity [160,161,163][32][33][35]. The metallic structures are known to increase the local electric field and the radiation decay rate up to a factor of 1000 [161][33]. The sensitivity of fluorophore detection due to combined local field increments and increased photostability is enhanced by 105 [164][36]. Metallic nanostructures are employed for enhanced fluorescence detection of DNA [165][37], protein microarrays [166][38], pathogens, cancer cells [167][39], and single species in tissue samples [168][40]. However, enhanced fluorescence detection using chemical method-based metallic nanoparticles is limited due to the inhomogeneity of the structure and non-proportion of fluorophore interaction with metals that provide enhancement. Plasmonic fluorescence enhancement by PVD-deposited metal nanostructures tends to provide increased enhancement due to the formation of periodic arrays over a large surface area. Controlled growth parameters and deposition rates may offer a large array of nano-columns with different shapes, sizes, periodicity, and interparticle separation, which can be regulated on the metal surface. Other applications of fabricated metal nanostructures in enhanced fluorescence-based bio-detection are discussed.
4. Colorimetric- and Wettability-Based Detection
Sensing based on the change in color and surface wettability has also been realized on GLAD substrates, especially plasmonic metallic arrays. Silver has the highest reflectance among all metals, at over 97% throughout most of the visible region and about 99% in the IR region. Pure silver looks shiny and whitish, as seen by the naked eye. However, silver nanoparticles exhibit a plasmonic effect with a very high extinction coefficient, and plasmonic absorption lies in the visible range and varies with particle size and shape, interparticle separation, and the refractive index of the surrounding medium [176][41]. A homogenous colloidal solution of nanoparticles of a particular size scatters a specific light wavelength, making them promising for different colorimetric visual readout sensors. Such properties of metal nanoparticles enable them to be exploited in various analytical tools, e.g., absorbance or fluorescence spectroscopy. Colorimetric-based assays have been developed by illustrating changes in the color associated with the aggregation of noble metal nanoparticles [177,178,179][42][43][44]. However, the employment of GLAD-fabricated silver nano-columnar thin film in such sensing applications is limited. The pristine aligned AgNR array fabricated by GLAD in a high vacuum looks bright due to the multiple scattering and multimode localized surface plasmon. In addition to optical-based sensing, these nanorod substrates have also been studied for gas and biosensing by exploiting their novel characteristic colorimetric properties. The chemistry between silver and sulfur has been studied by different groups [180,181,182][45][46][47]. Graedel et al. extensively explored the reaction between silver- and sulfur-containing gaseous molecules and found out the dependence of the reaction rate on relative humidity. After that, Chen et al. demonstrated using AgNP films as H2S gas sensors. According to their findings, the reaction between AgNPs and H2S gas follows a first-order reaction rate law and is proportional to the 1.3 power of the H2S gas concentration [182,183,184][47][48][49]. This relationship was used to determine the H2S gas concentration under ambient conditions. The intensity of the LSPR peak of the AgNP’s films decreases and exhibits a shift upon exposure to H2S gas. Though they have several applications in art conservation, AgNPs are used to detect the emission of H2S from degraded materials, e.g., aged wool fabrics, rubbers, etc., under ambient conditions. A GLAD-fabricated AgNR array was employed as an H2S gas sensor by Gahlaut et al. [185][50].
GLAD-fabricated sculptured thin films have been extensively used for chemical and biosensing, especially exploiting their LSPR and SERS properties. However, for colorimetric- and wettability-based applications, only a few reports are available; therefore, there is a gap yet to be filled. In recent years, considering the vast possibility of tuning the porosity of GLAD-fabricated substrates, there is enormous scope for surface-wetting modification in a wide hydrophilic to the superhydrophobic range. Some studies have been carried out on anisotropic wetting and water droplet evaporation on nano-columnar thin films for self-cleaning and the Leidenfrost effect [197,198,199,200][51][52][53][54]. Wettability-based sensing could be a promising method for the detection of analytes. Gahlaut et al. demonstrated a GLAD nanorod array of silver for H2S gas sensing by observing a rapid and drastic change in the water-wetting property of the array. As-grown AgNR arrays were found to be hydrophobic with a contact angle of 128°. Exposure to H2S gas led to the formation of Ag2S on the surface of the AgNR array and resulted in the enhancement of wettability with a water contact angle of 60° [185][50]. They also presented a novel method of bacterial viability detection using the wetting behavior of the AgNR array and further discriminated antibiotic resistance in bacterial species [38][55]. The AgNR array was reutilized as Ag-Ag2S nano-heterostructures for various energy and environmental applications, e.g., water purification, hydrogen evolution, and antibacterial activity [201][56]. All these novel applications of nano-sculptured thin films grown via oblique-angle deposition signify promising future scope in various domains.
4.5. Molecular Imaging
5. Molecular Imaging
The complex biological processes in the cell and its microenvironment can be visualized using a molecular technique. Using this technique, the internal mechanisms of living systems can be visualized. Additionally, certain specific molecules can be studied, and the pathways related to the molecules can be unveiled [202][57]. Disease progression and drug intervention can be effectively analyzed using imaging. The resolution and noise-to-sound ratio (NSR) challenge molecular imaging. To improve both of these features, the design and development of probes used in imaging in nanoscale regimes have a promising future [203][58]. Contrast materials show unique physical and chemical properties in the nanoscale zone, and this feature is exploited in this molecular imaging. Different chemical and biological methods are used to fabricate NPs in the molecular imaging technique. However, the fabrication cost and the complexity of its preparation have limited its use in biomedical applications. This current section will discuss the GLAD-based fabrication of NPs and their application in different molecular imaging applications [204,205][59][60].
6. In Vivo Application of GLAD
Biomimicking and biomaterial synthesis are new necessary fields in biomedical research. They have an enormous and substantial effect on healthcare, as new materials provide novel properties that can be used in prosthetics and drug carriers [232][61]. Biomaterials with an anisotropic composition are currently used in drug delivery for cancer treatment, as biomarkers, bactericidal agents, tissue engineering, and vaccine development [233][62]. Due to their multi-applicability, anisotropic NPs are currently in demand. They have varied in composition, functionality, shape, and size on the different surfaces of single NPs [234,235][63][64]. With this vast diversity, various in vivo applications are possible. Janus particles (JP) and patchy particles are the types of NPs used for such applications [236][65].
JPs or patchy particles are asymmetric colloidal particles with more than one composition and chemical modification at different sites [237][66]. Due to their multicomposition, JPs can be used for binding with specific molecules and probes [238][67]. Various methods are used for fabricating JPs, including physical deposition, chemical routes [239][68], electrochemical methods [240][69], microfluidics [241][70], electrohydrodynamic methods, and lithography [242][71].
As the position of the substrate and the angle can be varied using the GLAD setup, this vapor flux deposition method is used for obtaining different geometries required in Janus particles [243][72]. The thickness and geometric structures can be controlled by adjusting the flux rate and rotation of the substrate [244][73]. Xuan et al.’s group designed self-propelling Janus micromotors whereby GLAD was used for depositing Pt at a specific angle over the Si microparticles. Further, the micromotors were modified with biotin so that the charged organic dyes could be transported while maintaining the fast speed of the micromotor. This showed the efficacy of Janus particles in drug delivery applications [245][74].
Peng et al. combined both top-down and bottom-up fabrication techniques to prepare nanomotors, polymeric vesicles deposited with Pt, showing very high drug-loading efficiency. The Janus polymersome nanomotor showed enhanced permeability and retention (EPR) enhancement and released the encapsulated cargo in a controlled manner under external stimuli [246][75].
Tejeda-Rodriguez et al. and his group reported making a Janus nanomotor with the capsid from a plant virus on one hemisphere and Pt on the other hemisphere. The Janus viral nanomotor was found to carry and deliver the chemotherapeutic drug tamoxifen to breast tumor cells. The drug release was controlled by a pH-shift mechanism. As the capsid was a biomaterial, it showed an immense advantage over other materials; moreover, surface modification was easy to achieve in this case.
Zhiguang et al.’s group made an extraordinary development in this area by designing micro propellers that can penetrate the delicate vitreous humor of the eye and can perform drug delivery in the retina. The helical magnetic micro propellers were fabricated using GLAD. Ni was deposited onto the Si nanoparticles at an oblique angle. Then, Si was sequentially deposited upon rotation, forming a helical structure. Further, inspired by the sticky liquid layer found on the carnivorous Nepenthes pitcher plant, a non-toxic silicone oil, and fluorocarbon coating was used as a slippery surface. Under the influence of a magnetic field, the coated micro propellers showed controlled movement and could reach the retina within half an hour [247][76].
GLAD is a versatile and cheap fabrication method which has future potential in the fields of drug delivery and biomolecule transportation. Further research could be conducted on its use as a cargo transporter and gene delivery system. As a simple and cheap fabrication method, GLAD could be explored for various in vivo applications in biological fields. Minimal exploration has been done in this field.
7. Optical and Electrochemical GLAD-Based Sensors
Though GLAD-based biosensors are vastly employed in various sensing systems such as gas sensors [185][50], optical sensors [6][77], and electrochemical sensors [248][78], most of the work that has been conducted and reported employs optical and electrochemical systems due to the high porosity, plasmonic nature, and more excellent diffusion properties of these metallic nanostructures. Plasmonic metals such as Ag, Au, Cu, and Pt exhibit high plasmonic resonance in the optical region. The formation of dense electromagnetic hotspots among these nanostructures further increases their sensitivity as optical biosensors. A comparative study highlighting GLAD-based optical and electrochemical biosensors' advantages, LOD, and limitations are shown below (Table 1).
Table 1.
Comparative study of GLAD-based optical biosensors and electrochemical biosensors.
| GLAD-Based Optical Biosensors | GLAD-Based Electrochemical Biosensors |
|---|---|
| 1.The aspect ratio and morphology of the nanostructures are tuned and optimized to improve sensitivity to a variety of optical properties (fluorescence, absorption, etc.). | 1. An electrode surface is coated with biological sensing material for potentiometric, amperometric, or conductimetric measurements [249][79]. |
| 2. Enhanced sensitivity is provided by the plasmonic nature of metals such as Ag, Au, Cu, and Pt. | 2. Involves the modulation of electrical properties such as potential, current, or impedance associated with the interaction of biomolecules with the working electrodes [250][80]. |
| 3. By forming electromagnetic hotspots, GLAD-based nanostructures significantly improve optical spectroscopy (Raman, fluorescence, and infrared) and plasmon resonance sensing [24][81]. | 3. High porosity, large exposed areas, and excellent diffusion properties make GLAD-based metallic nanostructures excellent electrochemical sensors [251,252][82][83]. |
| 4. LOD ~ 1 fM [253][84] | 4. LOD ~ 1 µM [251][82] |
| 5. Nanostructures must be optimized to match optical measurements, must possess high sensitivity, and require trained personnel to operate [54][85] | 5. Comparatively low sensitivity, costly instruments, and trained personnel necessary for their operation [248][78]. |
References
- Homola, J. Present and Future of Surface Plasmon Resonance Biosensors. Anal. Bioanal. Chem. 2003, 377, 528–539.
- Liao, H.; Nehl, C.L.; Hafner, J.H. Biomedical Applications of Plasmon Resonant Metal Nanoparticles. Nanomedicine 2006, 1, 201–208.
- Willets, K.A.; Van Duyne, R.P. Localized Surface Plasmon Resonance Spectroscopy and Sensing. Annu. Rev. Phys. Chem. 2007, 58, 267–297.
- Schuck, P. Use of Surface Plasmon Resonance to Probe The Equilibrium and Dynamic Aspects of Interactions between Biological Macromolecules. Annu. Rev. Biophys. Biomol. Struct. 1997, 26, 541–566.
- Guedon, P.; Livache, T.; Martin, F.; Lesbre, F.; Roget, A.; Bidan, G.; Levy, Y. Characterization and Optimization of a Real-Time, Parallel, Label-Free, Polypyrrole-Based DNA Sensor by Surface Plasmon Resonance Imaging. Anal. Chem. 2000, 72, 6003–6009.
- Jordan, C.E.; Frutos, A.G.; Thiel, A.J.; Corn, R.M.; Peterlinz, K.A.; Georgiadis, R.M.; He, L.; Musick, M.D.; Nicewarner, S.R.; Salinas, F.G.; et al. Surface Plasmon Resonance Imaging Measurements of DNA and RNA Hybridization Adsorption onto DNA Microarrays. Gen. Anal. Biomol. Eng 1997, 69, 1–7.
- Homola, J.; Yee, S.S.; Gauglitz, G. Surface Plasmon Resonance Sensors: Review. Sens. Actuators B Chem. 1999, 54, 3–15.
- Bhalla, N.; Yu, Z.; Pauly, S.; Kumar, A.; Maddi, C.; Mariotti, D.; Zhao, P.; Payam, A.F.; Soin, N. Total Electrification of Large-Scale Nanophotonic Arrays by Frictional Charges. Nanoscale Horiz. 2022, 7, 1513–1522.
- Kumar Agrawal, A.; Das, A.; Dhawan, A. Enhanced Sensitivity of SPR Sensing and Imaging Using Plasmonic Nanopillar Arrays. In Proceedings of the IEEE Conference on Nanotechnology, Cork, Ireland, 23–26 July 2018; IEEE Computer Society: Washington, DC, USA, 2019; Volume 2018, pp. 1–4.
- Agrawal, A.; Gupta, N.; Das, A.; Ahmed, K.; Dhawan, A. Nanostructured Plasmonic Gold Films for Enhanced Sensitivity of SPR Biological Sensing and Imaging. In Proceedings of the Plasmonics in Biology and Medicine XVI, San Francisco, CA, USA, 7 March 2019; Vo-Dinh, T., Ho, H.-P.A., Ray, K., Eds.; SPIE: Bellingham, WA, USA, 2019; Volume 10894, p. 21.
- Moskovits, M. Surface-Enhanced Spectroscopy. Rev. Mod. Phys. 1985, 57, 783–826.
- Fleischmann, M.; Hendra, P.J.; McQuillan, A.J. Raman Spectra of Pyridine Adsorbed at a Silver Electrode. Chem. Phys. Lett. 1974, 26, 163–166.
- Kumar, S.; Taneichi, T.; Fukuoka, T.; Namura, K.; Suzuki, M. Study on Transport of Molecules in Gel by Surface-Enhanced Raman Spectroscopy. Cellulose 2021, 28, 10803–10813.
- Craig, A.P.; Franca, A.S.; Irudayaraj, J. Surface-Enhanced Raman Spectroscopy Applied to Food Safety. Annu. Rev. Food Sci. Technol. 2013, 4, 369–380.
- Granger, J.H.; Schlotter, N.E.; Crawford, A.C.; Porter, M.D. Prospects for Point-of-Care Pathogen Diagnostics Using Surface-Enhanced Raman Scattering (SERS). Chem. Soc. Rev. 2016, 45, 3865–3882.
- Kumar, S.; Goel, P.; Singh, J.P. Flexible and Robust SERS Active Substrates for Conformal Rapid Detection of Pesticide Residues from Fruits. Sens. Actuators B Chem. 2017, 241, 577–583.
- Gahlaut, S.K.; Savargaonkar, D.; Sharan, C.; Yadav, S.; Mishra, P.; Singh, J.P. SERS Platform for Dengue Diagnosis from Clinical Samples Employing a Hand Held Raman Spectrometer. Anal. Chem. 2020, 92, 2527–2534.
- Hughes, J.; Izake, E.L.; Lott, W.B.; Ayoko, G.A.; Sillence, M. Ultra Sensitive Label Free Surface Enhanced Raman Spectroscopy Method for the Detection of Biomolecules. Talanta 2014, 130, 20–25.
- Kong, K.V.; Leong, W.K.; Lam, Z.; Gong, T.; Goh, D.; Lau, W.K.O.; Olivo, M. A Rapid and Label-Free SERS Detection Method for Biomarkers in Clinical Biofluids. Small 2014, 10, 5030–5034.
- Radziuk, D.; Moehwald, H. Prospects for Plasmonic Hot Spots in Single Molecule SERS towards the Chemical Imaging of Live Cells. Phys. Chem. Chem. Phys. 2015, 17, 21072–21093.
- Hakonen, A.; Andersson, P.O.; Stenbæk Schmidt, M.; Rindzevicius, T.; Käll, M. Explosive and Chemical Threat Detection by Surface-Enhanced Raman Scattering: A Review. Anal. Chim. Acta 2015, 893, 1–13.
- Kumar, S.; Kumar, P.; Das, A.; Pathak, C.S.; Kumar, S.; Kumar, P.; Das, A.; Pathak, C.S. Surface-Enhanced Raman Scattering: Introduction and Applications. Recent Adv. Nanophotonics-Fundam. Appl. 2020.
- Sharma, B.; Fernanda Cardinal, M.; Kleinman, S.L.; Greeneltch, N.G.; Frontiera, R.R.; Blaber, M.G.; Schatz, G.C.; Van Duyne, R.P. High-Performance SERS Substrates: Advances and Challenges. MRS Bull. 2013, 38, 615–624.
- Kumar, S.; Tokunaga, K.; Namura, K.; Fukuoka, T.; Suzuki, M. Experimental Evidence of a Twofold Electromagnetic Enhancement Mechanism of Surface-Enhanced Raman Scattering. J. Phys. Chem. C 2020, 124, 21215–21222.
- Ko, H.; Singamaneni, S.; Tsukruk, V.V. Nanostructured Surfaces and Assemblies as SERS Media. Small 2008, 4, 1576–1599.
- Kumar, S.; Fukuoka, T.; Takahashi, R.; Yoshida, M.; Utsumi, Y.; Yamaguchi, A.; Namura, K.; Suzuki, M. Highly Stable and Reproducible Au Nanorod Arrays for Near-Infrared Optofluidic SERS Sensor. Mater. Lett. 2021, 286, 129106.
- Song, C.; Chen, J.; Zhao, Y.; Wang, L. Gold-Modified Silver Nanorod Arrays for SERS-Based Immunoassays with Improved Sensitivity. J. Mater. Chem. B 2014, 2, 7488–7494.
- Devor, E.J.; Behlke, M.A.; Huang, L.; Bogh, L.; Rose, S. Fluorescence and Fluorescence Applications; Integrated DNA Technologies: Coralville, IA, USA, 2005.
- Nanobiosensors and Fluorescence Based Biosensors: An Overview. Available online: http://www.ijnd.ir/article_660965.html (accessed on 19 July 2020).
- Hirshberg, M.; Henrick, K.; Lloyd Haire, L.; Vasisht, N.; Brune, M.; Corrie, J.E.T.; Webb, M.R. Crystal Structure of Phosphate Binding Protein Labeled with a Coumarin Fluorophore, a Probe for Inorganic Phosphate. Biochemistry 1998, 37, 10381–10385.
- Dillingham, M.S.; Tibbles, K.L.; Hunter, J.L.; Bell, J.C.; Kowalczykowski, S.C.; Webb, M.R. Fluorescent Single-Stranded DNA Binding Protein as a Probe for Sensitive, Real-Time Assays of Helicase Activity. Biophys. J. 2008, 95, 3330–3339.
- Aslan, K.; Gryczynski, I.; Malicka, J.; Matveeva, E.; Lakowicz, J.R.; Geddes, C.D. Metal-Enhanced Fluorescence: An Emerging Tool in Biotechnology. Curr. Opin. Biotechnol. 2005, 16, 55–62.
- Matveeva, E.G.; Gryczynski, Z.; Lakowicz, J.R. Myoglobin Immunoassay Based on Metal Particle-Enhanced Fluorescence. J. Immunol. Methods 2005, 302, 26–35.
- Singh, D.P.; Kumar, S.; Singh, J.P. Morphology Dependent Surface Enhanced Fluorescence Study on Silver Nanorod Arrays Fabricated by Glancing Angle Deposition. RSC Adv. 2015, 5, 31341–31346.
- Zhong, W. Nanomaterials in Fluorescence-Based Biosensing. Anal. Bioanal. Chem. 2009, 394, 47–59.
- Aslan, K.; Lakowicz, J.R.; Geddes, C.D. Metal-Enhanced Fluorescence Using Anisotropic Silver Nanostructures: Critical Progress to Date. Anal. Bioanal. Chem. 2005, 382, 926–933.
- Badshah, M.A.; Ju, J.; Lu, X.; Abbas, N.; Kim, S. min Enhancing the Sensitivity of DNA Microarrays by Metal-Enhanced Fluorescence Using Vertical Nanorod Structures. Sens. Actuators B Chem. 2018, 274, 451–457.
- Li, H.; Chen, C.Y.; Wei, X.; Qiang, W.; Li, Z.; Cheng, Q.; Xu, D. Highly Sensitive Detection of Proteins Based on Metal-Enhanced Fluorescence with Novel Silver Nanostructures. Anal. Chem. 2012, 84, 8656–8662.
- Xing, H.; Bu, W.; Zhang, S.; Zheng, X.; Li, M.; Chen, F.; He, Q.; Zhou, L.; Peng, W.; Hua, Y.; et al. Multifunctional Nanoprobes for Upconversion Fluorescence, MR and CT Trimodal Imaging. Biomaterials 2012, 33, 1079–1089.
- Zhang, J.; Fu, Y.; Li, G.; Zhao, R.Y.; Lakowicz, J.R. Direct Observation of Chemokine Receptors 5 on T-Lymphocyte Cell Surfaces Using Fluorescent Metal Nanoprobes 2: Approximation of CCR5 Populations. Biochem. Biophys. Res. Commun. 2011, 407, 63–67.
- Mayer, K.M.; Hafner, J.H. Localized Surface Plasmon Resonance Sensors. Chem. Rev. 2011, 111, 3828–3857.
- Fu, J.X.; Collins, A.; Zhao, Y.P. Optical Properties and Biosensor Application of Ultrathin Silver Films Prepared by Oblique Angle Deposition. J. Phys. Chem. C 2008, 112, 16784–16791.
- Zhang, X.; Zhou, W.; Yuan, Z.; Lu, C. Colorimetric Detection of Biological Hydrogen Sulfide Using Fluorosurfactant Functionalized Gold Nanorods. Analyst 2015, 140, 7443–7450.
- Jarosz, A.P.; Yep, T.; Mutus, B. Microplate-Based Colorimetric Detection of Free Hydrogen Sulfide. Anal. Chem. 2013, 85, 3638–3643.
- Lilienfeld, S.; White, C.E. A Study of the Reaction between Hydrogen Sulfide and Silver. J. Am. Chem. Soc. 1930, 52, 885–892.
- Graedel, T.E.; Franey, J.P.; Gualtieri, G.J.; Kammloty, G.W.; Malm, D.L. On the mechanism of silver and copper sulfidation by atmospheric h2s and ocs. Corros. Sci. 1985, 25, 1163–1180.
- Graedel, T.E. Corrosion Mechanisms for Silver Exposed to the Atmosphere. J. Electrochem. Soc. 1992, 139, 1963.
- Kim, H. Corrosion Process of Silver in Environments Containing 0.1 Ppm H2S and 1.2 Ppm NO2. Mater. Corros. 2003, 54, 243–250.
- Chen, R.; Morris, H.R.; Whitmore, P.M. Fast Detection of Hydrogen Sulfide Gas in the Ppmv Range with Silver Nanoparticle Films at Ambient Conditions. Sens. Actuators B Chem. 2013, 186, 431–438.
- Gahlaut, S.K.; Yadav, K.; Sharan, C.; Singh, J.P. Quick and Selective Dual Mode Detection of H2S Gas by Mobile App Employing Silver Nanorods Array. Anal. Chem. 2017, 89, 13582–13588.
- Kruse, C.; Anderson, T.; Wilson, C.; Zuhlke, C.; Alexander, D.; Gogos, G.; Ndao, S. Extraordinary Shifts of the Leidenfrost Temperature from Multiscale Micro/Nanostructured Surfaces. Langmuir 2013, 29, 9798–9806.
- Singh, D.P.; Singh, J.P. Delayed Freezing of Water Droplet on Silver Nanocolumnar Thin Film. Appl. Phys. Lett. 2013, 102, 243112.
- Tyrrell, J.W.G.; Attard, P. Images of Nanobubbles on Hydrophobic Surfaces and Their Interactions. Phys. Rev. Lett. 2001, 87, 176104.
- Li, C.; Wang, Z.; Wang, P.I.; Peles, Y.; Koratkar, N.; Peterson, G.P. Nanostructured Copper Interfaces for Enhanced Boiling. Small 2008, 4, 1084–1088.
- Gahlaut, S.K.; Kalyani, N.; Sharan, C.; Mishra, P.; Singh, J.P. Smartphone Based Dual Mode in Situ Detection of Viability of Bacteria Using Ag Nanorods Array. Biosens. Bioelectron. 2019, 126, 478–484.
- Gahlaut, S.K.; Devi, P.; Singh, J.P. Self-Sustainable and Recyclable Ag Nanorods for Developing Ag-Ag2S Nano Heterostructures Using Sewage Gas: Applications in Photocatalytic Water Purification, Hydrogen Evolution, SERS and Antibacterial Activity. Appl. Surf. Sci. 2020, 528, 147037.
- Cassidy, P.J.; Radda, G.K. Molecular Imaging Perspectives. J. R. Soc. Interface 2005, 2, 133–144.
- Minchin, R.F.; Martin, D.J. Minireview: Nanoparticles for Molecular Imaging—An Overview. Endocrinology 2010, 151, 474–481.
- Sheng, Y.; De Liao, L.; Thakor, N.V.; Tan, M.C. Nanoparticles for Molecular Imaging. J. Biomed. Nanotechnol. 2014, 10, 2641–2676.
- Padmanabhan, P.; Kumar, A.; Kumar, S.; Chaudhary, R.K.; Gulyás, B. Nanoparticles in Practice for Molecular-Imaging Applications: An Overview. Acta Biomater. 2016, 41, 1–16.
- Champion, J.A.; Katare, Y.K.; Mitragotri, S. Making Polymeric Micro- and Nanoparticles of Complex Shapes. Proc. Natl. Acad. Sci. USA 2007, 104, 11901–11904.
- Tirrell, D.A.; Langer, R. Materials for Biology and Medicine. Nature 2012, 428, 25.
- Love, J.C.; Gates, B.D.; Wolfe, D.B.; Paul, K.E.; Whitesides, G.M. Fabrication and Wetting Properties of Metallic Half-Shells with Submicron Diameters. Nano Lett. 2002, 2, 891–894.
- Sun, Y.; Xia, Y. Shape-Controlled Synthesis of Gold and Silver Nanoparticles. Science 2002, 298, 2176–2179.
- Pawar, A.B.; Kretzschmar, I. Fabrication, Assembly, and Application of Patchy Particles. Macromol. Rapid Commun. 2010, 31, 150–168.
- Zhang, J.; Grzybowski, B.A.; Granick, S. Janus Particle Synthesis, Assembly, and Application. Langmuir 2017, 33, 6964–6977.
- Yi, Y.; Sanchez, L.; Gao, Y.; Yu, Y. Janus Particles for Biological Imaging and Sensing. Analyst 2016, 141, 3526–3539.
- Wu, Z.; Li, L.; Liao, T.; Chen, X.; Jiang, W.; Luo, W.; Yang, J.; Sun, Z. Janus Nanoarchitectures: From Structural Design to Catalytic Applications. Nano Today 2018, 22, 62–82.
- Loget, G.; Roche, J.; Gianessi, E.; Bouffier, L.; Kuhn, A. Indirect Bipolar Electrodeposition. J. Am. Chem. Soc. 2012, 134, 20033–20036.
- Nie, Z.; Li, W.; Seo, M.; Xu, S.; Kumacheva, E. Janus and Ternary Particles Generated by Microfluidic Synthesis: Design, Synthesis, and Self-Assembly. J. Am. Chem. Soc. 2006, 128, 9408–9412.
- Roh, K.H.; Martin, D.C.; Lahann, J. Biphasic Janus Particles with Nanoscale Anisotropy. Nat. Mater. 2005, 4, 759–763.
- Pawar, A.B.; Kretzschmar, I. Multifunctional Patchy Particles by Glancing Angle Deposition Technique. In Proceedings of the 2008 Annual Meeting, Washington, DC, USA, 13 October 2008; pp. 355–358.
- Imura, Y.; Kato, M.; Kondo, T.; Kawai, T. Strings of Metal Half-Shells Fabricated Using Colloidal Particle Monolayer as a Template. Langmuir 2010, 26, 11314–11318.
- Xuan, M.; Shao, J.; Lin, X.; Dai, L.; He, Q. Self-Propelled Janus Mesoporous Silica Nanomotors with Sub-100 Nm Diameters for Drug Encapsulation and Delivery. ChemPhysChem 2014, 15, 2255–2260.
- Peng, F.; Men, Y.; Tu, Y.; Chen, Y.; Wilson, D.A. Nanomotor-Based Strategy for Enhanced Penetration across Vasculature Model. Adv. Funct. Mater. 2018, 28, 1706117.
- Wu, Z.; Troll, J.; Jeong, H.H.; Wei, Q.; Stang, M.; Ziemssen, F.; Wang, Z.; Dong, M.; Schnichels, S.; Qiu, T.; et al. A Swarm of Slippery Micropropellers Penetrates the Vitreous Body of the Eye. Sci. Adv. 2018, 4, eaat4388.
- Bochenkov, V.; Baumberg, J.; Noginov, M.; Benz, F.; Aldewachi, H.; Schmid, S.; Podolskiy, V.; Aizpurua, J.; Lin, K.; Ebbesen, T.; et al. Applications of Plasmonics: General Discussion. Faraday Discuss. 2015, 178, 435–466.
- Martín, M.; Salazar, P.; Álvarez, R.; Palmero, A.; López-Santos, C.; González-Mora, J.L.; González-Elipe, A.R. Cholesterol Biosensing with a Polydopamine-Modified Nanostructured Platinum Electrode Prepared by Oblique Angle Physical Vacuum Deposition. Sens. Actuators B Chem. 2017, 240, 37–45.
- El Kazzy, M.; Weerakkody, J.S.; Hurot, C.; Mathey, R.; Buhot, A.; Scaramozzino, N.; Hou, Y. An Overview of Artificial Olfaction Systems with a Focus on Surface Plasmon Resonance for the Analysis of Volatile Organic Compounds. Biosensors 2021, 11, 244.
- Wilson, A.D.; Baietto, M. Applications and Advances in Electronic-Nose Technologies. Sensors 2009, 9, 5099–5148.
- Gahlaut, S.K.; Pathak, A.; Gupta, B.D. Recent Advances in Silver Nanostructured Substrates for Plasmonic Sensors. Biosensors 2022, 12, 713.
- Salazar, P.; Rico, V.; González-Elipe, A.R. Nickel–Copper Bilayer Nanoporous Electrode Prepared by Physical Vapor Deposition at Oblique Angles for the Non-Enzymatic Determination of Glucose. Sens. Actuators B Chem. 2016, 226, 436–443.
- Yadav, J.; Raturi, P.; Yadav, S.; Singh, J.P. Zig-Zag Ag2S Nanostructures for Superior Optical Absorption and Photoelectrochemical Water Splitting Performance. Renew. Energy 2021, 179, 2256–2266.
- Norrod, K.L.; Sudnik, L.M.; Rousell, D.; Rowlen, K.L. Quantitative Comparison of Five SERS Substrates: Sensitivity and Limit of Detection. Appl. Spectrosc. 2016, 51, 994–1001.
- Yadav, S.; Senapati, S.; Desai, D.; Gahlaut, S.; Kulkarni, S.; Singh, J.P. Portable and Sensitive Ag Nanorods Based SERS Platform for Rapid HIV-1 Detection and Tropism Determination. Colloids Surf. B Biointerfaces 2021, 198, 111477.
More
