You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 2 by Beatrix Zheng and Version 1 by Saskia Braber.
Emerging antimicrobial resistance in respiratory infections requires novel intervention strategies. Non-digestible oligosaccharides (NDOs) are a diverse group of carbohydrates with broad protective effects. In addition to promoting the colonization of beneficial gut microbiota and maintaining the intestinal homeostasis, NDOs act as decoy receptors, effectively blocking the attachment of pathogens on host cells. NDOs also function as a bacteriostatic agent, inhibiting the growth of specific pathogenic bacteria. Based on this fact, NDOs potentiate the actions of antimicrobial drugs. Therefore, there is an increasing interest in characterizing the anti-infective properties of NDOs.
- oligosaccharides
- lung infections
- respiratory inflammation
1. Respiratory Infections
Respiratory infections are the largest cause of childhood deaths [1,2][1][2] and an are important cause of morbidity and mortality among adults worldwide [3,4][3][4]. Respiratory infections take responsibility for approximately 4 million deaths per year globally [3]. Among them, lung infections are a common and potentially life-threatening illness, being a major medical burden accounting for more than 15% of the deaths of children younger than 5 years of age [2]. Risk factors for the incidence and severity of lung infections in infants and children mostly include the lack of immunization, malnutrition, chronic underlying diseases, HIV infection and smoke exposure/indoor air pollution [5]. The Global Burden of Disease Study indicated that the most important risk factors were malnutrition, air pollution or sub-optimal breastfeeding [6]. In 2015, although the improvement of living conditions, nutrition and vaccines, 700,000 children younger than 5 years of age still died from lung infections worldwide [6]. In particular, the COVID-19 pandemic has increased the emergency of taking action to protect against respiratory infections.
Transmission of lung infections is thought to occur by airborne droplets/pathogens or through direct contact with colonized/infected individuals. The epithelial mucosal surface of the lungs is constantly exposed to invasive pathogens that have the potential to threaten the defense of susceptible hosts [7]. After the epithelial mucosa is invaded by pathogens, the inflammatory response occurs subsequently to recruit additional defenses. However, when these pathogens have the capacity to overwhelm the host defense, invasion of pathogens results in infections of the respiratory tract [7,8,9][7][8][9].
2. Non-Digestible Oligosaccharides (NDOs)
NDOs are low molecular weight carbohydrates, usually containing 3 to 10 sugar moieties. Food products that naturally contain NDOs, such as cereals, fruits, vegetables, nuts, beans, and seafood, but also breast milk and the application of prebiotics in functional food are the main sources of NDO intake [16][10]. Many NDOs are not digested by humans due to the lack of enzymes required to hydrolyze the β-links formed among the monosaccharide units. The most famous physicochemical and physiological properties of NDOs are related to their ability to behave like dietary fibers and prebiotics, including the improvement of gut microbial composition and gastrointestinal homeostasis. Moreover, due to the decrease in intestinal pH caused by their fermentation, NDOs exhibit the ability to reduce the growth of pathogenic bacteria, increase the populations of bifidobacteria and lactobacilli, and increase the utilization of minerals in the gut [16][10]. In addition, NDOs are associated with a lower risk of (gastrointestinal, respiratory, and urogenital) infections and exert anti-inflammatory and immunomodulatory properties [16,17][10][11]. In this revisew, wearch, the researchers mainly focused on several representative NDOs, including human milk oligosaccharides (HMOs), and galacto-oligosaccharides (GOS) and fructo-oligosaccharides (FOS) that mimic structures observed in mother milk.
2.1. HMOs
Human milk is the golden standard for infant nutrition, as most health experts, including the American Academy of Pediatrics, recommend exclusive breastfeeding for the first six months of life [18][12]. The average macromolecular profile of one liter of breast milk contains 9–12 g of proteins, 32–36 g of fats, 67–78 g of lactose, and 5–20 g of HMOs [19][13]. HMOs are the first group of NDOs consumed by humans after birth. Breastfed infants have a lower incidence of respiratory diseases, including respiratory infections, during early life [20,21,22,23][14][15][16][17]. In addition to the well-known prebiotic properties of HMOs and corresponding immunomodulatory effects [16][10], approximately 1–5% of HMOs are absorbed by the intestine into the systemic circulation [24][18], directly interacting with pathogens, immune cells, and epithelial cells outside the intestine to exert anti-inflammatory and anti-infective effects [16][10]. Moreover, breastfeeding infants ingest mother milk several times per day, bathing the nasopharynx and mouth for several minutes at each feeding with a solution high in HMOs, which might inhibit local adherence of airway pathogenic bacteria [20][14]. Bovine milk-derived infant formula is commonly used as an alternative to human breast milk. However, the concentration of oligosaccharides in bovine milk (0.7–1.2 g/L) is much lower compared to human milk (12–24 g/L) [25,26][19][20]. HMOs are composed of the five monosaccharide building blocks D-glucose, D-galactose, N-acetylglucosamine, L-fucose, and sialic acid. Currently, around 200 oligosaccharide structures have been identified in human milk compared to ± 50 identified oligosaccharide structures in bovine milk [27][21]. More than 70% of the oligosaccharides in bovine milk are composed of sialylated oligosaccharides, in contrast, human milk contains predominantly neutral oligosaccharides, and sialylated oligosaccharides account for approximately 10–30% of total HMOs [24,26,27,28,29][18][20][21][22][23]. Despite recent modern analytical techniques, the identification and biosynthesis of HMOs remain a challenge for researchers. The composition and content of HMOs can vary considerably between mothers. It depends both on their blood group and on the duration/length of the lactation period. Many manufacturers are trying to emulate HMOs; however, due to the lack of industrial production methods, the essential ingredients are mostly absent from infant formulas [30][24].
2.2. GOS and FOS
Various strategies have been used to mimic the beneficial effects of HMOs, including GOS and FOS, which have been supplemented in dietary products and infant formula [31,32][25][26]. Commercial production of GOS has been achieved from lactose by the transgalactosylation reactions, using β-galactosidases (EC 3.2.1.23) as biocatalysts [17,33][11][27]. FOS can be produced from the controlled enzymatic hydrolysis of the polysaccharide inulin, which can be extracted from roots of chicory, artichoke, yacon, dahlia or agave [33,34][27][28]. The process involves transfructosylation reactions in which fructosyltransferases (β-fructofuranosidase, EC 3.2.1.26 or β-D-fructosyltransferase, EC 2.4.1.9) act as biocatalysts [33][27]. GOS/FOS in a 9:1 ratio is commonly used in infant formula to mimic the molecular size distribution and beneficial functions of HMOs in breast milk [35][29].
Both GOS and FOS exert many beneficial properties. For example, both can stimulate the growth of
bifidobacteria
and
lactobacilli and support the development of the immune system. Moreover, both can inhibit the inflammatory responses and prevent epithelial barrier dysfunction in the intestine; in particular, GOS have the property of inhibiting the adhesion of pathogens to intestinal epithelial cells [16,32]. There is no doubt that GOS/FOS mixtures have similar properties, and even recently, a reduction in airway inflammation after oral administration of this mixture was demonstrated [36,37]. There are several studies investigating the supplementation of GOS and/or FOS in human respiratory infections mainly focusing on clinical parameters, observing a reduced frequency of respiratory infections and antibiotic prescriptions in infants, as well as a decreased duration and symptoms of cold or flu in university students [38,39,40]. These impressive observations encourage GOS and/or FOS to become attractive candidates in the prevention and clinical treatment of respiratory infections. Although not reported in respiratory infections, the effects of NDOs in some studies of inflammatory immune diseases are inconsistent; for example, a combination of probiotics and GOS showed no preventive effect on allergic diseases in infants [41]. In a study with mice, a mixture of FOS and inulin did not affect the immune response of delayed hypersensitivity in an influenza vaccination model [42]. In this focused review, we present a balanced overview of the role and mechanisms of NDOs in respiratory diseases (infections) (
and support the development of the immune system. Moreover, both can inhibit the inflammatory responses and prevent epithelial barrier dysfunction in the intestine; in particular, GOS have the property of inhibiting the adhesion of pathogens to intestinal epithelial cells [10][26]. There is no doubt that GOS/FOS mixtures have similar properties, and even recently, a reduction in airway inflammation after oral administration of this mixture was demonstrated [30][31]. There are several studies investigating the supplementation of GOS and/or FOS in human respiratory infections mainly focusing on clinical parameters, observing a reduced frequency of respiratory infections and antibiotic prescriptions in infants, as well as a decreased duration and symptoms of cold or flu in university students [32][33][34]. These impressive observations encourage GOS and/or FOS to become attractive candidates in the prevention and clinical treatment of respiratory infections. Although not reported in respiratory infections, the effects of NDOs in some studies of inflammatory immune diseases are inconsistent; for example, a combination of probiotics and GOS showed no preventive effect on allergic diseases in infants [35]. In a study with mice, a mixture of FOS and inulin did not affect the immune response of delayed hypersensitivity in an influenza vaccination model [36]. In this focused research, the researchers present a balanced overview of the role and mechanisms of NDOs in respiratory diseases (infections) (
Figure 1).
).
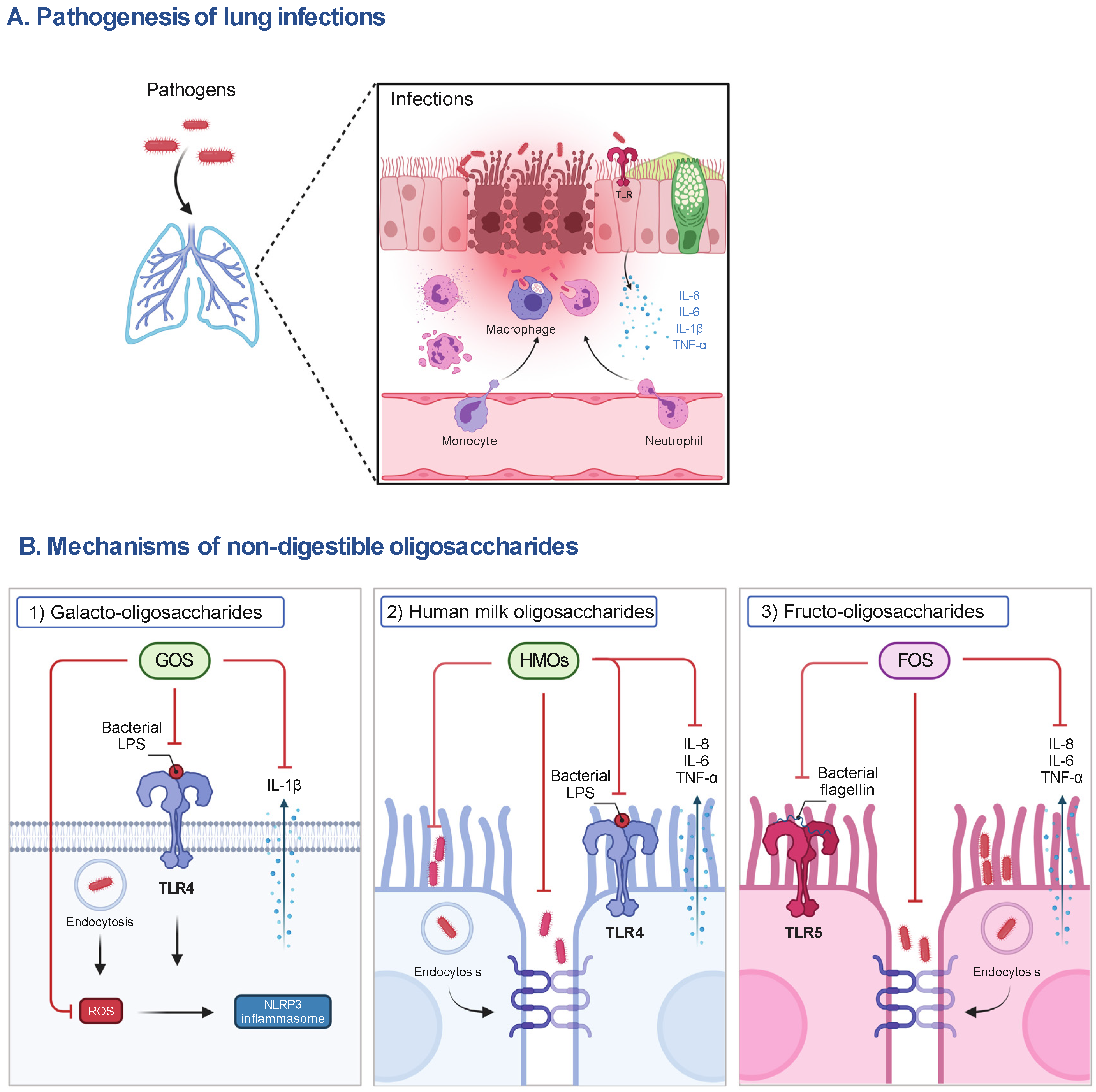
Figure 1. The pathogenesis of lung infections and the postulated mechanisms of representative NDOs on host cells. (A) During lung infections, pathogens can induce the release of pro-inflammatory mediators (e.g., IL-8, IL-6, IL-1β and TNF-α) by activating TLR signaling to recruit immune cells (e.g., neutrophils, macrophages), contributing to the phagocytosis of pathogens and elimination of inflammation in the early stage of infections. However, the impairment of the airway epithelial barrier, the accumulation of pro-inflammatory mediators, the depletion of macrophages, and the infiltration of neutrophils caused by excessive pathogens and their released virulence factors (e.g., LPS), lead to lung injury and organ dysfunction and even death of susceptible hosts. (B) (1) The anti-inflammatory mechanisms of NDOs (e.g., GOS) may include the inhibition of NLRP3 inflammasome activation via the interference with TLR-4 signaling and the decrease in ROS production, subsequently reducing IL-1β release, and (2) the decrease in adhesion to and invasion of airway epithelial cells by pathogens or the direct killing of pathogens induced by NDOs (e.g., HMOs). (3) Anti-inflammatory effects of NDOs (e.g., FOS) may be related to the interference with TLR-5 pro-inflammatory signaling and protection of airway epithelial barrier function. FOS, fructo-oligosaccharides; GOS, galacto-oligosaccharides; IL, interleukin; LPS, lipopolysaccharides; NLRP3, NLR family pyrin domain containing 3; ROS, reactive oxygen species; TLR, Toll-like receptor; TNF-α, tumor necrosis factor-α.
References
- Ravi Kumar, S.; Paudel, S.; Ghimire, L.; Bergeron, S.; Cai, S.; Zemans, R.L.; Downey, G.P.; Jeyaseelan, S. Emerging Roles of Inflammasomes in Acute Pneumonia. Am. J. Respir. Crit. Care Med. 2018, 197, 160–171.
- WHO. Pneumonia Fact Sheet. . Available online: http://www.who.int/mediacentre/factsheets/fs331/en/ (accessed on 2 May 2021).
- Ferkol, T.; Schraufnagel, D. The global burden of respiratory disease. Ann. Am. Thorac. Soc. 2014, 11, 404–406.
- Dwyer, L.L.; Harris-Kojetin, L.D.; Valverde, R.H.; Frazier, J.M.; Simon, A.E.; Stone, N.D.; Thompson, N.D. Infections in long-term care populations in the United States. J. Am. Geriatr. Soc. 2013, 61, 342–349.
- Marangu, D.; Zar, H.J. Childhood pneumonia in low-and-middle-income countries: An update. Paediatr. Respir. Rev. 2019, 32, 3–9.
- Collaborators, G.L. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory tract infections in 195 countries: A systematic analysis for the Global Burden of Disease Study 2015. Lancet Infect. Dis. 2017, 17, 1133–1161.
- Leiva-Juárez, M.M.; Kolls, J.K.; Evans, S.E. Lung epithelial cells: Therapeutically inducible effectors of antimicrobial defense. Mucosal Immunol. 2018, 11, 21–34.
- Waites, K.B.; Xiao, L.; Liu, Y.; Balish, M.F.; Atkinson, T.P. Mycoplasma pneumoniae from the Respiratory Tract and Beyond. Clin. Microbiol. Rev. 2017, 30, 747–809.
- Caswell, J.L. Failure of respiratory defenses in the pathogenesis of bacterial pneumonia of cattle. Vet. Pathol. 2014, 51, 393–409.
- Cai, Y.; Folkerts, J.; Folkerts, G.; Maurer, M.; Braber, S. Microbiota-dependent and -independent effects of dietary fibre on human health. Br. J. Pharmacol. 2020, 177, 1363–1381.
- Mussatto, S.I.; Mancilha, I.M. Non-digestible oligosaccharides: A review. Carbohydr. Polym. 2007, 68, 587–597.
- Gartner, L.M.; Morton, J.; Lawrence, R.A.; Naylor, A.J.; O’Hare, D.; Schanler, R.J.; Eidelman, A.I.; American Academy of Pediatrics Section on Breastfeeding. Breastfeeding and the use of human milk. Pediatrics 2005, 115, 496–506.
- Moore, R.E.; Xu, L.L.; Townsend, S.D. Prospecting Human Milk Oligosaccharides as a Defense Against Viral Infections. ACS Infect. Dis. 2021, 7, 254–263.
- Zopf, D.; Roth, S. Oligosaccharide anti-infective agents. Lancet 1996, 347, 1017–1021.
- César, J.A.; Victora, C.G.; Barros, F.C.; Santos, I.S.; Flores, J.A. Impact of breast feeding on admission for pneumonia during postneonatal period in Brazil: Nested case-control study. BMJ 1999, 318, 1316–1320.
- Howie, P.W.; Forsyth, J.S.; Ogston, S.A.; Clark, A.; Florey, C.D. Protective effect of breast feeding against infection. BMJ 1990, 300, 11–16.
- Oddy, W.H.; Sly, P.D.; de Klerk, N.H.; Landau, L.I.; Kendall, G.E.; Holt, P.G.; Stanley, F.J. Breast feeding and respiratory morbidity in infancy: A birth cohort study. Arch. Dis. Child. 2003, 88, 224–228.
- Bode, L. Human milk oligosaccharides: Every baby needs a sugar mama. Glycobiology 2012, 22, 1147–1162.
- Newburg, D.S.; Neubauer, S.H. CHAPTER 4-Carbohydrates in Milks: Analysis, Quantities, and Significance. In Handbook of Milk Composition; Jensen, R.G., Ed.; Academic Press: San Diego, CA, USA, 1995; pp. 273–349.
- Tao, N.; DePeters, E.J.; German, J.B.; Grimm, R.; Lebrilla, C.B. Variations in bovine milk oligosaccharides during early and middle lactation stages analyzed by high-performance liquid chromatography-chip/mass spectrometry. J. Dairy Sci. 2009, 92, 2991–3001.
- Robinson, R.C. Structures and Metabolic Properties of Bovine Milk Oligosaccharides and Their Potential in the Development of Novel Therapeutics. Front. Nutr. 2019, 6, 50.
- Tao, N.; DePeters, E.J.; Freeman, S.; German, J.B.; Grimm, R.; Lebrilla, C.B. Bovine milk glycome. J. Dairy Sci. 2008, 91, 3768–3778.
- Nwosu, C.C.; Aldredge, D.L.; Lee, H.; Lerno, L.A.; Zivkovic, A.M.; German, J.B.; Lebrilla, C.B. Comparison of the human and bovine milk N-glycome via high-performance microfluidic chip liquid chromatography and tandem mass spectrometry. J. Proteome Res. 2012, 11, 2912–2924.
- Intanon, M.; Arreola, S.L.; Pham, N.H.; Kneifel, W.; Haltrich, D.; Nguyen, T.H. Nature and biosynthesis of galacto-oligosaccharides related to oligosaccharides in human breast milk. FEMS Microbiol. Lett. 2014, 353, 89–97.
- Zivkovic, A.; Barile, D. Bovine milk as a source of functional oligosaccharides for improving human health. Adv. Nutr. 2011, 2, 284–289.
- Akkerman, R.; Faas, M.M.; and de Vos, P. Non-digestible carbohydrates in infant formula as substitution for human milk oligosaccharide functions: Effects on microbiota and gut maturation. Crit. Rev. Food Sci. Nutr. 2019, 59, 1486–1497.
- Martins, G.N.; Ureta, M.M.; Tymczyszyn, E.E.; Castilho, P.C.; Gomez-Zavaglia, A. Technological Aspects of the Production of Fructo and Galacto-Oligosaccharides. Enzymatic Synthesis and Hydrolysis. Front. Nutr. 2019, 6, 78.
- Crittenden, R.; Playne, M. Production, properties and applications of food-grade oligosaccharides. Trends Food Sci. Technol. 1996, 7, 353–361.
- Knol, J.; Scholtens, P.; Kafka, C.; Steenbakkers, J.; Gro, S.; Helm, K.; Klarczyk, M.; Schöpfer, H.; Böckler, H.M.; Wells, J. Colon microflora in infants fed formula with galacto- and fructo-oligosaccharides: More like breast-fed infants. J. Pediatr. Gastroenterol. Nutr. 2005, 40, 36–42.
- Janbazacyabar, H.; Bergenhenegouwen, J.V.; Verheijden, K.A.; Leusink-Muis, T.; Helvoort, A.A.; Garssen, J.; Folkerts, G.; Braber, S. Non-digestible oligosaccharides partially prevent the development of LPS-induced lung emphysema in mice. PharmaNutrition 2019, 10, 100163.
- Sagar, S.; Vos, A.P.; Morgan, M.E.; Garssen, J.; Georgiou, N.A.; Boon, L.; Kraneveld, A.D.; Folkerts, G. The combination of Bifidobacterium breve with non-digestible oligosaccharides suppresses airway inflammation in a murine model for chronic asthma. Biochim. Biophys. Acta 2014, 1842, 573–583.
- Arslanoglu, S.; Moro, G.E.; Boehm, G. Early supplementation of prebiotic oligosaccharides protects formula-fed infants against infections during the first 6 months of life. J. Nutr. 2007, 137, 2420–2424.
- Arslanoglu, S.; Moro, G.E.; Schmitt, J.; Tandoi, L.; Rizzardi, S.; Boehm, G. Early dietary intervention with a mixture of prebiotic oligosaccharides reduces the incidence of allergic manifestations and infections during the first two years of life. J. Nutr. 2008, 138, 1091–1095.
- Hughes, C.; Davoodi-Semiromi, Y.; Colee, J.C.; Culpepper, T.; Dahl, W.J.; Mai, V.; Christman, M.C.; Langkamp-Henken, B. Galactooligosaccharide supplementation reduces stress-induced gastrointestinal dysfunction and days of cold or flu: A randomized, double-blind, controlled trial in healthy university students. Am. J. Clin. Nutr. 2011, 93, 1305–1311.
- Kukkonen, K.; Savilahti, E.; Haahtela, T.; Juntunen-Backman, K.; Korpela, R.; Poussa, T.; Tuure, T.; Kuitunen, M. Probiotics and prebiotic galacto-oligosaccharides in the prevention of allergic diseases: A randomized, double-blind, placebo-controlled trial. J. Allergy Clin. Immunol. 2007, 119, 192–198.
- Vos, A.P.; Haarman, M.; Buco, A.; Govers, M.; Knol, J.; Garssen, J.; Stahl, B.; Boehm, G.; M’Rabet, L. A specific prebiotic oligosaccharide mixture stimulates delayed-type hypersensitivity in a murine influenza vaccination model. Int. Immunopharmacol. 2006, 6, 1277–1286.
More
