Ferroptosis involves cell metabolism, regulations of reactive oxygen species (ROS), and iron metabolism. To trigger ferroptosis, specific lipids must undergo peroxidation, and the natural defense mechanisms that prevent the accumulation of peroxidized lipids must be compromised. Acyl CoA synthetase (ACSLs) play an important role in tissue cell metabolism, and different isoforms have different tissue distributions and substrate preferences, which regulate different intracellular lipid compositions. Among these five isoforms, ACSL3 and ACSL4 have been shown to participate in ferroptosis. In addition, ACSL4 is a positive regulator in ferroptosis, whereas ACSL3 contributes to cancer cells acquiring ferroptosis resistance.
- ACSL3
- ACSL4
- ferroptosis
1. Introduction
2. ACSL3 and ACSL4
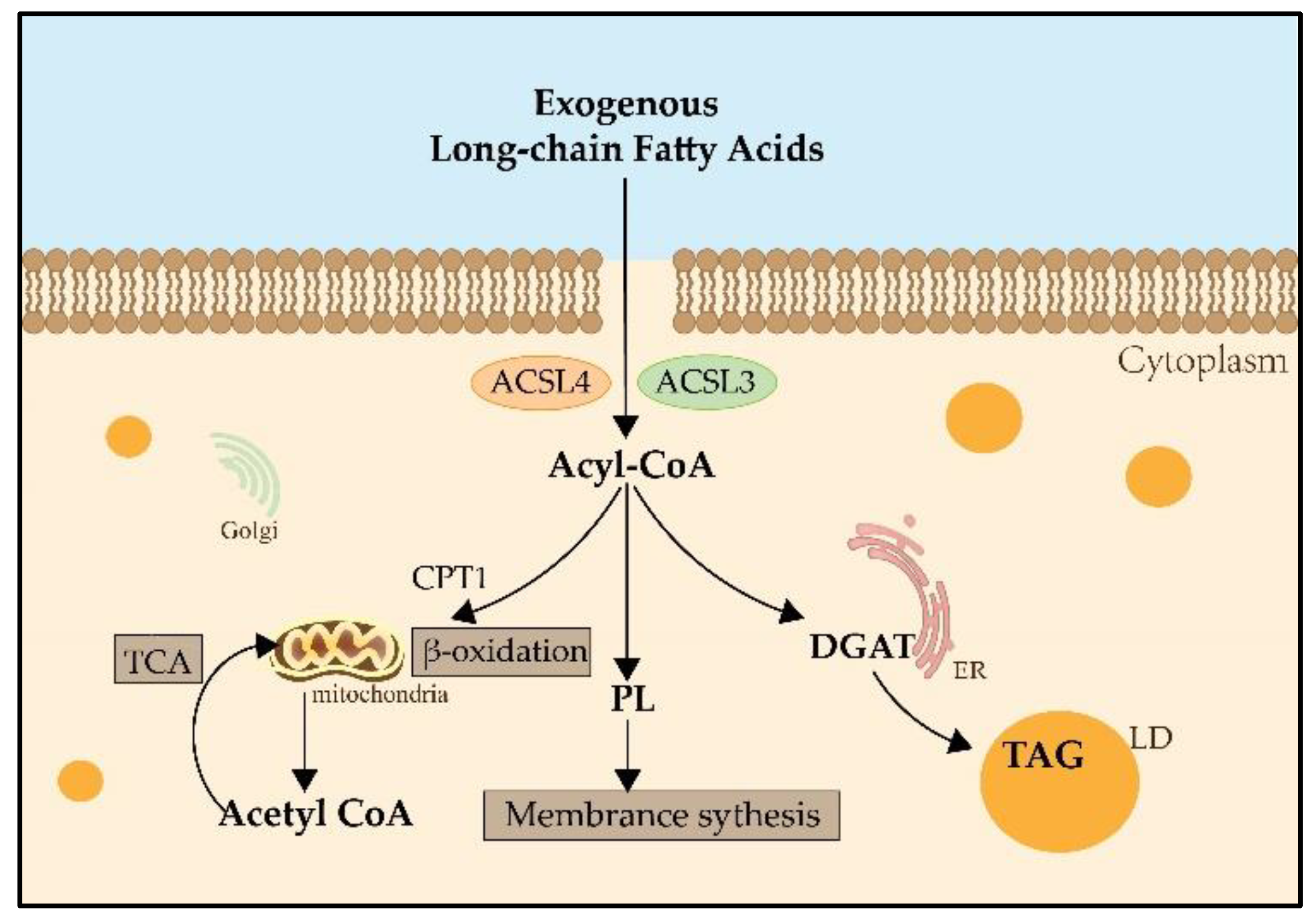
During lipid metabolism, the activation of fatty acids by esterification with coenzyme A is indispensable. ACSLs catalyze this reaction which is the first step in the utilization of fatty acids in mammals [7] (Figure 1). The main process of catalysis and the metabolic pathways of long-chain fatty acids are described in Figure 2 [8][9]. Researchers have already found that ACSL3 and ACSL4 are involved in ferroptosis [10]. However, information is limited regarding the specific roles of ACSL3 and 4 in ferroptosis.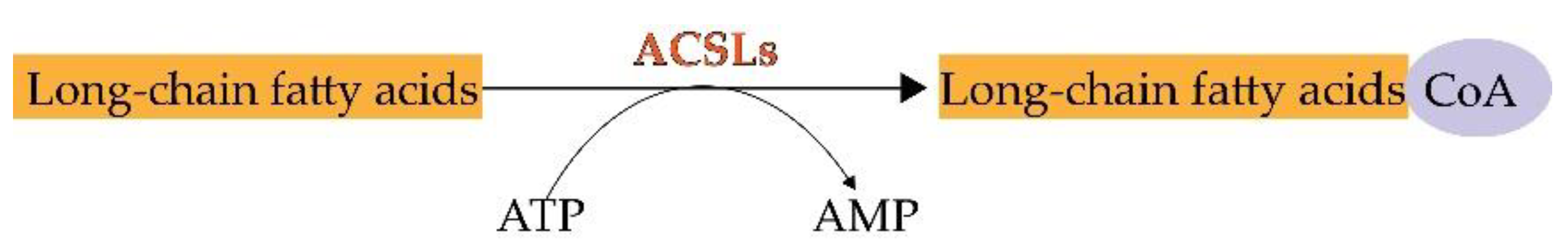
3. ACSL3 and ACSL4 in Ferroptosis
Ferroptosis is characterized by excessive accumulation and failure to eliminate iron-dependent lethal toxic lipid ROS, thereby initiating cell death. As a type of RCD, ferroptosis is either directed by intracellular signals or induced by external factors, leading to the initiation of suicidal protective strategies. It is considered a highly complicated and strictly orchestrated process that is regulated by a series of signals from distinct cell organelles, such as mitochondria, ER, and lysosomes [14]. Morphologically, distinct from other types of RCD such as apoptosis, necroptosis, and pyroptosis, ferroptosis shows swelling of cells, formation of pores in the cell membrane, smaller mitochondria, reduced mitochondrial cristae, increased mitochondrial membrane density, normal-sized nuclei and non-cohesive chromatin [15]. Ferroptosis is initiated by the accumulation of iron, lipid peroxidation, and subsequent plasma membrane rupture. Lipid peroxidation is a free radical-driven reaction that primarily affects polyunsaturated fatty acids (PUFA) in the cell membrane and undergoes three stages including initiation, propagation, and termination. Finally, lipid peroxidation produces lipid hydroperoxides [14]. Iron can favor tumor incidence and growth, as it is an important nutrient for cell proliferation and a key cofactor of metabolic enzymes [16]. Cellular or organelle membranes are particularly susceptible to lipid peroxidation because of their high PUFA content [17]. Cystatin-GSH-GPX4, CoQ10-FSP1, and GCH1-BH4-DHFR can effectively resist ferroptosis by inhibiting lipid peroxidation [18] (Figure 3).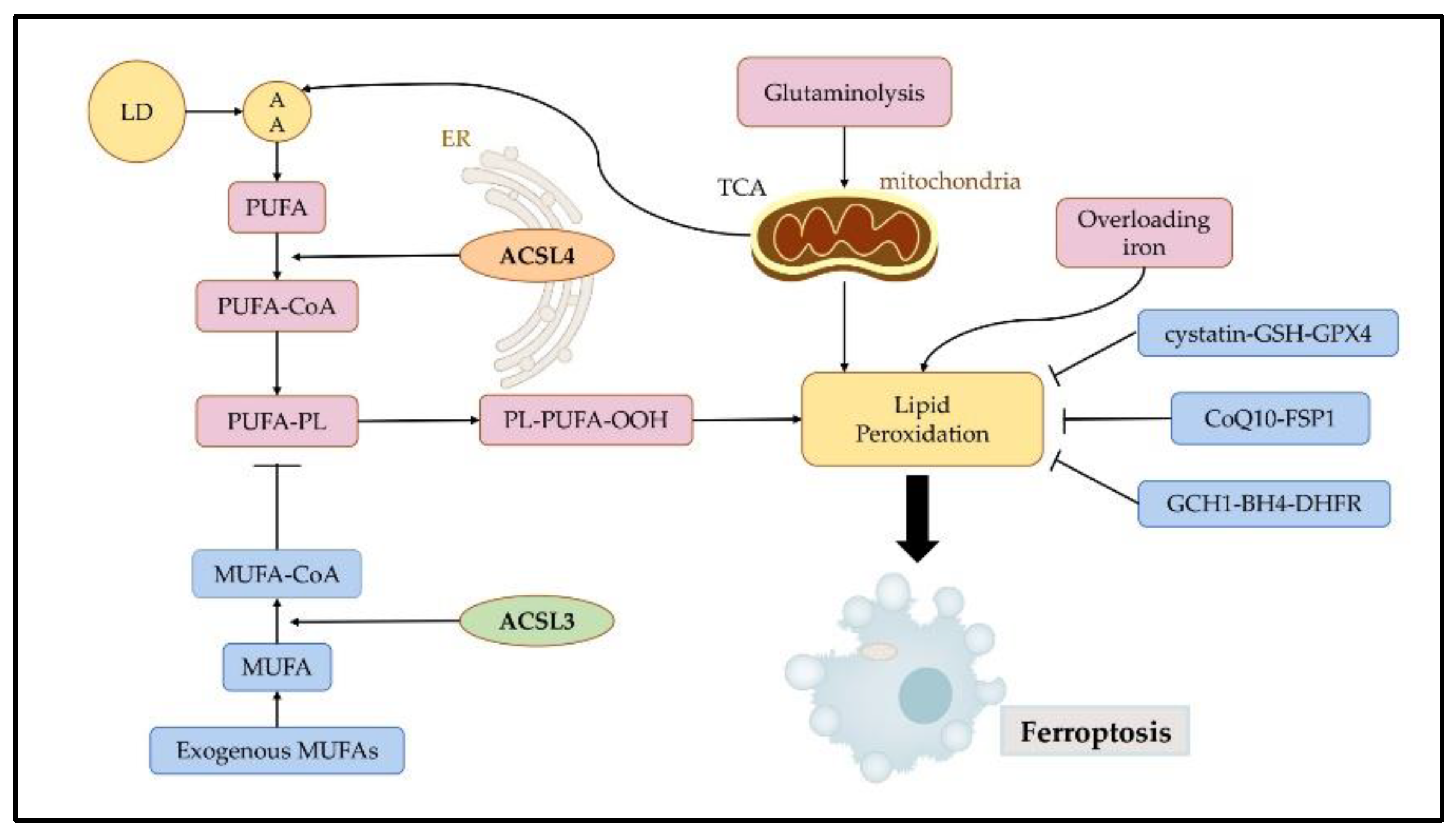
3.1. ACSL3 in Ferroptosis
ACSL3 may be involved in intracellular lipid metabolism by promoting LD formation and maturation. LDs are commonly found in the organelles of various cells, and they store neutral lipids, such as TAG and cholesteryl ester. ACSL3’s N-terminal domain controls the position of LD and FA absorption [3]. ACSL3 grows LD; in the absence of LD, ACSL3 may be present in the ER and translocated to the LD surface during LD formation or after LD is reconnected to ER by membrane bridges [21]. The transfer of ACSL3 from the ER to the LD occurs because of the strong affinity between ACSL3 and LD [22]. Fujimoto et al. found that the addition of an oleic acid medium increased intracellular LD accumulation, which was accompanied by an increase in ACSL3 expression. Meanwhile, with the use of ACSL3 inhibitor triacsin C, the expression of the ACSL3 correspondingly decreased, accompanied by a significant decrease in intracellular LD content. This suggests that ACSL3 plays an important role in the process of ER budding LD growth and maturation after the cellular uptake of exogenous fatty acids [23]. Further research has revealed that the mechanism of action may be that the LD-associated protein Rab18 (low molecular weight GTP-binding protein) interacts with perilipin 2 (PLIN2), a major LD protein, and forms a complex with PLIN2 and ACSL3 [24]. Although the study on ACSL3 is limited, some evidence shows that ACSL3 is highly relevant to ferroptosis and that ACSL3 expression is correlated with ferroptosis sensitivity [5]. ACSL3 is essential for activating MUFA, which reduces the susceptibility of plasma membrane lipids to oxidation over a period of several hours. MUFA can modify cell membrane properties by replacing PUFAs. It inhibits iron-dependent oxidative cell death by preventing the accumulation of lipid ROS in the plasma membranes, and effectively inhibits the process of iron-dependent oxidative cell death. Based on the findings of Magtanong et al., treatment with exogenous MUFA reduces the sensitization of plasma membrane lipids to lethal oxidation within a few hours, and this process entails the activation of MUFA by ACSL3 [12]. Their study demonstrated that exogenous MUFAs and ACSL3 activities promote cellular resistance to ferroptosis. It has also been found that a combination of the anesthetic drug propofol or propofol injectable emulsion (PIE) and the chemotherapeutic drug paclitaxel inhibited cell viability and augmented the sensitivity of paclitaxel to cervical cancer cells. Mechanistically, ferroptosis-related pathways were influenced by drug treatments, including the SLC7A11/GPX4 ubiquinol/CoQ10/FSP1 and YAP/ACSL4/TFRC pathways [25].3.2. ACSL4 in Ferroptosis
As a specific biomarker and driver of ferroptosis, ACSL4 dictates ferroptosis sensitivity by altering the composition of cellular lipids [13][26]. Ferroptosis is induced by the iron-dependent peroxidation of lipids, mostly PLs containing PUFAs [13]. ACSL4 is not only a sensitive monitor for ferroptosis but an essential regulator of lipid metabolism in ferroptosis. The ACSL4 gene-encoded enzyme has a strong preference for 20-carbon PUFA substrates, such as arachidonic acid (AA) and adrenaline (ADA), catalyzing their conversion to AA-CoA and ADA-CoA, producing LPO, and finally promoting ferroptosis [27][28]. Notably, oxidized AA and epinephrine phosphatidylethanolamines (Pes) have been proven to induce ferroptosis in cells [29]. A mechanistic study showed that ACSL4 is a target gene of the oncoprotein YAP. Such an interaction between E-cadherin-mediated cell-cell contacts affecting the cell density on ferroptosis in epithelial cells could activate intracellular Hippo signaling via the NF2/merlin tumor suppressor, and thus inhibit the transcription of various target genes of YAP, including ACSL4, TfR1, and others [30]. This discovery indicates that additional potential signaling pathways regulating ferroptosis by influencing ACSL4 levels are worthy of investigation and targeting these pathways may be useful in regulating cell death. An analysis revealed that ACSL4 and GPX4 are novel predictive and prognostic biomarkers for patients receiving neoadjuvant chemotherapy, which provided promising evidence for the regulation of ferroptosis in breast cancer (BC) treatment [31]. Currently, ACSL4 is studied in cervical cancer mostly in relation to drug therapy. Oleanolic acid (OA) is an anti-cancer compound found naturally in the leaves, fruits, and rhizomes of plants. Research has shown that OA activates ferroptosis in cervical cancer cells by enhancing ACSL4 expression and then a significant decrease in the viability and proliferative capacity of cancer cells was observed after exposure to OA [32]. Curcumin, a yellow polyphenol compound derived from turmeric plants, has also been shown to induce ferroptosis with increasing levels of ACSL4, which presents a theoretical basis for the application of ferroptotic inducers in the treatment of lung cancer [33]. Clinically, radiotherapy upregulates the expression of ACSL4, thereby increasing lipid synthesis and subsequent oxidative damage, thus inducing ferroptosis [34]. In addition to its involvement in tumor progression, ACSL4 serves as a biomarker for sorafenib in the treatment of hepatocellular carcinoma (HCC) [35]. The results of these contents demonstrate that the ETS1/miR-23a-3p/ACSL4 axis contributes to sorafenib resistance in HCC by regulating ferroptosis. ACSL4 is upregulated in ovarian cancer (OC) and is a direct target of miR-424-5p, which inhibits ferroptosis in OC cells [36]. The results of these studies have identified a potential therapeutic strategy for treating OC. These studies clearly indicate that there is a relationship between ACSL4 and ferroptosis in various cancers; however, more mechanisms among them remain to be further investigated and may contribute to more effective clinical treatments.References
- Magtanong, L.; Ko, P.J.; Dixon, S.J. Emerging roles for lipids in non-apoptotic cell death. Cell Death Differ. 2016, 23, 1099–1109.
- Bian, X.; Liu, R.; Meng, Y.; Xing, D.; Xu, D.; Lu, Z. Lipid metabolism and cancer. J. Exp. Med. 2021, 218, e20201606.
- Quan, J.; Bode, A.M.; Luo, X. ACSL family: The regulatory mechanisms and therapeutic implications in cancer. Eur. J. Pharmacol. 2021, 909, 174397.
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An Iron-Dependent Form of Nonapoptotic Cell Death. Cell 2012, 149, 1060–1072.
- Li, D.; Li, Y. The interaction between ferroptosis and lipid metabolism in cancer. Signal Transduct. Target. Ther. 2020, 5, 108.
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46.
- Grevengoed, T.J.; Klett, E.L.; Coleman, R.A. Acyl-CoA Metabolism and Partitioning. Annu. Rev. Nutr. 2014, 34, 1–30.
- Currie, E.; Schulze, A.; Zechner, R.; Walther, T.C.; Farese, R.V., Jr. Cellular Fatty Acid Metabolism and Cancer. Cell Metab. 2013, 18, 153–161.
- Yamashita, A.; Hayashi, Y.; Nemoto-Sasaki, Y.; Ito, M.; Oka, S.; Tanikawa, T.; Waku, K.; Sugiura, T. Acyltransferases and transacylases that determine the fatty acid composition of glycerolipids and the metabolism of bioactive lipid mediators in mammalian cells and model organisms. Prog. Lipid Res. 2014, 53, 18–81.
- Chen, X.; Kang, R.; Kroemer, G.; Tang, D. Targeting ferroptosis in pancreatic cancer: A double-edged sword. Trends Cancer 2021, 7, 891–901.
- Kassan, A.; Herms, A.; Fernández-Vidal, A.; Bosch, M.; Schieber, N.L.; Reddy, B.J.N.; Fajardo, A.; Gelabert-Baldrich, M.; Tebar, F.; Enrich, C.; et al. Acyl-CoA synthetase 3 promotes lipid droplet biogenesis in ER microdomains. J. Cell Biol. 2013, 203, 985–1001.
- Magtanong, L.; Ko, P.-J.; To, M.; Cao, J.Y.; Forcina, G.C.; Tarangelo, A.; Ward, C.C.; Cho, K.; Patti, G.J.; Nomura, D.K.; et al. Exogenous Monounsaturated Fatty Acids Promote a Ferroptosis-Resistant Cell State. Cell Chem. Biol. 2019, 26, 420–432.e9.
- Doll, S.; Proneth, B.; Tyurina, Y.; Panzilius, E.; Kobayashi, S.; Ingold, I.; Irmler, M.; Beckers, M.I.J.; Aichler, M.; Walch, M.A.A.; et al. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat. Chem. Biol. 2017, 13, 91–98.
- Chen, X.; Li, J.; Kang, R.; Klionsky, D.J.; Tang, D. Ferroptosis: Machinery and regulation. Autophagy 2021, 17, 2054–2081.
- Xie, Y.; Hou, W.; Song, X.; Yu, Y.; Huang, J.; Sun, X.; Kang, R.; Tang, D. Ferroptosis: Process and function. Cell Death Differ. 2016, 23, 369–379.
- Morales, M.; Xue, X. Targeting iron metabolism in cancer therapy. Theranostics 2021, 11, 8412–8429.
- Xu, H.; Ye, D.; Ren, M.; Zhang, H.; Bi, F. Ferroptosis in the tumor microenvironment: Perspectives for immunotherapy. Trends Mol. Med. 2021, 27, 856–867.
- Zheng, J.; Conrad, M. The Metabolic Underpinnings of Ferroptosis. Cell Metab. 2020, 32, 920–937.
- Kagan, V.; Tyurina, Y.; Sun, W.; Vlasova, I.; Dar, H.; Tyurin, V.; Amoscato, A.; Mallampalli, R.; van der Wel, P.; He, R.; et al. Redox phospholipidomics of enzymatically generated oxygenated phospholipids as specific signals of programmed cell death. Free Radic. Biol. Med. 2020, 147, 231–241.
- Tang, D.; Chen, X.; Kang, R.; Kroemer, G. Ferroptosis: Molecular mechanisms and health implications. Cell Res. 2021, 31, 107–125.
- Fujino, T.; Kang, M.-J.; Suzuki, H.; Iijima, H.; Yamamoto, T.; Michell, B.J.; Stapleton, D.; Mitchelhill, K.I.; House, C.M.; Katsis, F.; et al. Molecular Characterization and Expression of Rat Acyl-CoA Synthetase 3. J. Biol. Chem. 1996, 271, 16748–16752.
- Poppelreuther, M.; Rudolph, B.; Du, C.; Großmann, R.; Becker, M.; Thiele, C.; Ehehalt, R.; Füllekrug, J. The N-terminal region of acyl-CoA synthetase 3 is essential for both the localization on lipid droplets and the function in fatty acid uptake. J. Lipid Res. 2012, 53, 888–900.
- Fujimoto, Y.; Itabe, H.; Kinoshita, T.; Homma, K.J.; Onoduka, J.; Mori, M.; Yamaguchi, S.; Makita, M.; Higashi, Y.; Yamashita, A.; et al. Involvement of ACSL in local synthesis of neutral lipids in cytoplasmic lipid droplets in human hepatocyte HuH7. J. Lipid Res. 2007, 48, 1280–1292.
- Deng, Y.; Zhou, C.; Mirza, A.H.; Bamigbade, A.T.; Zhang, S.; Xu, S.; Liu, P. Rab18 binds PLIN2 and ACSL3 to mediate lipid droplet dynamics. Biochim. Et Biophys. Acta Mol. Cell Biol. Lipids 2021, 1866, 158923.
- Zhao, M.-Y.; Liu, P.; Sun, C.; Pei, L.-J.; Huang, Y.-G. Propofol Augments Paclitaxel-Induced Cervical Cancer Cell Ferroptosis In Vitro. Front. Pharmacol. 2022, 13, 816432.
- Yuan, H.; Li, X.; Zhang, X.; Kang, R.; Tang, D. Identification of ACSL4 as a biomarker and contributor of ferroptosis. Biochem. Biophys. Res. Commun. 2016, 478, 1338–1343.
- Küch, E.-M.; Vellaramkalayil, R.; Zhang, I.; Lehnen, D.; Brügger, B.; Stremmel, W.; Ehehalt, R.; Poppelreuther, M.; Füllekrug, J. Differentially localized acyl-CoA synthetase 4 isoenzymes mediate the metabolic channeling of fatty acids towards phosphatidylinositol. Biochim. Biophys. Acta 2013, 1841, 227–239.
- Soupene, E.; Kuypers, F.A. Mammalian Long-Chain Acyl-CoA Synthetases. Exp. Biol. Med. 2008, 233, 507–521.
- Kagan, V.E.; Mao, G.; Qu, F.; Angeli, J.P.F.; Doll, S.; Croix, C.S.; Dar, H.H.; Liu, B.; Tyurin, V.A.; Ritov, V.B.; et al. Oxidized Arachidonic and Adrenic PEs Navigate Cells to Ferroptosis. Nat. Chem. Biol. 2017, 13, 81–90.
- Wu, J.; Minikes, A.M.; Gao, M.; Bian, H.; Li, Y.; Stockwell, B.R.; Chen, Z.-N.; Jiang, X. Intercellular interaction dictates cancer cell ferroptosis via NF2–YAP signalling. Nature 2019, 572, 402–406.
- Sha, R.; Xu, Y.; Yuan, C.; Sheng, X.; Wu, Z.; Peng, J.; Wang, Y.; Lin, Y.; Zhou, L.; Xu, S.; et al. Predictive and prognostic impact of ferroptosis-related genes ACSL4 and GPX4 on breast cancer treated with neoadjuvant chemotherapy. eBioMedicine 2021, 71, 103560.
- Xiaofei, J.; Mingqing, S.; Miao, S.; Yizhen, Y.; Shuang, Z.; Qinhua, X.; Kai, Z. Oleanolic acid inhibits cervical cancer Hela cell proliferation through modulation of the ACSL4 ferroptosis signaling pathway. Biochem. Biophys. Res. Commun. 2021, 545, 81–88.
- Tang, X.; Ding, H.; Liang, M.; Chen, X.; Yan, Y.; Wan, N.; Chen, Q.; Zhang, J.; Cao, J. Curcumin induces ferroptosis in non-small-cell lung cancer via activating autophagy. Thorac. Cancer 2021, 12, 1219–1230.
- Lei, G.; Zhang, Y.; Koppula, P.; Liu, X.; Zhang, J.; Lin, S.H.; Ajani, J.A.; Xiao, Q.; Liao, Z.; Wang, H.; et al. The role of ferroptosis in ionizing radiation-induced cell death and tumor suppression. Cell Res. 2020, 30, 146–162.
- Lu, Y.; Chan, Y.-T.; Tan, H.-Y.; Zhang, C.; Guo, W.; Xu, Y.; Sharma, R.; Chen, Z.-S.; Zheng, Y.-C.; Wang, N.; et al. Epigenetic regulation of ferroptosis via ETS1/miR-23a-3p/ACSL4 axis mediates sorafenib resistance in human hepatocellular carcinoma. J. Exp. Clin. Cancer Res. 2022, 41, 3.
- Ma, L.-L.; Liang, L.; Zhou, D.; Wang, S.-W. Tumor suppressor miR-424-5p abrogates ferroptosis in ovarian cancer through targeting ACSL4. Neoplasma 2021, 68, 165–173.
