Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Vivi Li and Version 1 by Marina Mat Baki.
Glottic insufficiency is widespread in the elderly population and occurs as a result of secondary damage or systemic disease. Tissue engineering is a viable treatment for glottic insufficiency since it aims to restore damaged nerve tissue and revitalize aging muscle. After injection into the biological system, injectable biomaterial delivers cost- and time-effectiveness while acting as a protective shield for cells and biomolecules.
- glottic insufficiency
- vocal fold injection
- incorporation
- cell-laden
- biomolecule
1. Introduction
Voice disorders affect 16.9% of the adult population (aged 18 and more) and 13.1% more of the elderly population (aged 85 and more) [1,2,3][1][2][3]. Glottic insufficiency is diagnosed when the vocal fold does not entirely close during phonation [4]. It impairs voice production and the protection of the lower airway, resulting in impaired social function, decreased work performance, and an increased risk of aspiration. Due to the unique characteristics of the human vocal fold and the numerous causes of glottic insufficiency, it is difficult to recommend the optimal treatment for this illness. Tissue engineering has advantages in this field since it strives to enhance regeneration and provides longer-lasting or even permanent vocal fold augmentation [5,6][5][6]. Tissue engineering has been researched extensively in several regenerative techniques, including cartilage, neuron, cardiac, and bone regeneration [7,8,9,10][7][8][9][10]. Nonetheless, previous studies [11,12,13,14][11][12][13][14] have identified vocal fold fibroblasts, muscle progenitor cells, embryonic stem cells (ESCs), bone marrow mesenchymal stem cells (BMMSCs), and adipose stem cells (ASCs) with or without the use of a scaffold as a delivery vehicle for vocal fold regeneration. This sentudry seeks to outline the most recent advancements in injectable biomaterials that transport biomolecules and cells for regeneration purposes and to identify future directions for tissue engineering–based treatment of glottic insufficiency.
1.1. Structure of Vocal Fold
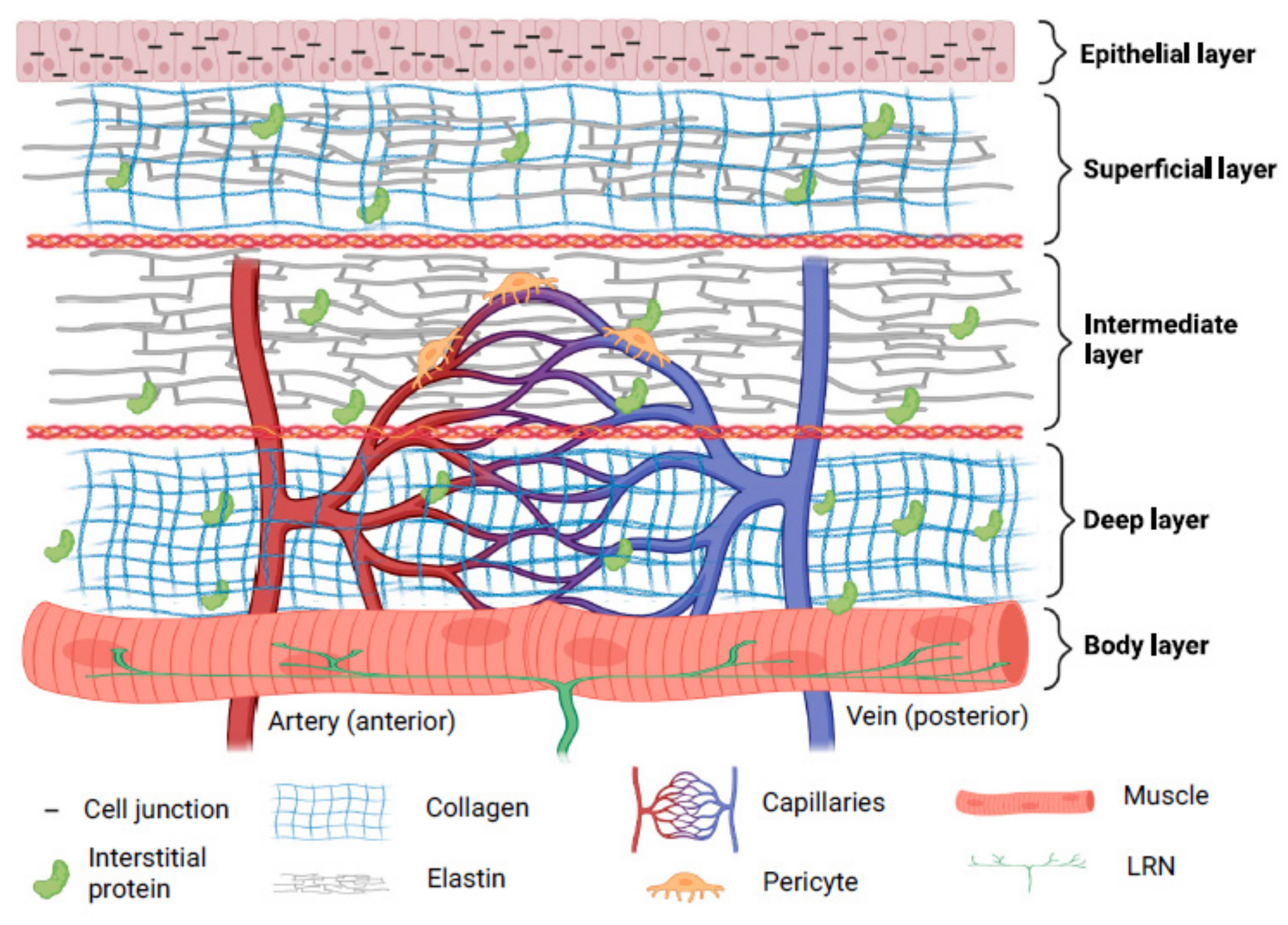
Three layers—cover, transition, and body—are thought to make up a human vocal fold [15]. Collagen, elastin, hyaluronic acid (HA), decorin, and fibronectin make up the majority of the extracellular matrix (ECM) proteins found in the lamina propria [16]. The superficial layers of lamina propria and epithelium in the cover have vibratory qualities which are crucial for phonation. Superficial lamina propria comprises loosely packed connective tissue [17]. The transition layer is made up of an intermediate layer primarily of elastin and deep layer of collagen. Collagen provides mechanical support to the vocal fold while elastin maintains the elasticity of the vocal fold [18,19][18][19]. The vocalis muscle makes up the body layer, which forms the base of this intricate three-dimensional structure [15]. Interestingly, cell junctions hold stratified squamous epithelium, which serves as a protective layer around membranous vocal folds [20]. Compared to newborn epithelial cells, adult epithelial cells displayed more significant intercellular gaps, greater mechanical strength, and more excellent elasticity [21]. In the lamina propria, the vascular network disperses differently. Only capillaries are seen in the superficial lamina propria; arterioles and venules are located in the intermediate and deep lamina propria. In muscular tissue, bigger vessels are more prevalent. Pericytes have been seen on capillaries, and it is thought that pericytes can shield capillaries from lamina propria vibration. Additionally, pericytes are found to be crucial for angiogenesis [22].
The paraglottic area, which houses intrinsic laryngeal muscle, nerve connections, and adipose tissue, is connected laterally to the vocal fold [23]. Intrinsic laryngeal muscles include thyroarytenoid (TA), lateral-cricoarytenoid (LCA) and interarytenoid (IA) muscles for adduction, and the posterior cricoarytenoid (PCA) muscle for abduction [24]. It is suggested that the TA, cricothyroid (CT), and LCA function as a single muscle stimulated by a motor unit [25]. Recurrent laryngeal nerves (RLN) and superior laryngeal nerves (SLN) provide intrinsic and extrinsic impulses, respectively [26]. RLN perform adduction and abduction functions [27]. The inability to change vocal pitch due to CT motor loss is directly related to SLN dysfunction [28]. The RLN innervates the thyroarytenoid-lateral cricoarytenoid (TA-LCA) for adduction, whereas the SLN innervates the CT. It can be summarized that the composition of the vocal fold includes elastic components which enable its phonation properties, mechanically strong components to support its structure, blood capillaries to provide nutrients to cells, and nerves to control its movement. The structure of the human vocal fold is briefly demonstrated in Figure 1.
1.2. Etiologies of Glottic Insufficiency
It is vital for the clinician to differentiate the causes of glottic insufficiency in opting for suitable treatments. The main symptoms of glottic insufficiency include being unable to generate an effective voice and being unable to protect the lower airway during swallowing. The most common cause of glottic insufficiency is vocal fold paralysis/paresis. Simply put, dysfunctional nerves or muscles are the primary causes of the vocal fold’s inadequate closure. Figure 2 explains the physiological characteristics of the normal condition and glottic insufficiency.
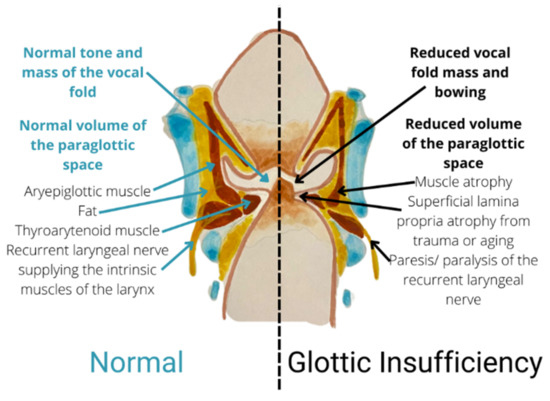
Figure 2.
Coronal section showing a comparison between normal condition and glottic insufficiency.
Vocal fold paralysis/paresis is identified when the RLN or SLN are damaged, causing the inability of the intrinsic laryngeal muscles to contract [24,29,30][24][29][30], contributing to the inability to move the vocal fold. Vocal fold paresis is defined when the nerve is partially damaged, causing incomplete signaling or abnormal signaling of nerve; paralysis is diagnosed when the vocal fold is not able to move completely [26]. Vocal fold paresis/paralysis has many etiologies, including scarring, iatrogenic disorders, malignancy, central nervous system pathology, and systemic illnesses [31]. An idiopathic cause is searched for when an aetiology cannot be determined after a comprehensive study.
Vocal fold atrophy is characterized when there is dissipation of muscle and loss of intonation even though the TA-LCA complex is mobile within a certain range [32]. Presbyphonia, child/adolescent, and inborn vocal fold scar are the three distinct types of vocal fold atrophy [33,34,35,36][33][34][35][36]. Commonly, the decreased sensitivity or malfunction of the contractile components inside the TA muscle is linked to the pathophysiology of vocal fold atrophy [1]. One of the possible causes of an ageing voice is structural changes in the vocal fold’s lamina propria [37]. The lamina propria became stiffer from increased collagen density and decreased elastin and HA density. Additionally, it was discovered that the activity of collagenase decreased in ageing vocal folds [16,38][16][38].
2. Tissue Engineering as a Promising Treatment for Glottic Insufficiency
2.1. Tissue Engineering in Vocal Fold Injection
Regenerative medicine is the approach of reinstating human cells, tissue or organs to their usual role [52][39]. Tissue engineering is application of biomaterial with or without cell transplantation to encourage endogenous regeneration and regain functional tissues or organs [53][40]. Fillers such as Teflon, polydimethysilicone and calcium hydroxyapatite are commonly injected into the vocal folds to improve glottal closure but are linked with the risk of inflammation, migration, and granuloma development [54][41]. Moreover, current clinical trial research for vocal fold injection focuses primarily on biomaterial alone (ClinicalTrials.gov number: NCT04700566, NCT03790956, NCT02163772) or direct injection of biomolecules or cells (ClinicalTrials.gov number: NCT05354544, NCT05385159, NCT03749863, NCT02622464, NCT02120781, NCT02904824, NCT04839276). It is suggested that combining biomaterials with biomolecules such as growth factors can improve the efficacy [55][42]. The combination of biomolecules, cells, and a scaffold serves as a unique delivery system. As cells proliferate to generate new tissue, biomolecules promote the growth of new tissue, and the scaffold serves as an environment for the regeneration of new tissue [56,57][43][44]. The comparison between injection of cells and biomolecules without and with a scaffold is shown in Figure 3.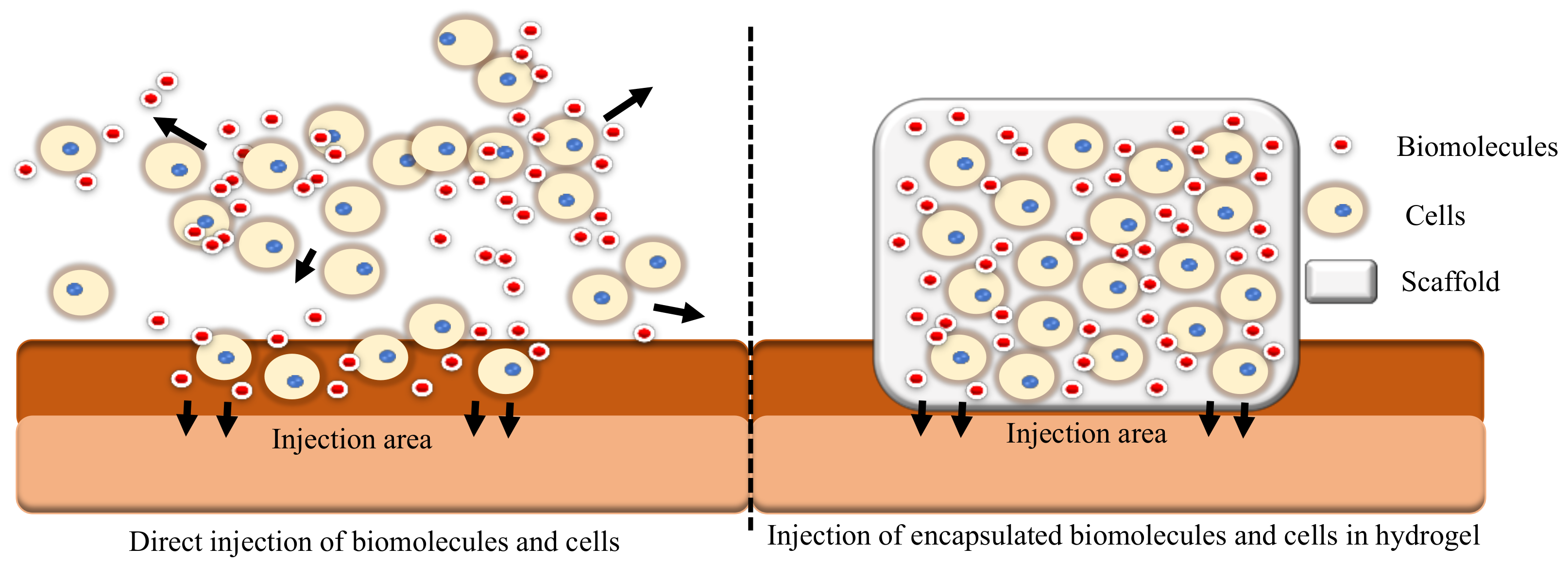
Figure 3.
A comparison between direct injection of cells and biomolecules versus injection of cells and biomolecules encapsulated in a hydrogel.
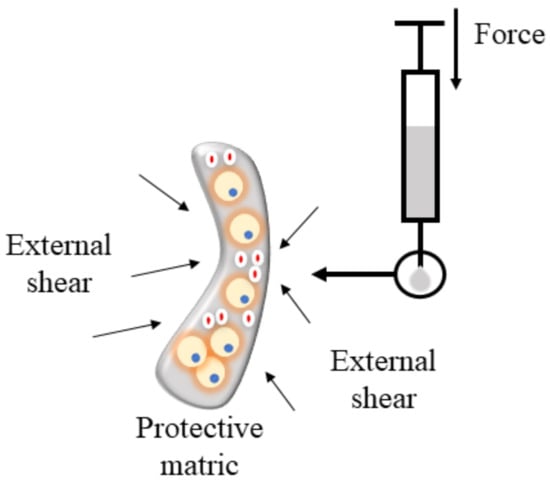
Figure 4.
Hydrogel protects cells during injection.
2.2. Injectable Hydrogel as Cell Delivery Vehicle
The majority of research develops encapsulation techniques for broad regeneration objectives. Muscle regeneration is followed by angiogenesis, nerve regeneration, and adipose tissue engineering in terms of the number of relevant studies. Comparatively, more research tried to encapsulate cells alone in biomaterials, followed by encapsulation of growth factor, MSC-extracellular vehicle (EV), and siRNA (siRNA). As illustrated in Supplementary Figure S3, three major categories of cells were examined. In clinical studies, direct injection of adipose-derived stem cells (ASCs) improved voice outcomes in patients with vocal fold scarring and glottic insufficiency [84,85][56][57]. Numerous in vivo investigations involving direct injection of ASCs have shown that ASCs can upregulate HA while downregulating collagen type I, type III, matrix metalloproteinase (Mmp1), and Mmp8 expression [86,87,88][58][59][60]. ASCs were also intimately linked to the secretion of FGF2, HGF, and basic fibroblast growth factor (bFGF). However, with direct injection, ASCs were only able to survive for 14 days; encapsulation helps to circumvent this problem [89,90][61][62]. It is uncertain whether ASCs or BMMSCs are more effective for augmenting vocal folds. Hiwatashi and colleagues recommended ASCs because they would increase HA control more effectively than BMMSCs [91][63]. Bartlett and colleagues recommended otherwise [92][64]. By stimulating BMMSCs with transforming growth factor beta (TGF-β), differentiation of vocal fold fibroblast into myofibroblast is inhibited [93][65]. Few investigations demonstrated that the qualities of ASCs are superior to those of BMMSCs because they are more stable, anti-inflammatory, proliferative, and have the same capacity to differentiate into various lineages [94,95,96,97][66][67][68][69]. The anti-fibrosis function of ASCs is depicted in Figure 5.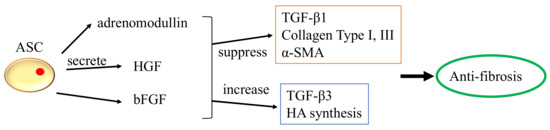
Figure 5.
The anti-fibrosis function of ASCs.
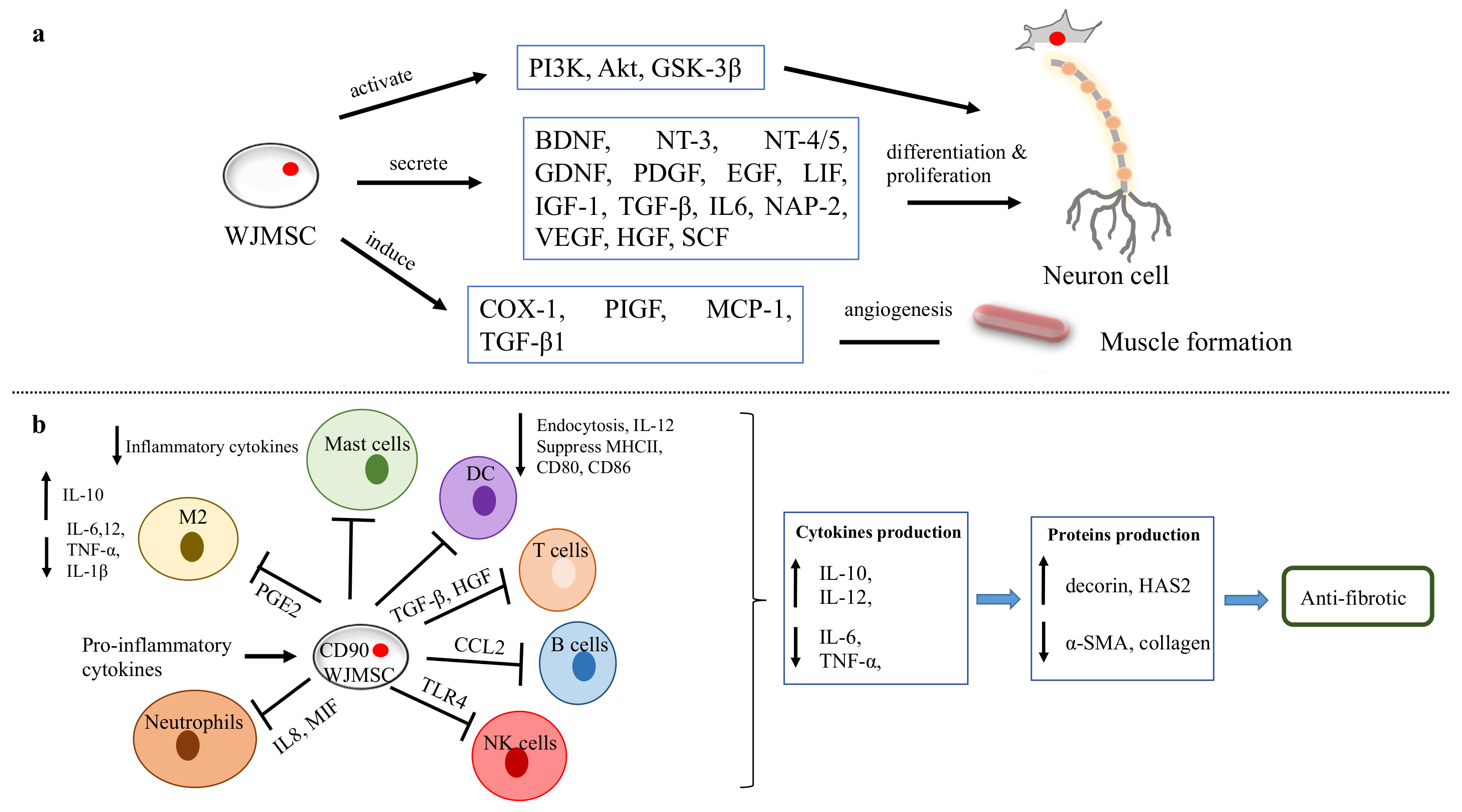
Figure 6.
WJMSC functions. (
a
) The function of WJMSCs in neuronal and muscular regeneration. (
b
) Immunomodulatory properties of WJMSCs in preventing fibrosis.
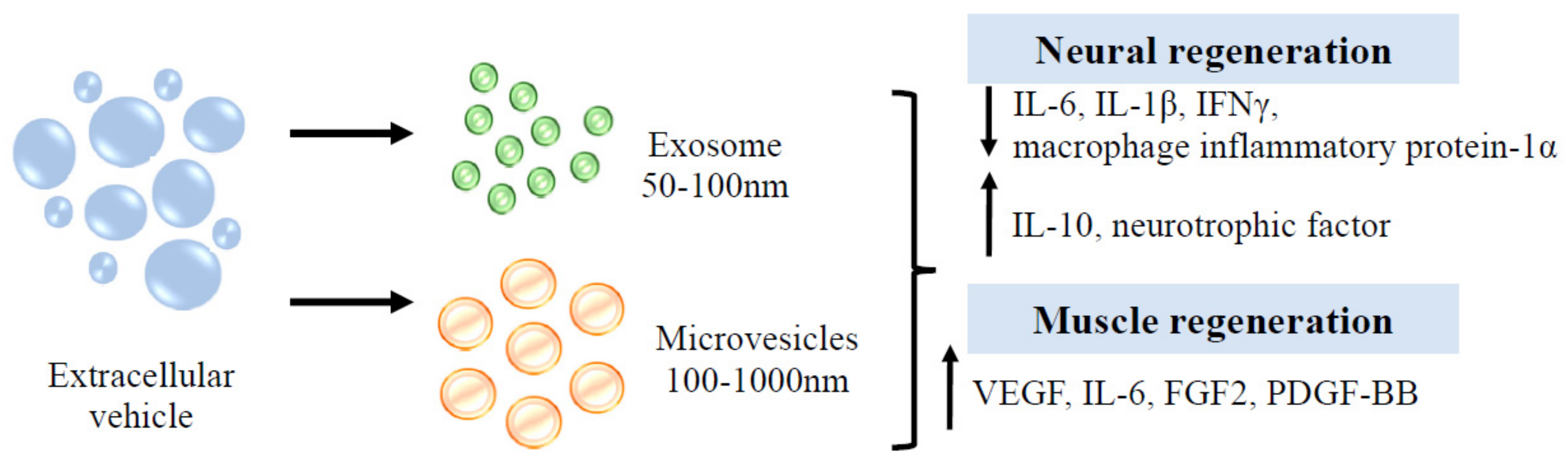
Figure 7.
Function of EVs in neuron and muscle regeneration.
2.3. Injectable Hydrogel as Biomolecule Delivery Vehicle
As hydrogel has a special affinity for water, it can be used as a hydrophilic growth factor delivery system. Based on the application (slow and extended or fast and short release), protein retention and delivery can be modified [117][79]. A hydrogel with low crosslinking, small particle size and susceptibility to enzymatic degradation will result in quicker growth factor release [118][80]. Growth factor incorporation will increase the bioactivity of the hydrogel [119][81]. For instance, Walters and colleagues demonstrated [120][82] that combining platelet-derived growth factor AB (PDGF-AB) and TGF-1 in collagen hydrogel promotes the differentiation of ASCs into smooth muscle cells. The thermosensitive heparin-poloxamer hydrogel containing bFGF and NGF enhances Schwann cell proliferation via the PI3K/Akt, JAK/STAT and MAPK/ERK signalling pathways [121][83]. To provide multiple regenerative aims, three different types of growth factors, namely VEGF, PDGF and BMP2, were released from a collagen hydrogel over a 28-day period in order to stimulate angiogenesis in a rat model [122][84]. As described in Supplementary Figure S4, tThe biomolecules employed in biomaterials can be categorised into four categories: neurotrophic growth factor, growth factor, proteins, and extracellular vesicles. In a rabbit model, a collagen scaffold containing NGF and human umbilical MSC was administered. After eight weeks, a positive response was obtained in the RLN injury model [100][72]. In vocal fold regeneration, brain-derived neurotrophic factor (BDNF), ciliary neurotrophic factor (CNF), and stromal cell-derived factor-1 (SDF-1) have not been researched. The SDF-1/CXCR4-mediated FAK/PI3K/Akt pathway [123][85] is thought to protect neuron tissue by avoiding cell death and inflammation. Following nerve damage, BDNF and SDF-1 increase in order to repair and modulate cells [124][86]. Ciliary neurotrophic factor enhances axon regeneration by binding to ciliary neurotrophic factor receptor α and then activating STAT3 [125][87]. Glottic insufficiency may have various causes, including muscle atrophy, nerve degeneration or damage, and anatomical alterations to the lamina propria. Consequently, treatment with just nerve growth factor may not yield optimal results. As the vocal fold is composed of epithelium and fibroblasts, EGF can reconstitute functional mucosa by promoting epithelium regeneration and HA synthesis. Several clinical trials [126,127,128][88][89][90] have demonstrated that direct injection of bFGF into the vocal fold has a beneficial effect on functional voice results. The most effective therapeutic impact on vocal fold atrophy was produced by this treatment. As the majority of instances were caused by ageing, bFGF use was shown to increase fibroblast synthesis of HA, hence lowering collagen deposition It was believed that bFGF treatment was more effective than biomaterial implantation because it altered the vibratory characteristics of the vocal fold and increased its volume [129][91]. bFGF was not only able to restore the flexibility of the vocal fold but also increased the density of the thyroarytenoid muscle in aged vocal folds [130][92]. Hepatocyte growth factor (HGF) has anti-fibrotic and angiogenesis properties and is produced by mesenchymal cells such as fibroblasts, macrophages, renal mesangium, etc. A clinical investigation demonstrated the efficacy and regeneration potential of HGF in patients with vocal fold scarring and sulcus. By repeatedly injecting the vocal fold with HGF, the patients exhibited a considerably improved outcome while maintaining a high level of safety. An earlier pre-clinical investigation demonstrated that HGF could boost fibroblast synthesis of HA and decrease collagen deposition [131][93]. In a second in-vitro study of a damaged vocal fold in rabbits, HGF delivered in a hyaluronic/alginate hydrogel was more effective than HGF injection alone [132][94]. With immediate treatment of HGF after vocal fold injury, collagen synthesis was decreased and angiogenesis was stimulated [133][95]. There is paucity of data on the effect of PDGF-BB on vocal fold regeneration. One study, however, showed that PDGF-BB can stimulate vessel development in rat models [134][96]. Similar to PDGF-BB, angiogenin has not been researched in the regeneration of vocal folds. By blocking the TGF-1/Smad pathway, it has been shown to alter fibroblast scar formation [135][97]. VEGF stimulates the development of blood vessels, which is essential for tissue regeneration. Then, numerous investigations on VEGF in various areas, including skeletal, peripheral nerve, dental pulp, and heart regeneration, were conducted [136,137,138,139,140][98][99][100][101][102]. VEGF promotes the development of HUVEC and neurite cells through the Erk/Akt pathway [141][103]. IGF-1 is one of the key proteins that regulate skeletal muscle metabolism pathways such as PI3K/Akt/mTOR and PI3K/Akt/GSK3β. IGF-1 suppresses cytokines that produce muscle atrophy and myostatin via these signalling pathways by suppressing nuclear factor-kappa beta (NF-ĸB) and Smad pathways. It can also promote skeletal muscle stem cells for the regeneration of skeletal muscle [142][104]. Insulin growth factor-1 (IGF-1) was induced by myokine/cytokine Meteorin-like that promotes myogenesis [143][105]. Researchers are currently interested in the exosome from umbilical cord MSC, since it can prevent tumorigenic concerns. It has great promise for use in a variety of conditions, including wounds, type 2 diabetes, inflammatory bowel disease, Alzheimer’s disease, spinal cord injury, myocardial ischemia injury, and graft-versus-host disease [144][106]. Extracellular vesicles (EVs) can be subdivided into numerous categories. Microvesicles and exosomes are among the subgroups. Microvesicles transport phosphatidylserine-containing proteins, mRNAs, miRNAs, and lipids, while exosomes transport DNA, lipids, RNAs, and proteins [145][107]. Human umbilical cord EVs have been demonstrated to exert anti-inflammatory qualities (interleukin-10, IL-10) and reduced pro-inflammatory responses (IL-1β, IL-6), followed by improved motor, axon, and Schwann cell regeneration [146][108]. Myoblasts release EVs containing HGF, IGF-1, TGF-3, VEGF, fibroblast growth factor-β3 (TGF-β3) and fibroblast growth factor 2 (FGF2). These secretions promote cell interaction between myoblasts for proliferation [147][109]. EVs enhance cross-talk in skeletal muscle to induce glucose release and adipose dissociation [148][110]. Interestingly, EVs that are secreted by astrocyte support a variety of tasks that range from delivering angiogenic factors such as FGF2, and VEGF to heat shock proteins, synapsin 1 and apolipoprotein-D, which aid in neuronal protection in hazardous settings [149][111]. EVs play a role in neuronal cell communication and in response to external stimuli such as inflammatory responses and the central nervous system [150][112]. Although EV can be created from a variety of cell sources, EV derived from MSC is persuasive in its ability to regenerate the central nervous system [151][113]. Figure 8 summarises the regenerative properties of EVs in neuronal and muscular tissue.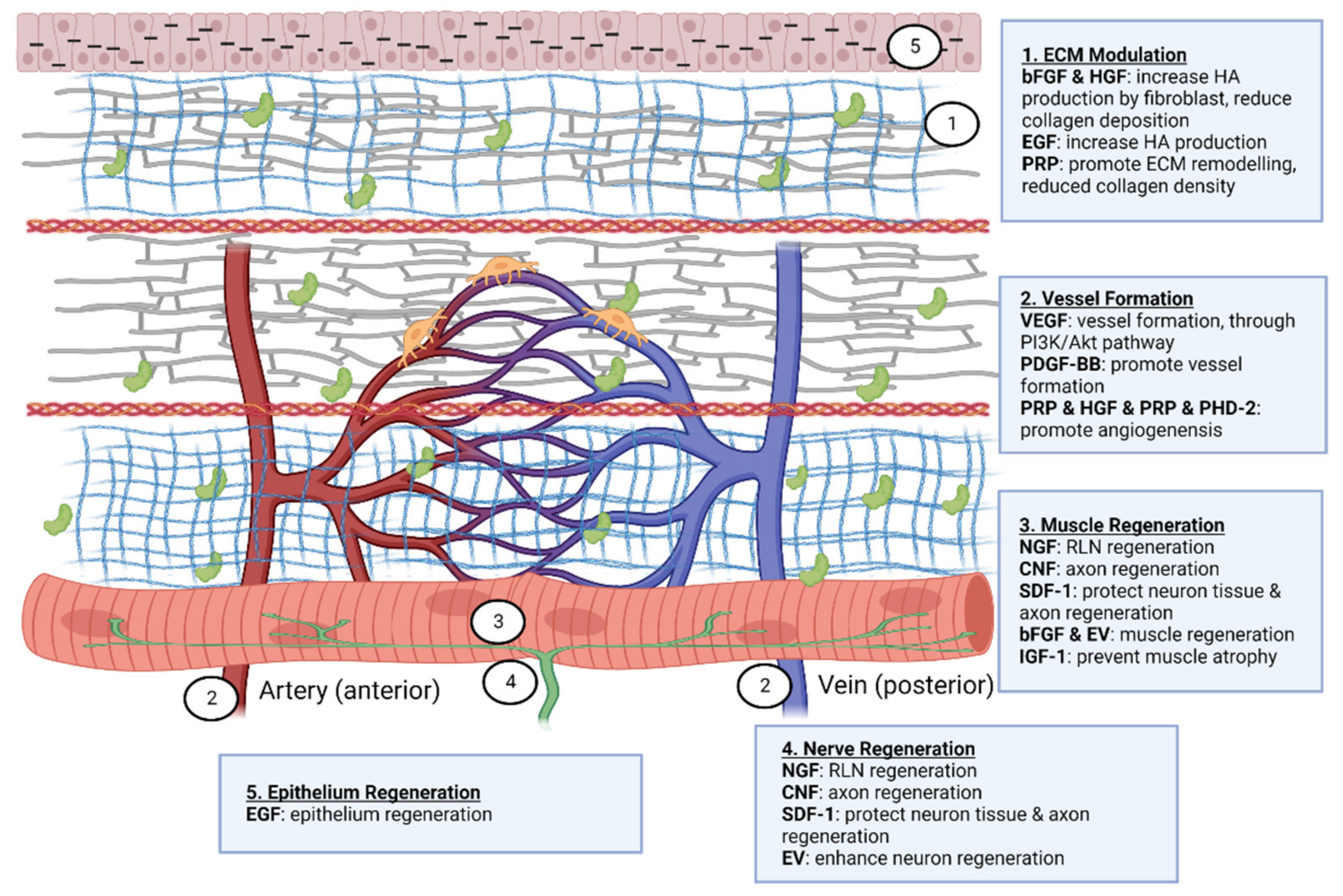
Figure 8.
Therapeutic effect of biomolecules on vocal fold regeneration. Created with Biorender.com (accessed on 24 October 2022).
References
- Rosow, D.E.; Pan, D.R. Presbyphonia and Minimal Glottic Insufficiency. Otolaryngol. Clin. N. Am. 2019, 52, 617–625.
- De Araújo Pernambuco, L.; Espelt, A.; Balata, P.M.M.; de Lima, K.C. Prevalence of voice disorders in the elderly: A systematic review of population-based studies. Eur. Arch. Oto-Rhino-Laryngol. 2015, 272, 2601–2609.
- Lyberg-Åhlander, V.; Rydell, R.; Fredlund, P.; Magnusson, C.; Wilén, S. Prevalence of Voice Disorders in the General Population, Based on the Stockholm Public Health Cohort. J. Voice 2019, 33, 900–905.
- Onwordi, L.N.; Al Yaghchi, C. Airway Glottic Insufficiency; StatPearls Publishing: Treasure Island, FL, USA, 2022.
- Lee, S.W.; Park, K.N. A long-term comparative prospective study between reinnervation and injection laryngoplasty. Laryngoscope 2018, 128, 1893–1897.
- Ban, M.J.; Lee, S.C.; Park, J.H.; Park, K.N.; Kim, H.K.; Lee, S.W. Regenerative efficacy of fibroblast growth factor for the treatment of aged vocal fold: From animal model to clinical application. Clin. Otolaryngol. 2021, 46, 131–137.
- Sharma, V.; Dash, S.K.; Govarthanan, K.; Gahtori, R.; Negi, N.; Barani, M.; Tomar, R.; Chakraborty, S.; Mathapati, S.; Bishi, D.K.; et al. Recent Advances in Cardiac Tissue Engineering for the Management of Myocardium Infarction. Cells 2021, 10, 2538.
- Walczak, P.A.; Perez-Esteban, P.; Bassett, D.C.; Hill, E.J. Modelling the central nervous system: Tissue engineering of the cellular microenvironment. Emerg. Top. life Sci. 2021, 5, 507–517.
- Pedrero, S.G.; Llamas-Sillero, P.; Serrano-López, J. A Multidisciplinary Journey towards Bone Tissue Engineering. Materials 2021, 14, 4896.
- Stampoultzis, T.; Karami, P.; Pioletti, D.P. Thoughts on cartilage tissue engineering: A 21st century perspective. Curr. Res. Transl. Med. 2021, 69, 103299.
- Fishman, J.M.; Long, J.; Gugatschka, M.; De Coppi, P.; Hirano, S.; Hertegard, S.; Thibeault, S.L.; Birchall, M.A. Stem cell approaches for vocal fold regeneration. Laryngoscope 2016, 126, 1865–1870.
- Kumai, Y. Pathophysiology of Fibrosis in the Vocal Fold: Current Research, Future Treatment Strategies, and Obstacles to Restoring Vocal Fold Pliability. Int. J. Mol. Sci. 2019, 20, 2551.
- Tian, H.; Pan, J.; Chen, L.; Wu, Y. A narrative review of current therapies in unilateral recurrent laryngeal nerve injury caused by thyroid surgery. Gland Surg. 2022, 11, 270–278.
- Mattei, A.; Magalon, J.; Bertrand, B.; Philandrianos, C.; Veran, J.; Giovanni, A. Cell therapy and vocal fold scarring. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2017, 134, 339–345.
- Benboujja, F.; Greenberg, M.; Nourmahnad, A.; Rath, N.; Hartnick, C. Evaluation of the Human Vocal Fold Lamina Propria Development Using Optical Coherence Tomography. Laryngoscope 2021, 131, E2558–E2565.
- Wrona, E.A.; Peng, R.; Amin, M.R.; Branski, R.C.; Freytes, D.O. Extracellular Matrix for Vocal Fold Lamina Propria Replacement: A Review. Tissue Eng. Part B Rev. 2016, 22, 421–429.
- Saran, M.; Georgakopoulos, B.; Bordoni, B. Anatomy, Head and Neck, Larynx Vocal Cords; StatPearls Publishing: Treasure Island, FL, USA, 2022.
- Tang, S.S.; Mohad, V.; Gowda, M.; Thibeault, S.L. Insights Into the Role of Collagen in Vocal Fold Health and Disease. J. Voice 2017, 31, 520–527.
- Moore, J.; Thibeault, S. Insights into the role of elastin in vocal fold health and disease. J. Voice 2012, 26, 269–275.
- Levendoski, E.E.; Leydon, C.; Thibeault, S.L. Vocal fold epithelial barrier in health and injury: A research review. J. Speech Lang. Hear. Res. 2014, 57, 1679–1691.
- Sato, K.; Chitose, S.-I.; Sato, K.; Sato, F.; Ono, T.; Umeno, H. Epithelium of the human vocal fold as a vibrating tissue. Auris Nasus Larynx 2021, 48, 704–709.
- Sato, K. Pericytes in the Human Vocal Fold Mucosa. Adv. Exp. Med. Biol. 2018, 1109, 79–93.
- Jones, C.L.; Achuthan, A.; Erath, B.D. Modal response of a computational vocal fold model with a substrate layer of adipose tissue. J. Acoust. Soc. Am. 2015, 137, EL158–EL164.
- Chhetri, D.K.; Neubauer, J.; Sofer, E. Posterior cricoarytenoid muscle dynamics in canines and humans. Laryngoscope 2014, 124, 2363–2367.
- Manriquez, R.; Peterson, S.D.; Prado, P.; Orio, P.; Galindo, G.E.; Zanartu, M. Neurophysiological Muscle Activation Scheme for Controlling Vocal Fold Models. IEEE Trans. Neural Syst. Rehabil. Eng. 2019, 27, 1043–1052.
- Ivey, C.M. Vocal Fold Paresis. Otolaryngol. Clin. N. Am. 2019, 52, 637–648.
- Weissbrod, P.; Pitman, M.J.; Sharma, S.; Bender, A.; Schaefer, S.D. Quantity and three-dimensional position of the recurrent and superior laryngeal nerve lower motor neurons in a rat model. Ann. Otol. Rhinol. Laryngol. 2011, 120, 761–768.
- Dekhou, A.S.; Morrison, R.J.; Gemechu, J.M. The Superior Laryngeal Nerve and Its Vulnerability in Surgeries of the Neck. Diagnostics 2021, 11, 1243.
- Dewan, K.; Vahabzadeh-Hagh, A.; Soofer, D.; Chhetri, D.K. Neuromuscular compensation mechanisms in vocal fold paralysis and paresis. Laryngoscope 2017, 127, 1633–1638.
- Marina, M.B.; Marie, J.-P.; Birchall, M.A. Laryngeal reinnervation for bilateral vocal fold paralysis. Curr. Opin. Otolaryngol. Head Neck Surg. 2011, 19, 434–438.
- Salik, I.; Winters, R. Bilateral Vocal Cord Paralysis; StatPearls Publishing: Treasure Island, FL, USA, 2022.
- Carroll, T.L.; Rosen, C.A. Trial vocal fold injection. J. Voice 2010, 24, 494–498.
- Martins, R.H.G.; Gonçalvez, T.M.; Pessin, A.B.B.; Branco, A. Aging voice: Presbyphonia. Aging Clin. Exp. Res. 2014, 26, 1–5.
- Samlan, R.A.; Kunduk, M.; Ikuma, T.; Black, M.; Lane, C. Vocal Fold Vibration in Older Adults With and Without Age-Related Dysphonia. Am. J. Speech-Lang. Pathol. 2018, 27, 1039–1050.
- Bouhabel, S.; Hartnick, C.J. Current trends in practices in the treatment of pediatric unilateral vocal fold immobility: A survey on injections, thyroplasty and nerve reinnervation. Int. J. Pediatr. Otorhinolaryngol. 2018, 109, 115–118.
- Padia, R.; Smith, M.E. Posterior Glottic Insufficiency in Children. Ann. Otol. Rhinol. Laryngol. 2017, 126, 268–273.
- Bruzzi, C.; Salsi, D.; Minghetti, D.; Negri, M.; Casolino, D.; Sessa, M. Presbiphonya. Acta Biomed. 2017, 88, 6–10.
- Chen, X.; Thibeault, S.L. Characteristics of age-related changes in cultured human vocal fold fibroblasts. Laryngoscope 2008, 118, 1700–1704.
- Baptista, P.M.; Atala, A. Chapter 1—Regenerative Medicine: The Hurdles and Hopes; Academic Press: Boston, MA, USA, 2016; pp. 3–7. ISBN 978-0-12-800548-4.
- Furth, M.E.; Atala, A. Chapter 6—Tissue Engineering: Future Perspectives; Academic Press: Boston, MA, USA, 2014; pp. 83–123. ISBN 978-0-12-398358-9.
- Walimbe, T.; Panitch, A.; Sivasankar, P.M. A Review of Hyaluronic Acid and Hyaluronic Acid-based Hydrogels for Vocal Fold Tissue Engineering. J. Voice 2017, 31, 416–423.
- Bakhshandeh, B.; Zarrintaj, P.; Oftadeh, M.O.; Keramati, F.; Fouladiha, H.; Sohrabi-Jahromi, S.; Ziraksaz, Z. Tissue engineering; strategies, tissues, and biomaterials. Biotechnol. Genet. Eng. Rev. 2017, 33, 144–172.
- Akter, F. Chapter 2—Principles of Tissue Engineering; Academic Press: Cambridge, MA, USA, 2016; pp. 3–16. ISBN 978-0-12-805361-4.
- Koh, B.; Sulaiman, N.; Fauzi, M.B.; Law, J.X.; Ng, M.H.; Idrus, R.B.H.; Yazid, M.D. Three dimensional microcarrier system in mesenchymal stem cell culture: A systematic review. Cell Biosci. 2020, 10, 1–16.
- Sheikh, I.; Dahman, Y. Chapter 2—Applications of Nanobiomaterials in Hard Tissue Engineering; William Andrew Publishing: Norwich, NY, USA, 2016; pp. 33–62. ISBN 978-0-323-42862-0.
- Li, L.; Stiadle, J.M.; Lau, H.K.; Zerdoum, A.B.; Jia, X.; Thibeault, S.L.; Kiick, K.L. Tissue engineering-based therapeutic strategies for vocal fold repair and regeneration. Biomaterials 2016, 108, 91–110.
- Kannan, R.; Wei, G.; Ma, P.X. Chapter 2—Synthetic polymeric biomaterials for tissue engineering. In Woodhead Publishing Series in Biomaterials; Woodhead Publishing: Sawston, UK, 2022; pp. 41–74. ISBN 978-0-12-820508-2.
- Heris, H.K.; Latifi, N.; Vali, H.; Li, N.; Mongeau, L. Investigation of Chitosan-glycol/glyoxal as an Injectable Biomaterial for Vocal Fold Tissue Engineering. Procedia Eng. 2015, 110, 143–150.
- Li, L.; Stiadle, J.M.; Levendoski, E.E.; Lau, H.K.; Susan, L.; Kiick, K.L.; Surgery, N. Biocompatibility of Injectable Resilin-based Hydrogels. J. Biomed. Mater. Res. B Appl. Biomater. 2018, 106, 2229–2242.
- Kwon, S.; Choi, H.; Park, C.; Choi, S.; Kim, E.; Kim, S.W.; Kim, C.-S.; Koo, H. In vivo vocal fold augmentation using an injectable polyethylene glycol hydrogel based on click chemistry. Biomater. Sci. 2021, 9, 108–115.
- Yap, L.-S.; Yang, M.-C. Thermo-reversible injectable hydrogel composing of pluronic F127 and carboxymethyl hexanoyl chitosan for cell-encapsulation. Colloids Surf. B Biointerfaces 2020, 185, 110606.
- Tong, X.; Yang, F. Recent Progress in Developing Injectable Matrices for Enhancing Cell Delivery and Tissue Regeneration. Adv. Healthc. Mater. 2018, 7, e1701065.
- Oliva, N.; Conde, J.; Wang, K.; Artzi, N. Designing Hydrogels for On-Demand Therapy. Acc. Chem. Res. 2017, 50, 669–679.
- Ma, J.; Huang, C. Composition and Mechanism of Three-Dimensional Hydrogel System in Regulating Stem Cell Fate. Tissue Eng. Part B Rev. 2020, 26, 498–518.
- Hamilton, M.; Harrington, S.; Dhar, P.; Stehno-Bittel, L. Hyaluronic Acid Hydrogel Microspheres for Slow Release Stem Cell Delivery. ACS Biomater. Sci. Eng. 2021, 7, 3754–3763.
- Mattei, A.; Bertrand, B.; Jouve, E.; Blaise, T.; Philandrianos, C.; Grimaud, F.; Giraudo, L.; Aboudou, H.; Dumoulin, C.; Arnaud, L.; et al. Feasibility of First Injection of Autologous Adipose Tissue-Derived Stromal Vascular Fraction in Human Scarred Vocal Folds: A Nonrandomized Controlled Trial. JAMA Otolaryngol. Head Neck Surg. 2020, 146, 355–363.
- Lasso, J.M.; Poletti, D.; Scola, B.; Gómez-Vilda, P.; García-Martín, A.I.; Fernández-Santos, M.E. Injection Laryngoplasty Using Autologous Fat Enriched with Adipose-Derived Regenerative Stem Cells: A Safe Therapeutic Option for the Functional Reconstruction of the Glottal Gap after Unilateral Vocal Fold Paralysis. Stem Cells Int. 2018, 2018, 8917913.
- Li, X.; Wang, H.; Xu, W. HGF and bFGF Secreted by Adipose-Derived Mesenchymal Stem Cells Revert the Fibroblast Phenotype Caused by Vocal Fold Injury in a Rat Model. J. Voice 2020, 36, 622–629.
- Valerie, A.; Vassiliki, K.; Irini, M.; Nikolaos, P.; Karampela, E.; Apostolos, P. Adipose-Derived Mesenchymal Stem Cells in the Regeneration of Vocal Folds: A Study on a Chronic Vocal Fold Scar. Stem Cells Int. 2016, 2016, 9010279.
- Morisaki, T.; Kishimoto, Y.; Tateya, I.; Kawai, Y.; Suzuki, R.; Tsuji, T.; Hiwatashi, N.; Nakamura, T.; Omori, K.; Kitano, H.; et al. Adipose-derived mesenchymal stromal cells prevented rat vocal fold scarring. Laryngoscope 2018, 128, E33–E40.
- Huang, D.; Wang, R.; Yang, S. Cogels of Hyaluronic Acid and Acellular Matrix for Cultivation of Adipose-Derived Stem Cells: Potential Application for Vocal Fold Tissue Engineering. BioMed Res. Int. 2016, 2016, 6584054.
- Goel, A.N.; Gowda, B.S.; Veena, M.S.; Shiba, T.L.; Long, J.L. Adipose-Derived Mesenchymal Stromal Cells Persist in Tissue-Engineered Vocal Fold Replacement in Rabbits. Ann. Otol. Rhinol. Laryngol. 2018, 127, 962–968.
- Hiwatashi, N.; Hirano, S.; Suzuki, R.; Kawai, Y.; Mizuta, M.; Kishimoto, Y.; Tateya, I.; Kanemaru, S.-I.; Nakamura, T.; Dezawa, M.; et al. Comparison of ASCs and BMSCs combined with atelocollagen for vocal fold scar regeneration. Laryngoscope 2016, 126, 1143–1150.
- Bartlett, R.S.; Gaston, J.D.; Ye, S.; Kendziorski, C.; Thibeault, S.L. Mechanotransduction of vocal fold fibroblasts and mesenchymal stromal cells in the context of the vocal fold mechanome. J. Biomech. 2019, 83, 227–234.
- Hiwatashi, N.; Bing, R.; Kraja, I.; Branski, R.C. Mesenchymal stem cells have antifibrotic effects on transforming growth factor-β1-stimulated vocal fold fibroblasts. Laryngoscope 2017, 127, E35–E41.
- Strioga, M.; Viswanathan, S.; Darinskas, A.; Slaby, O.; Michalek, J. Same or not the same? Comparison of adipose tissue-derived versus bone marrow-derived mesenchymal stem and stromal cells. Stem Cells Dev. 2012, 21, 2724–2752.
- Zhou, W.; Lin, J.; Zhao, K.; Jin, K.; He, Q.; Hu, Y.; Feng, G.; Cai, Y.; Xia, C.; Liu, H.; et al. Single-Cell Profiles and Clinically Useful Properties of Human Mesenchymal Stem Cells of Adipose and Bone Marrow Origin. Am. J. Sports Med. 2019, 47, 1722–1733.
- Bacakova, L.; Zarubova, J.; Travnickova, M.; Musilkova, J.; Pajorova, J.; Slepicka, P.; Kasalkova, N.S.; Svorcik, V.; Kolska, Z.; Motarjemi, H.; et al. Stem cells: Their source, potency and use in regenerative therapies with focus on adipose-derived stem cells—A review. Biotechnol. Adv. 2018, 36, 1111–1126.
- Si, Z.; Wang, X.; Sun, C.; Kang, Y.; Xu, J.; Wang, X.; Hui, Y. Adipose-derived stem cells: Sources, potency, and implications for regenerative therapies. Biomed. Pharmacother. 2019, 114, 108765.
- Nagamura-Inoue, T.; He, H. Umbilical cord-derived mesenchymal stem cells: Their advantages and potential clinical utility. World J. Stem Cells 2014, 6, 195–202.
- Ding, D.-C.; Chang, Y.-H.; Shyu, W.-C.; Lin, S.-Z. Human umbilical cord mesenchymal stem cells: A new era for stem cell therapy. Cell Transplant. 2015, 24, 339–347.
- Pan, Y.; Jiao, G.; Yang, J.; Guo, R.; Li, J.; Wang, C. Insights into the Therapeutic Potential of Heparinized Collagen Scaffolds Loading Human Umbilical Cord Mesenchymal Stem Cells and Nerve Growth Factor for the Repair of Recurrent Laryngeal Nerve Injury. Tissue Eng. Regen. Med. 2017, 14, 317–326.
- Li, N.; Hua, J. Interactions between mesenchymal stem cells and the immune system. Cell. Mol. Life Sci. 2017, 74, 2345–2360.
- Han, K.-H.; Kim, A.-K.; Kim, M.-H.; Kim, D.-H.; Go, H.-N.; Kim, D.-I. Enhancement of angiogenic effects by hypoxia-preconditioned human umbilical cord-derived mesenchymal stem cells in a mouse model of hindlimb ischemia. Cell Biol. Int. 2016, 40, 27–35.
- Mishra, S.; Sevak, J.K.; Das, A.; Arimbasseri, G.A.; Bhatnagar, S.; Gopinath, S.D. Umbilical cord tissue is a robust source for mesenchymal stem cells with enhanced myogenic differentiation potential compared to cord blood. Sci. Rep. 2020, 10, 18978.
- Li, J.; Gao, F.; Ma, S.; Zhang, Y.; Zhang, J.; Guan, F.; Yao, M. Control the fate of human umbilical cord mesenchymal stem cells with dual-enzymatically cross-linked gelatin hydrogels for potential applications in nerve regeneration. J. Tissue Eng. Regen. Med. 2020, 14, 1261–1271.
- Li, Y.; Yang, J.; Fu, G.; Zhou, P.; Liu, Y.; Li, Z.; Jiao, G. Human umbilical cord mesenchymal stem cells differentiate into neuron-like cells after induction with B27-supplemented serum-free medium. J. South. Med. Univ. 2020, 40, 1340–1345.
- Guo, Z.-Y.; Sun, X.; Xu, X.-L.; Zhao, Q.; Peng, J.; Wang, Y. Human umbilical cord mesenchymal stem cells promote peripheral nerve repair via paracrine mechanisms. Neural Regen. Res. 2015, 10, 651–658.
- Caballero Aguilar, L.M.; Silva, S.M.; Moulton, S.E. Growth factor delivery: Defining the next generation platforms for tissue engineering. J. Control. Release 2019, 306, 40–58.
- Buie, T.; McCune, J.; Cosgriff-Hernandez, E. Gelatin Matrices for Growth Factor Sequestration. Trends Biotechnol. 2020, 38, 546–557.
- Sarrigiannidis, S.O.; Rey, J.M.; Dobre, O.; González-García, C.; Dalby, M.J.; Salmeron-Sanchez, M. A tough act to follow: Collagen hydrogel modifications to improve mechanical and growth factor loading capabilities. Mater. Today Bio 2021, 10, 100098.
- Walters, B.; Turner, P.A.; Rolauffs, B.; Hart, M.L.; Stegemann, J.P. Controlled Growth Factor Delivery and Cyclic Stretch Induces a Smooth Muscle Cell-like Phenotype in Adipose-Derived Stem Cells. Cells 2021, 10, 3123.
- Li, R.; Li, Y.; Wu, Y.; Zhao, Y.; Chen, H.; Yuan, Y.; Xu, K.; Zhang, H.; Lu, Y.; Wang, J.; et al. Heparin-Poloxamer Thermosensitive Hydrogel Loaded with bFGF and NGF Enhances Peripheral Nerve Regeneration in Diabetic Rats. Biomaterials 2018, 168, 24–37.
- Subbiah, R.; Ruehle, M.A.; Klosterhoff, B.S.; Lin, A.S.P.; Hettiaratchi, M.H.; Willett, N.J.; Bertassoni, L.E.; García, A.J.; Guldberg, R.E. Triple growth factor delivery promotes functional bone regeneration following composite musculoskeletal trauma. Acta Biomater. 2021, 127, 180–192.
- Ma, S.; Zhou, J.; Huang, T.; Zhang, Z.; Xing, Q.; Zhou, X.; Zhang, K.; Yao, M.; Cheng, T.; Wang, X.; et al. Sodium alginate/collagen/stromal cell-derived factor-1 neural scaffold loaded with BMSCs promotes neurological function recovery after traumatic brain injury. Acta Biomater. 2021, 131, 185–197.
- Said, M.F.; Islam, A.A.; Massi, M.N. Prihantono Effect of erythropoietin administration on the expression of brain-derived neurotrophic factor, stromal cell-derived Factor-1, and neuron-specific enolase in traumatic brain injury: A literature review. Ann. Med. Surg. 2021, 69, 102666.
- Lee, N.; Spearry, R.P.; Rydyznski, C.E.; MacLennan, A.J. Muscle ciliary neurotrophic factor receptor α contributes to motor neuron STAT3 activation following peripheral nerve lesion. Eur. J. Neurosci. 2019, 49, 1084–1090.
- Takeharu, K.; Kurakami, K.; Konomi, U.; Komazawa, D.; Misawa, K.; Imayoshi, S.; Watanabe, Y. Safety and short-term outcomes of basic fibroblast growth factor injection for sulcus vocalis. Acta Otolaryngol. 2018, 138, 1014–1019.
- Hirano, S.; Tateya, I.; Kishimoto, Y.; Kanemaru, S.; Ito, J. Clinical trial of regeneration of aged vocal folds with growth factor therapy. Laryngoscope 2012, 122, 327–331.
- Kanazawa, T.; Komazawa, D.; Indo, K.; Akagi, Y.; Lee, Y.; Nakamura, K.; Matsushima, K.; Kunieda, C.; Misawa, K.; Nishino, H.; et al. Single injection of basic fibroblast growth factor to treat severe vocal fold lesions and vocal fold paralysis. Laryngoscope 2015, 125, E338–E344.
- Sueyoshi, S.; Umeno, H.; Kurita, T.; Fukahori, M.; Chitose, S.-I. Long-term outcomes of basic fibroblast growth factor treatments in patients with vocal fold scarring, aged vocal fold, and sulcus vocalis. Auris Nasus Larynx 2021, 48, 949–955.
- Okui, A.; Konomi, U.; Kanazawa, T.; Komazawa, D.; Nakamura, K.; Matsushima, K.; Watanabe, Y. Therapeutic Efficacy of Basic Fibroblast Growth Factor in Patients With Vocal Fold Atrophy. Laryngoscope 2020, 130, 2847–2852.
- Hirano, S.; Kawamoto, A.; Tateya, I.; Mizuta, M.; Kishimoto, Y.; Hiwatashi, N.; Kawai, Y.; Tsuji, T.; Suzuki, R.; Kaneko, M.; et al. A phase I/II exploratory clinical trial for intracordal injection of recombinant hepatocyte growth factor for vocal fold scar and sulcus. J. Tissue Eng. Regen. Med. 2018, 12, 1031–1038.
- Choi, J.-S.; Heang Oh, S.; Kim, Y.-M.; Lim, J.-Y. Hyaluronic Acid/Alginate Hydrogel Containing Hepatocyte Growth Factor and Promotion of Vocal Fold Wound Healing. Tissue Eng. Regen. Med. 2020, 17, 651–658.
- Dias Garcia, R.I.; Tsuji, D.H.; Imamura, R.; Mauad, T.; Ferraz da Silva, L.F. Effects of hepatocyte growth factor injection and reinjection on healing in the rabbit vocal fold. J. Voice 2012, 26, e7–e12.
- Minardi, S.; Pandolfi, L.; Taraballi, F.; Wang, X.; De Rosa, E.; Mills, Z.D.; Liu, X.; Ferrari, M.; Tasciotti, E. Enhancing Vascularization through the Controlled Release of Platelet-Derived Growth Factor-BB. ACS Appl. Mater. Interfaces 2017, 9, 14566–14575.
- Pan, S.-C.; Lee, C.-H.; Chen, C.-L.; Fang, W.-Y.; Wu, L.-W. Angiogenin Attenuates Scar Formation in Burn Patients by Reducing Fibroblast Proliferation and Transforming Growth Factor β1 Secretion. Ann. Plast. Surg. 2018, 80, S79–S83.
- Li, X.W.; Sun, H.C.; Liu, X.H. Vascular endothelial growth factor-loaded microspheres promote dental pulp regeneration and vascularization. Zhonghua Kou Qiang Yi Xue Za Zhi 2018, 53, 42–48.
- Zhu, L.; Dissanayaka, W.L.; Zhang, C. Dental pulp stem cells overexpressing stromal-derived factor-1α and vascular endothelial growth factor in dental pulp regeneration. Clin. Oral Investig. 2019, 23, 2497–2509.
- Räsänen, M.; Sultan, I.; Paech, J.; Hemanthakumar, K.A.; Yu, W.; He, L.; Tang, J.; Sun, Y.; Hlushchuk, R.; Huan, X.; et al. VEGF-B Promotes Endocardium-Derived Coronary Vessel Development and Cardiac Regeneration. Circulation 2021, 143, 65–77.
- Rao, F.; Wang, Y.; Zhang, D.; Lu, C.; Cao, Z.; Sui, J.; Wu, M.; Zhang, Y.; Pi, W.; Wang, B.; et al. Aligned chitosan nanofiber hydrogel grafted with peptides mimicking bioactive brain-derived neurotrophic factor and vascular endothelial growth factor repair long-distance sciatic nerve defects in rats. Theranostics 2020, 10, 1590–1603.
- Hu, K.; Olsen, B.R. Vascular endothelial growth factor control mechanisms in skeletal growth and repair. Dev. Dyn. 2017, 246, 227–234.
- Gnavi, S.; di Blasio, L.; Tonda-Turo, C.; Mancardi, A.; Primo, L.; Ciardelli, G.; Gambarotta, G.; Geuna, S.; Perroteau, I. Gelatin-based hydrogel for vascular endothelial growth factor release in peripheral nerve tissue engineering. J. Tissue Eng. Regen. Med. 2017, 11, 459–470.
- Yoshida, T.; Delafontaine, P. Mechanisms of IGF-1-Mediated Regulation of Skeletal Muscle Hypertrophy and Atrophy. Cells 2020, 9, 1970.
- Baht, G.S.; Bareja, A.; Lee, D.E.; Rao, R.R.; Huang, R.; Huebner, J.L.; Bartlett, D.B.; Hart, C.R.; Gibson, J.R.; Lanza, I.R.; et al. Meteorin-like facilitates skeletal muscle repair through a Stat3/IGF-1 mechanism. Nat. Metab. 2020, 2, 278–289.
- Yaghoubi, Y.; Movassaghpour, A.; Zamani, M.; Talebi, M.; Mehdizadeh, A.; Yousefi, M. Human umbilical cord mesenchymal stem cells derived-exosomes in diseases treatment. Life Sci. 2019, 233, 116733.
- Abbaszadeh, H.; Ghorbani, F.; Derakhshani, M.; Movassaghpour, A.; Yousefi, M. Human umbilical cord mesenchymal stem cell-derived extracellular vesicles: A novel therapeutic paradigm. J. Cell. Physiol. 2020, 235, 706–717.
- Ma, Y.; Dong, L.; Zhou, D.; Li, L.; Zhang, W.; Zhen, Y.; Wang, T.; Su, J.; Chen, D.; Mao, C.; et al. Extracellular vesicles from human umbilical cord mesenchymal stem cells improve nerve regeneration after sciatic nerve transection in rats. J. Cell. Mol. Med. 2019, 23, 2822–2835.
- Wang, W.; Li, M.; Chen, Z.; Xu, L.; Chang, M.; Wang, K.; Deng, C.; Gu, Y.; Zhou, S.; Shen, Y.; et al. Biogenesis and function of extracellular vesicles in pathophysiological processes of skeletal muscle atrophy. Biochem. Pharmacol. 2022, 198, 114954.
- Vechetti, I.J.J.; Valentino, T.; Mobley, C.B.; McCarthy, J.J. The role of extracellular vesicles in skeletal muscle and systematic adaptation to exercise. J. Physiol. 2021, 599, 845–861.
- Upadhya, R.; Zingg, W.; Shetty, S.; Shetty, A.K. Astrocyte-derived extracellular vesicles: Neuroreparative properties and role in the pathogenesis of neurodegenerative disorders. J. Control. Release 2020, 323, 225–239.
- Delpech, J.-C.; Herron, S.; Botros, M.B.; Ikezu, T. Neuroimmune Crosstalk through Extracellular Vesicles in Health and Disease. Trends Neurosci. 2019, 42, 361–372.
- Andjus, P.; Kosanović, M.; Milićević, K.; Gautam, M.; Vainio, S.J.; Jagečić, D.; Kozlova, E.N.; Pivoriūnas, A.; Chachques, J.-C.; Sakaj, M.; et al. Extracellular Vesicles as Innovative Tool for Diagnosis, Regeneration and Protection against Neurological Damage. Int. J. Mol. Sci. 2020, 21, 6859.
More