You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 2 by Sirius Huang and Version 1 by Yeseul Choi.
Group B Streptococcus (GBS, Streptococcus agalactiae) is a Gram-positive bacterium that is commonly found in the gastrointestinal and urogenital tracts. Since the emergence of GBS as a predominant pathogen causing sepsis or meningitis in newborns in the US and worldwide, it has been investigated widely, especially in relation to invasive GBS disease in neonates.
- Group B Streptococcus
- obstetrics
- early-onset disease
- late-onset disease
1. What Is Group B Streptococcus (GBS)?
1.1. Microbiology
Group B Streptococcus (GBS, Streptococcus agalactiae) is a Gram-positive, β-hemolytic facultative anaerobic bacterium, comprised of cocci arranged in chains, that primarily colonizes the gastrointestinal and urogenital tracts [1,2][1][2]. In 1933, Lancefield identified different species of Streptococci based on their serological properties and hemolytic patterns; among these different species, S. agalactiae was found to belong to Group B [3]. Later, Group B was further divided into 10 serotypes (Ia, Ib, II, III, IV, V, VI, VII, VIII, and IX) based on the composition of the capsular polysaccharides (CPS) [4,5][4][5].
1.2. Virulence Factors
GBS has several virulence factors that facilitate its adherence to the host cell, evasion of the host immune system, colonization, and eventual progression to invasive GBS disease [6]. First, GBS has pili that play a role in its attachment to the host cell and further invasion into the cell [7]. In addition, GBS produces β-hemolysin, a pore-forming toxin that destroys the red blood cells of the host and causes hemolysis. Moreover, enzymes produced by GBS, such as C5a-ase, assist the bacterium in the evasion of the human immune system and further evolution to GBS infection. Lastly, the polysaccharide layer that encapsulates GBS is rich in sialic acid, which is also found in human cells. Therefore, the naïve immune cells of a newborn may recognize the sialic acid of GBS as that of human cells and allow the bacterium to survive in the body, leading to infection [8].
1.3. Epidemiology
In the 1970s, GBS emerged as a predominant pathogen causing sepsis or meningitis in newborns in the US and worldwide [9,10,11,12][9][10][11][12]. Since then, it has been investigated widely, especially in relation to invasive GBS disease in neonates. According to the US Centers for Disease Control and Prevention (CDC), approximately one in every four pregnant women carries GBS [13]. Although most of them are asymptomatic GBS carriers, its colonization in the maternal urogenital tract at the time of delivery is an important risk factor for neonatal GBS infection. In a recent report, approximately 19.7 million pregnant women (posterior median; an updated predictive median value after taking consideration of currently available data) were estimated to have rectovaginal GBS colonization in 2020, resulting in an estimated 58,300 infant deaths and 46,200 stillbirths [14]. In addition to perinatal or infantile death, invasive GBS infection in neonates was associated with long-term neurodevelopment impairments [14]. In terms of GBS incidence, there is a variance in GBS colonizer estimates by regions around the world. Overall, 18% of the world is estimated to be colonized, ranging from a high incidence in the Caribbean of 35% to a much lower prevalence in Southern Asia (13%) and Eastern Asia (11%) [15]. Among regions including North America, Europe, and Latin America, Sub-Saharan Africa had the highest burden of invasive GBS infection with 20,300, 90,800, 78,100, and 50,600 cases of stillbirth, early-onset disease (EOD), late-onset disease (LOD), and infant death, respectively, accounting for nearly half of all global GBS-related events [14].
2. GBS-Related Clinical Diseases
2.1. GBS and Non-Pregnant Women
The incidence of GBS disease in non-pregnant women or immunocompromised adults is increasing, especially in elders with underlying diseases [16]. In total, 20 to 70% of these infections are nosocomial [17]. Several clinical diseases result from GBS infection in non-pregnant adults. The first such disease is skin and soft tissue infection, which is the most frequently reported clinical manifestation associated with invasive GBS disease. This infection mostly presents as cellulitis, decubitus ulcers, and infected foot ulcers [16]. GBS pneumonia usually occurs in older adults with neurological impairments, such as dementia and cerebrovascular disease [16]. Approximately 5 to 23% of non-pregnant women with GBS infection have urinary tract infections, and most such infections occur in elderly adults [4]. Meningitis is another significant but not uncommon clinical manifestation in adults. Bone and joint infections, such as osteomyelitis and septic arthritis, and recurrent invasive GBS infection are other clinical manifestations in non-pregnant women [16].
2.2. GBS in Pregnancy
Maternal and fetal GBS results range from asymptomatic colonization to sepsis. It causes maternal bacteriuria, pyelonephritis, postpartum mastitis, and endometritis [8,18,19,20,21][8][18][19][20][21]. Although heterogeneity was noted globally, GBS serotypes Ia, III, and VI accounted for the majority of cases of maternal systemic GBS disease [15,22,23,24][15][22][23][24]. It may also reach the amniotic fluid by overcoming the normally protective cervical barrier during pregnancy. These ascending GBS infections have been involved in preterm labor, prelabor rupture of membranes (PROM), chorioamnionitis, fetal infection, and stillbirth [25]. About 98% of colonized newborns show a good prognosis while 1–3% of colonized newborns have early-onset disease (EOD), which is defined as neonatal infection within 7 days after birth [26]. The main causes of EOD in neonates are vertical transmission from the mother and amniotic GBS infection [8,27][8][27]. In the US, more than 95% of cases of EOD are related to GBS serotypes Ia, Ib, II, III, IV, and V [28]. In neonates with EOD, sepsis occurs in 80 to 85%, and 10% of EOD cases show pneumonia [18,28][18][28]. Lastly, meningitis is found in about 5 to 10% of EOD [28].
However, meningitis is a common manifestation in late-onset disease (LOD), an infection beyond 6 days through 90 days after birth. Although the pathogenesis of LOD is less understood, it is believed to be acquired from vertical transmission, nosocomial sources, or horizontal transmission from household or community settings [29,30][29][30]. The six aforementioned serotypes responsible for most cases of EOD are also present in more than 97% of cases of LOD, and among them, serotype III is known to be highly related to meningitis [28]. Although the risk factors of LOD are not yet well-established, the expected risk factors are maternal GBS colonization, prematurity, young maternal age, HIV exposure, and black maternal ethnicity [30]. Among them, prematurity was identified as a major risk factor in a recent study [31]. The clinical manifestations of LOD other than meningitis are bacteremia without a focus, bone-joint infection, and cellulitis-adenitis. Unlike EOD, the evaluation and initiation of empiric treatment for LOD are mainly based on the clinical appearance and signs of illness [32]. At present, there are no effective preventive measures for LOD.
Very late onset GBS disease (VLOD) is identified as GBS infection in infants aged 3 months or older. The risk factors of VLOD are believed to be similar to those of LOD [33]. However, most cases of VLOD occur in preterm babies or those with very low birth weight. Infants with VLOD are more likely to acquire immunodeficiency or HIV infection, and the most common clinical manifestations of VLOD are bacteremia without a focus and meningitis [8].
In infants, the mortality rate for EOD is about 2 to 3%, and that for LOD is 1 to 3% [8]. In preterm babies, the mortality rate for EOD is approximately 20 to 30%, and that of LOD is 5 to 8% [8]. Although GBS-infected infants survive and leave the hospital, their survival rate in the first decade is low, and they experience repetitive hospitalization in their first five years of life [18]. The findings of Yeo et al. support this high mortality rate in infants with EOD. Children with GBS infection are 3-fold more likely to die and require hospitalization during the first 11 years of life [34]. Long-term morbidity is another serious issue associated with GBS infection in infants. GBS infection can lead to high risks of permanent neurodevelopmental disorders, such as cerebral palsy and epilepsy [34]. For instance, only 51% of infants with GBS meningitis develop appropriately with age [35]. Meanwhile, 25% of such infants develop mild to moderate neurodevelopmental disorders, and the remaining infants experience severe neurological or functional impairments [35]. With its concerning and long-lasting comorbidities, it is critical to detect GBS infection at an early stage and prevent the manifestations of invasive GBS disease in neonates and infants.
In recognition of its clinical significance in maternal and neonatal health, the CDC and the American College of Obstetricians and Gynecologists (ACOG) have provided guidelines and recommendations for GBS screening and management in pregnant women focusing on the prevention of EOD (Figure 1). The ACOG recommends performing universal GBS screening between 36 0/7 and 37 6/7 weeks of gestation, which is approximately 5 weeks before their delivery [27,36][27][36]. If positive GBS culture results are obtained from the screening, intrapartum antibiotic prophylaxis (IAP) is indicated. They also commented that GBS bacteriuria at any concentration identified at any time in pregnancy represents the heavy maternal vaginal-rectal GBS colonization, which indicates the need for IAP without a subsequent GBS screening vaginal-rectal culture at 36 0/7–37 6/7 weeks of gestation. When bacteriuria with a concentration higher than 105 CFU/mL is identified at any time during pregnancy, acute maternal antibiotic therapy should be administered during the antepartum period, and IAP is required at the time of birth [26]. If the concentration is below 105 CFU/mL, maternal antibiotic therapy during the antepartum period is not required but IAP at the time of birth is warranted.
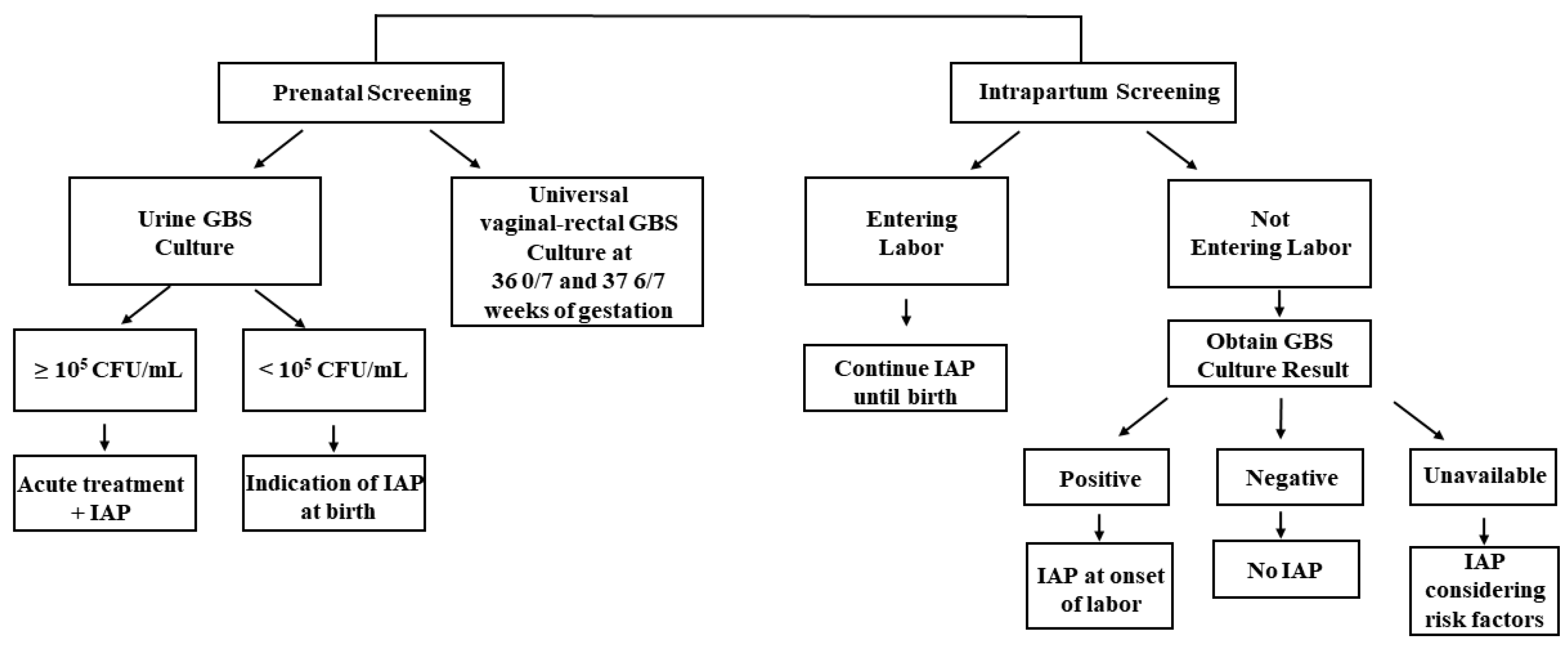
Figure 1.
An overall schema of the CDC recommended IAP strategy for GBS screening and IAP administration [26].
IAP is also administered during intrapartum screening, especially in pregnant women without GBS screening results who developed their labor or rupture of membranes before 36 weeks of gestation (Figure 1). When such women enter labor, IAP is continued until the birth of the baby. For women who have not entered labor, a different strategy is applied. In particular, IAP administration is discontinued, and further administration is decided based on the result of the universal vaginal-rectal GBS screening culture. IAP is administered at the onset of labor if the GBS culture result is positive. IAP is not administered to mothers with negative culture results, and another vaginal-rectal GBS culture is recommended when the 5-week screening accuracy window has passed. Lastly, when culture results are unavailable at the onset of labor, IAP is administered to mothers before 37 weeks of gestation. For those with more than 37 weeks of gestation, IAP is administered after considering the following risk factors: longer duration of ROM (>18 h), the occurrence of PROM, and maternal intrapartum temperature exceeding 38 °C [26]. The indications for IAP administration to prevent EOD recommended by the CDC are described in Table 1 below [26].
Table 1.
| GBS IAP Indicated | GBS IAP Not Indicated |
|---|---|
Maternal History
|
|
Current Pregnancy
|
|
Intrapartum
|
|
Abbreviations: GBS, Group B Streptococcus; NAAT, nucleic acid amplification test.
The detection of GBS colonization in the maternal rectovaginal area by universal GBS screening culture in pregnant women at 36 0/7–37 6/7 weeks, identification of candidates of IAP based on risk factors, and appropriate IAP administration are the mainstream procedures for the prevention of EOD [27]. These methods have substantially reduced the prevalence of neonatal EOD to approximately 0.25 cases per 100 live births, representing a nearly 85% decrease compared with that in 1990 [28,30,36,37,38,39,40][28][30][36][37][38][39][40]. However, LOD has not been prevented, and its rate remains stable, at approximately 0.27 per 1000 live births [30,41][30][41]. Acknowledging the significance of such guidelines for the prevention of GBS disease, many other countries and clinical institutions have also proposed guidelines for treating maternal GBS infections as well [42,43,44,45,46,47][42][43][44][45][46][47].
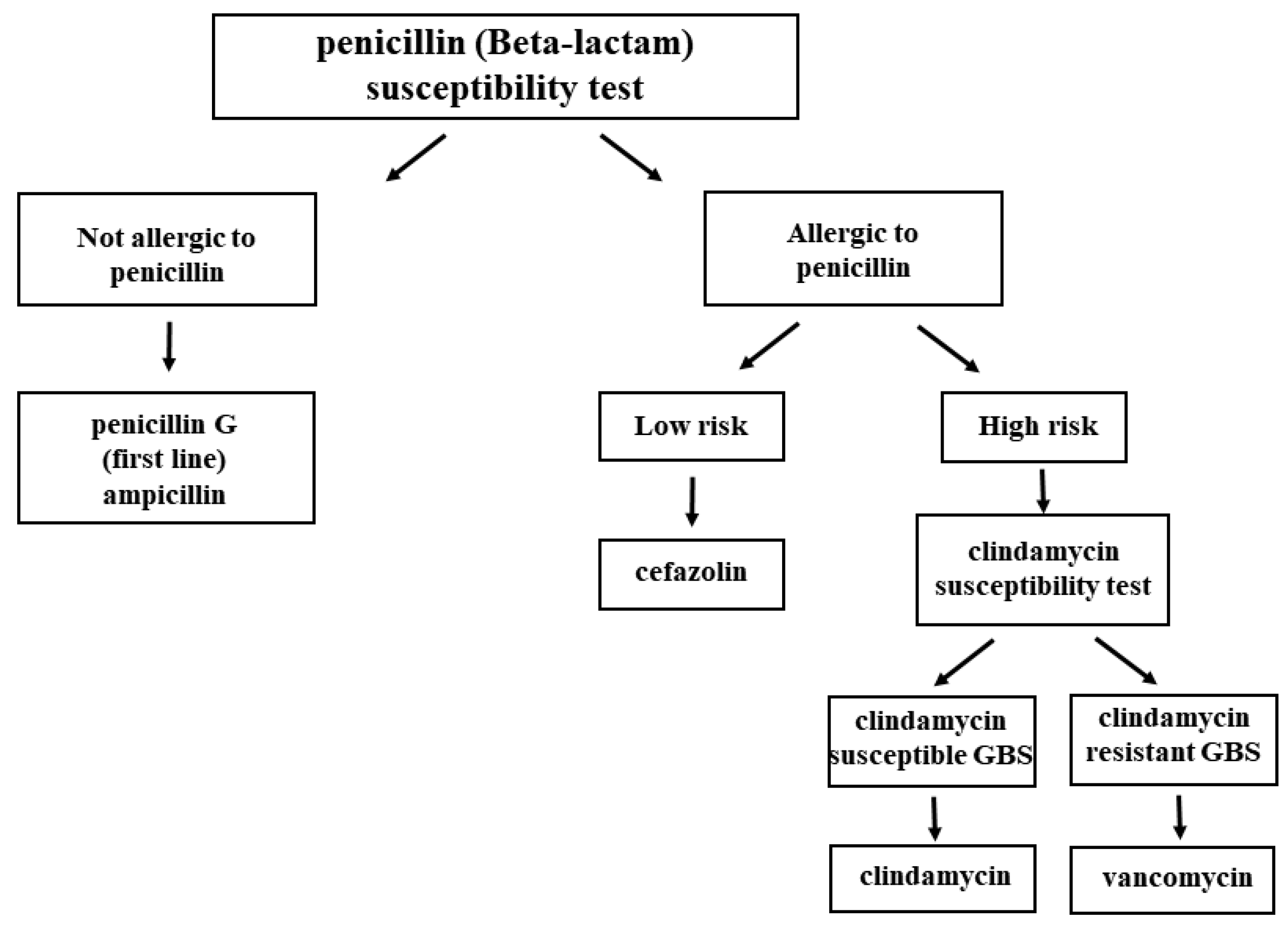
Penicillin G is usually the first-line agent for GBS IAP because of its low cost, low toxicity, and narrow-spectrum activity [2]. According to the American Academy of Pediatrics, antibiotics should be administered at least 4 h before delivery to achieve a sufficient concentration in the amniotic fluid and in the placenta circulation, which would reduce maternal-fetal GBS transmission [48]. However, for those who are allergic to penicillin G, or β-lactam antibiotics, other antibiotics are alternatively given based on the risk-based algorithm. If pregnant women are allergic to β-lactam antibiotics but have a low risk of anaphylaxis, cefazolin is given. For those with a high risk of anaphylaxis, a susceptibility test for clindamycin should first be performed via a GBS culture method. Clindamycin should be administered to pregnant women with clindamycin-susceptible GBS infection, and those with clindamycin-resistant GBS infection should be administered vancomycin. The treatment algorithm involving the usage of antibiotics is described in Figure 2 [49,50][49][50].
Unlike other general antibiotic therapies, IAP is only given as a “partial antibiotics treatment”. For example, “full antibiotics therapy” has been employed for treating gastric cancer to eradicate Helicobacter pylori, a bacterium that was classified as a group 1 human carcinogen by the International Agency for Research on Cancer of the WHO [51,52,53,54,55,56,57][51][52][53][54][55][56][57]. Such full doses of antibiotics would destroy H. pylori colonization and therefore treat gastric cancer. However, in the field of obstetrics, such “full antibiotics treatment” cannot be performed because it can cause severe harm to the health of both the mother and fetus, including fatal disease or chronic disabilities.
Similarly, IAP has some limitations. There has been an increasing necessity for antimicrobial susceptibility tests for pregnant women because of the risk of anaphylaxis [58,59][58][59]. Some researchers reported that the ratio of maternally transferred antibodies to newborns is approximately 0.5–0.7, indicating the inefficiency of IAP treatment [60]. As previously mentioned, IAP possibly disrupts the microbiota of newborns, and treatment in that neonatal period has inherent risks [1,2,61,62,63][1][2][61][62][63].
2.3. GBS and Maternal Microbiome
As the vaginal flora is one of the microbiomes in the maternal vaginal environment, many studies have investigated its association with GBS [64,65][64][65]. Moreover, many studies have examined the association of the vaginal microbiome with adverse obstetric outcomes in the context of vaginosis, an imbalance of the vaginal microbiome composition that usually involves the loss of Lactobacillus and the overgrowth of other pathogenic microbiomes [66,67,68,69,70,71][66][67][68][69][70][71]. There are mainly two types of vaginosis: bacterial vaginosis (BV) and aerobic vaginosis (AV). BV is defined as the replacement of normal lactobacilli by a large number of anaerobic microbes, such as Gardnerella, Prevotella, or Bacteroides [72]. AV is characterized by the disruption of lactobacilli accompanied by increases in the number of aerobic facultative pathogenic microbes, such as GBS or Escherichia coli [73]. Both BV and AV are considered to be associated with various serious obstetric clinical complications, such as preterm birth, miscarriage, prelabor rupture of membrane (PROM), fetal infection, and low birth weight [74]. Donders et al. reported that both BV and AV along with an abnormal vaginal microbiome in early pregnancy are associated with such complications [75,76][75][76]. The same group further confirmed an abnormal vaginal flora can influence cervical shortening, thereby leading to preterm birth [77].
Recently, Mohamed et al. discovered that dysbiosis in pregnant women with BV was accompanied by an increased prevalence of Streptococcus. In particular, pregnant BV showed a significant decline in the abundance of Lactobacillus (34.7% vs. 88.4% in the healthy group) along with an increase in the abundance of Streptococcus (29.7% in the BV group vs. 3.8% in the healthy group) [78]. However, Daskalakis et al. reported that although BV was associated with an increased risk of preterm delivery, GBS colonization in the second trimester of pregnancy was negatively correlated with the risk of preterm birth [79].
Hypothesizing that the presence of GBS in pregnant women influences and perhaps leads to obstetrics-related complications, such as PROM or preterm birth, researchers conducted a 16S rRNA metagenomics study using swab samples from nine pregnant Korean women as a pilot study. Contrary to expectation, there was no significant change in alpha and beta diversity between the GBS-positive and GBS-negative groups. This finding was consistent with that of other studies on pregnant Korean and Guatemalan cohorts [80,81,82][80][81][82]. However, researchers identified four relatively abundant pathogens in the GBS-positive group: Actinomyces, Shigella, Fenollaria, and Gemella. Researchers presume that these genera could serve as potential indicators of pregnancy management and stratification [unpublished data]. The findings might further support the dynamics between GBS and other diverse vaginal microbiomes in the vaginal environment, which collectively influence pregnancy-related adverse outcomes.
References
- Marió, M.J.S.; Valenzuela, I.; Vásquez, A.E.; Illanes, S.E. Prevention of Early-Onset Neonatal Group B Streptococcal Disease. Rev. Obstet. Gynecol. 2013, 6, 63–68.
- Ben-Taleb, H.; Nayme, K.; Lebrazi, H.; Chamekh, M.; SAILE, R.; Zerouali, K.; Timinouni, M.; Nayeme, K.; Lebrazi, H.; Chamekh, M.; et al. Review A Review of Group B Streptococcus Maternal-Fetal Infection. Moroc. J. Public Heath 2021, 3, 32–39.
- Lancefield, B.R.C. A Serological Differentiation of Human and Other Groups of Hemolytic Streptococci. J. Exp. Med. 1933, 1919, 571–595.
- Edwards, M.S.; Baker, C.J. Group B Streptococcal Infections in Elderly Adults. Clin. Infect. Dis. 2005, 41, 839–847.
- Slotved, H.C.; Kong, F.; Lambertsen, L.; Sauer, S.; Gilbert, G.L. Serotype IX, a Proposed New Streptococcus Agalactiae Serotype. J. Clin. Microbiol. 2007, 45, 2929–2936.
- Armistead, B.; Oler, E.; Adams Waldorf, K.; Rajagopal, L. The Double Life of Group B Streptococcus: Asymptomatic Colonizer and Potent Pathogen. J. Mol. Biol. 2019, 431, 2914–2931.
- Paoletti, L.C.; Kasper, D.L. Surface Structures of Group B Streptococcus Important in Human Immunity. Gram Positive Pathog. 2019, 204–227.
- Hanna, M.; Noor, A. Streptococcus, Group B. In StatPearls; StatPearls Publishing: Tampa, FL, USA, 2022.
- Randis, T.M.; Baker, J.A.; Ratner, A.J. Group B Streptococcal Infections. Pediatr. Rev. 2017, 38, 254–262.
- Raabe, V.N.; Shane, A.L. Group b Streptococcus (Streptococcus Agalactiae). Gram Positive Pathog. 2019, 228–238.
- Wilkinson, H.W. Group B Streptococcal Infection in Humans. Annu. Rev. Microbiol. 1978, 32, 41–57.
- Anthony, B.F.; Okada, D.M. The Emergence of GBS in Infections of the Newborn Infant. Annu. Rev. Med. 1977, 28, 355–369.
- Group B Strep: Fast Facts and Statistics|CDC. Available online: https://www.cdc.gov/groupbstrep/about/fast-facts.html (accessed on 11 November 2022).
- Gonçalves, B.P.; Procter, S.R.; Paul, P.; Chandna, J.; Lewin, A.; Seedat, F.; Koukounari, A.; Dangor, Z.; Leahy, S.; Santhanam, S.; et al. Group B Streptococcus Infection during Pregnancy and Infancy: Estimates of Regional and Global Burden. Lancet Glob. Health 2022, 10, e807–e819.
- Russell, N.J.; Seale, A.C.; O’Driscoll, M.; O’Sullivan, C.; Bianchi-Jassir, F.; Gonzalez-Guarin, J.; Lawn, J.E.; Baker, C.J.; Bartlett, L.; Cutland, C.; et al. Maternal Colonization with Group B Streptococcus and Serotype Distribution Worldwide: Systematic Review and Meta-Analyses. Clin. Infect. Dis. 2017, 65, S100–S111.
- Farley, M.M. Group B Streptococcal Disease in Nonpregnant Adults. Clin. Infect. Dis. 2001, 33, 556–561.
- Group B Streptococcal Disease in Nonpregnant Adults-UpToDate. Available online: https://www.uptodate.com/contents/group-b-streptococcal-infections-in-nonpregnant-adults (accessed on 15 November 2022).
- Group B Streptococcal Infection in Neonates and Young Infants-UpToDate. Available online: https://www.uptodate.com/contents/group-b-streptococcal-infection-in-neonates-and-young-infants (accessed on 15 November 2022).
- Collin, S.M.; Shetty, N.; Guy, R.; Nyaga, V.N.; Bull, A.; Richards, M.J.; van der Kooi, T.I.I.; Koek, M.B.G.; De Almeida, M.; Roberts, S.A.; et al. Group B Streptococcus in Surgical Site and Non-Invasive Bacterial Infections Worldwide: A Systematic Review and Meta-Analysis. Int. J. Infect. Dis. 2019, 83, 116–129.
- Krohn, M.A.; Hillier, S.L.; Baker, C.J. Maternal Peripartum Complications Associated with Vaginal Group B Streptococci Colonization. J. Infect. Dis. 1999, 179, 1410–1415.
- Menichini, D.; Chiossi, G.; Monari, F.; De Seta, F.; Facchinetti, F. Supplementation of Probiotics in Pregnant Women Targeting Group B Streptococcus Colonization: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 4520.
- Hall, J.; Adams, N.H.; Bartlett, L.; Seale, A.C.; Lamagni, T.; Bianchi-Jassir, F.; Lawn, J.E.; Baker, C.J.; Cutland, C.; Heath, P.T.; et al. Maternal Disease with Group B Streptococcus and Serotype Distribution Worldwide: Systematic Review and Meta-Analyses. Clin. Infect. Dis. 2017, 65, S112–S124.
- Lee, C.C.; Hsu, J.F.; Prasad Janapatla, R.; Chen, C.L.; Zhou, Y.L.; Lien, R.; Chiu, C.H. Clinical and Microbiological Characteristics of Group B Streptococcus from Pregnant Women and Diseased Infants in Intrapartum Antibiotic Prophylaxis Era in Taiwan. Sci. Rep. 2019, 9, 13525.
- Kwatra, G.; Cunnington, M.C.; Merrall, E.; Adrian, P.V.; Ip, M.; Klugman, K.P.; Tam, W.H.; Madhi, S.A. Prevalence of Maternal Colonisation with Group B Streptococcus: A Systematic Review and Meta-Analysis. Lancet Infect. Dis. 2016, 16, 1076–1084.
- Group B Streptococcal Infection in Pregnant Individuals-UpToDate. Available online: https://www.uptodate.com/contents/group-b-streptococcal-infection-in-pregnant-individuals (accessed on 15 November 2022).
- Prevention of Group B Streptococcal Early-Onset Disease in Newborns: ACOG Committee Opinion, Number 797. Obstet. Gynecol. 2020, 135, e51–e72.
- Puopolo, K.M.; Lynfield, R.; Cummings, J.J. Management of Infants at Risk for Group B Streptococcal Disease. Pediatrics 2019, 144, e20191881.
- Nanduri, S.A.; Petit, S.; Smelser, C.; Apostol, M.; Alden, N.B.; Harrison, L.H.; Lynfield, R.; Vagnone, P.S.; Burzlaff, K.; Spina, N.L.; et al. Epidemiology of Invasive Early-Onset and Late-Onset Group B Streptococcal Disease in the United States, 2006 to 2015: Multistate Laboratory and Population-Based Surveillance. JAMA Pediatr. 2019, 173, 224–233.
- Berardi, A.; Tzialla, C.; Riva, M.; Cerbo, R.M.; Creti, R. Group B Streptococcus: Early- and Late-Onset Infections. J. Chemother. 2007, 19 (Suppl. S2), 24–27.
- Berardi, A.; Trevisani, V.; Di Caprio, A.; Bua, J.; China, M.; Perrone, B.; Pagano, R.; Lucaccioni, L.; Fanaro, S.; Iughetti, L.; et al. Understanding Factors in Group b Streptococcus Late-Onset Disease. Infect. Drug Resist. 2021, 14, 3207–3218.
- Lin, F.Y.C.; Weisman, L.E.; Troendle, J.; Adams, K. Prematurity Is the Major Risk Factor for Late-Onset Group B Streptococcus Disease. J. Infect. Dis. 2003, 188, 267–271.
- Le Doare, K.; Heath, P.T.; Plumb, J.; Owen, N.A.; Brocklehurst, P.; Chappell, L.C. Uncertainties in Screening and Prevention of Group B Streptococcus Disease. Clin. Infect. Dis. 2019, 69, 720–725.
- Bartlett, A.W.; Smith, B.; George, C.R.R.; McMullan, B.; Kesson, A.; Lahra, M.M.; Palasanthiran, P. Epidemiology of Late and Very Late Onset Group B Streptococcal Disease: Fifteen-Year Experience from Two Australian Tertiary Pediatric Facilities. Pediatr. Infect. Dis. J. 2017, 36, 20–24.
- Yeo, K.T.; Lahra, M.; Bajuk, B.; Hilder, L.; Abdel-Latif, M.E.; Wright, I.M.; Oei, J.L. Long-Term Outcomes after Group B Streptococcus Infection: A Cohort Study. Arch. Dis. Child. 2019, 104, 172–178.
- Libster, R.; Edwards, K.M.; Levent, F.; Edwards, M.S.; Rench, M.A.; Castagnini, L.A.; Cooper, T.; Sparks, R.C.; Baker, C.J.; Shah, P.E. Long-Term Outcomes of Group B Streptococcal Meningitis. Pediatrics 2012, 130, e8–e15.
- Verani, J.R.; McGee, L.; Schrag, S.J. Prevention of Perinatal Group B Streptococcal Disease—Revised Guidelines from CDC, 2010. MMWR Recomm. Rep. 2010, 59, 1–36.
- Schrag, S.; Gorwitz, R.; Fultz-Butts, K.; Schuchat, A. Prevention of Perinatal Group B Streptococcal Disease. Revised Guidelines from CDC. MMWR Recomm. Rep. 2002, 51, 1–22.
- Schrag, S.J.; Zywicki, S.; Farley, M.M.; Reingold, A.L.; Harrison, L.H.; Lefkowitz, L.B.; Hadler, J.L.; Danila, R.; Cieslak, P.R.; Schuchat, A. Group B Streptococcal Disease in the Era of Intrapartum Antibiotic Prophylaxis. N. Engl. J. Med. 2000, 342, 15–20.
- Dermer, P.; Lee, C.; Eggert, J.; Few, B. A History of Neonatal Group B Streptococcus with Its Related Morbidity and Mortality Rates in the United States. J. Pediatr. Nurs. 2004, 19, 357–363.
- Edmond, K.M.; Kortsalioudaki, C.; Scott, S.; Schrag, S.J.; Zaidi, A.K.; Cousens, S.; Heath, P.T. Group B Streptococcal Disease in Infants Aged Younger than 3 Months: Systematic Review and Meta-Analysis. Lancet 2012, 379, 547–556.
- Kristeva, M.; Tillman, C.; Goordeen, A. Immunization Against Group B Streptococci vs. Intrapartum Antibiotic Prophylaxis in Peripartum Pregnant Women and Their Neonates: A Review. Cureus 2017, 9, e1775.
- University Hospitals of Leicester NHS. Group B Streptococcus in Pregnancy and the Newborn UHL Obstetric Guideline. 2008. Available online: https://secure.library.leicestershospitals.nhs.uk/PAGL/Shared%20Documents/Group%20B%20Streptococcus%20in%20Pregnancy%20and%20the%20Newborn%20UHL%20Obstetric%20Guideline.pdf (accessed on 31 August 2022).
- Group B Strep Suppprt. Group B Streptococcus (GBS) in Pregnancy and Newborn Babies. 2017, Volume 2017. Available online: https://gbss.org.uk/wp-content/uploads/2018/01/2017-Joint-RCOG-GBSS-PIL_final.pdf (accessed on 13 September 2022).
- Group B Streptococcus (GBS) Infections Guidelines: GBS Prophylaxis in Preterm Labor. Available online: https://emedicine.medscape.com/article/229091-guidelines (accessed on 15 November 2022).
- Pangerl, S.; Sundin, D.; Geraghty, S. Group B Streptococcus Screening Guidelines in Pregnancy: A Critical Review of Compliance. Matern. Child Health J. 2021, 25, 257–267.
- NHS Joint Clinical Guideline for: Group B Sreptococcus in Pregnancy. The Management of Women known to be carriers of Group B Streptococcus. Ver 6.4. 2021; 1–7.
- Group B Strep Suppprt. Updated Group B Strep Guidelines. Available online: https://gbss.org.uk/wp-content/uploads/2018/06/2018_06_RCOG_Summary_Leaflet.pdf (accessed on 22 October 2022).
- Committee on Infectious Diseases; Committee on Fetus and Newborn; Baker, C.J.; Byington, C.L.; Polin, R.A. Policy Statement-Recommendations for the Prevention of Perinatal Group B Streptococcal (GBS) Disease. Pediatrics 2011, 128, 611–616.
- Ahmadzia, H.K.; Heine, R.P. Diagnosis and Management of Group B Streptococcus in Pregnancy. Obstet. Gynecol. Clin. North Am. 2014, 41, 629–647.
- Dhudasia, M.B.; Flannery, D.D.; Pfeifer, M.R.; Puopolo, K.M. Updated Guidance: Prevention and Management of Perinatal Group b Streptococcus Infection. Neoreviews 2021, 22, e177–e188.
- Liou, J.M.; Malfertheiner, P.; Lee, Y.C.; Sheu, B.S.; Sugano, K.; Cheng, H.C.; Yeoh, K.G.; Hsu, P.I.; Goh, K.L.; Mahachai, V.; et al. Screening and Eradication of Helicobacter Pylori for Gastric Cancer Prevention: The Taipei Global Consensus. Gut 2020, 69, 2093–2112.
- Fallone, C.A.; Chiba, N.; van Zanten, S.V.; Fischbach, L.; Gisbert, J.P.; Hunt, R.H.; Jones, N.L.; Render, C.; Leontiadis, G.I.; Moayyedi, P.; et al. The Toronto Consensus for the Treatment of Helicobacter Pylori Infection in Adults. Gastroenterology 2016, 151, 51–69.e14.
- Goderska, K.; Agudo Pena, S.; Alarcon, T. Helicobacter Pylori Treatment: Antibiotics or Probiotics. Appl. Microbiol. Biotechnol. 2018, 102, 1–7.
- Piscione, M.; Mazzone, M.; Di Marcantonio, M.C.; Muraro, R.; Mincione, G. Eradication of Helicobacter Pylori and Gastric Cancer: A Controversial Relationship. Front. Microbiol. 2021, 12, 630852.
- Freedberg, D.E.; Abrams, J.A.; Wang, T.C. Prevention of Gastric Cancer with Antibiotics: Can It Be Done without Eradicating Helicobacter Pylori? J. Natl. Cancer Inst. 2014, 106, 1995–1996.
- Gao, Y.; Shang, Q.; Li, W.; Guo, W.; Stojadinovic, A.; Mannion, C.; Man, Y.G.; Chen, T. Antibiotics for Cancer Treatment: A Double-Edged Sword. J. Cancer 2020, 11, 5135–5149.
- Schistosomes, liver flukes and Helicobacter pylori. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; International Agency for Research on Cancer: Lyon, France, 1994; Volume 61, pp. 1–241.
- Hayes, K.; O’Halloran, F.; Cotter, L. A Review of Antibiotic Resistance in Group B Streptococcus: The Story so Far. Crit. Rev. Microbiol. 2020, 46, 253–269.
- Davies, H.G.; Carreras-Abad, C.; Le Doare, K.; Heath, P.T. Group B Streptococcus: Trials and Tribulations. Pediatr. Infect. Dis. J. 2019, 38, S72–S76.
- Madhi, S. Group B Streptococcus (GBS) Vaccine. Int. J. Infect. Dis. 2018, 73, 31.
- Dominguez, K.; Randis, T.M. Toward the Development of a Protein-Based Group B Streptococcus Vaccine. Cell Rep. Med. 2022, 3, 100536.
- Garcia, V.R. Impact of Intrapartum Antibiotic Prophylaxis for Group B Streptococcus on the Term Infant Gut Microbiome: A State of the Science Review. J. Midwifery Women’s Health 2021, 66, 351–359.
- Ainonen, S.; Tejesvi, M.V.; Mahmud, M.R.; Paalanne, N.; Pokka, T.; Li, W.; Nelson, K.E.; Salo, J.; Renko, M.; Vänni, P.; et al. Antibiotics at Birth and Later Antibiotic Courses: Effects on Gut Microbiota. Pediatr. Res. 2022, 91, 154–162.
- Rosen, G.H.; Randis, T.M.; Desai, P.V.; Sapra, K.J.; Ma, B.; Gajer, P.; Humphrys, M.S.; Ravel, J.; Gelber, S.E.; Ratner, A.J. Group B Streptococcus and the Vaginal Microbiota. J. Infect. Dis. 2017, 216, 744–751.
- Brzychczy-Wloch, M.; Pabian, W.; Majewska, E.; Zuk, M.; Kielbik, J.; Gosiewski, T.; Bulanda, M. Dynamics of Colonization with Group B Streptococci in Relation to Normal Flora in Women during Subsequent Trimesters of Pregnancy. New Microbiol. 2014, 37, 307–319.
- Svare, J.A.; Schmidt, H.; Hansen, B.B.; Lose, G. Bacterial Vaginosis in a Cohort of Danish Pregnant Women: Prevalence and Relationship with Preterm Delivery, Low Birthweight and Perinatal Infections. BJOG An Int. J. Obstet. Gynaecol. 2006, 113, 1419–1425.
- Fettweis, J.M.; Serrano, M.G.; Brooks, J.P.; Edwards, D.J.; Girerd, P.H.; Parikh, H.I.; Huang, B.; Arodz, T.J.; Edupuganti, L.; Glascock, A.L.; et al. The Vaginal Microbiome and Preterm Birth. Nat. Med. 2019, 25, 1012–1021.
- Leitich, H.; Kiss, H. Asymptomatic Bacterial Vaginosis and Intermediate Flora as Risk Factors for Adverse Pregnancy Outcome. Best Pract. Res. Clin. Obstet. Gynaecol. 2007, 21, 375–390.
- Hillier, S.L.; Nugent, R.P.; Eschenbach, D.A.; Krohn, M.A.; Gibbs, R.; Martin, D.H.; Cotch, M.F.; Edelman, R. Association between Bacterial Vaginosis and Preterm Delivery of a Low- Birth-Weight Infant. Stud. Fam. Plann. 1996, 27, 57.
- Guerra, B.; Ghi, T.; Quarta, S.; Morselli-Labate, A.M.; Lazzarotto, T.; Pilu, G.; Rizzo, N. Pregnancy Outcome after Early Detection of Bacterial Vaginosis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2006, 128, 40–45.
- Guaschino, S.; De Seta, F.; Piccoli, M.; Maso, G.; Alberico, S. Aetiology of Preterm Labour: Bacterial Vaginosis. BJOG An Int. J. Obstet. Gynaecol. 2006, 113, 46–51.
- Campisciano, G.; Zanotta, N.; Petix, V.; Giangreco, M.; Ricci, G.; Maso, G.; Comar, M.; De Seta, F. Vaginal Dysbiosis and Partial Bacterial Vaginosis: The Interpretation of the “Grey Zones” of Clinical Practice. Diagnostics 2021, 11, 191.
- Kaambo, E.; Africa, C.; Chambuso, R.; Passmore, J.A.S. Vaginal Microbiomes Associated with Aerobic Vaginitis and Bacterial Vaginosis. Front. Public Health 2018, 6, 78.
- Klebanoff, M.A.; Hillier, S.L.; Nugent, R.P.; MacPherson, C.A.; Hauth, J.C.; Carey, J.C.; Harper, M.; Wapner, R.J.; Trout, W.; Moawad, A.; et al. Is Bacterial Vaginosis a Stronger Risk Factor for Preterm Birth When It Is Diagnosed Earlier in Gestation? Am. J. Obstet. Gynecol. 2005, 192, 470–477.
- Donders, G.G.; Van Calsteren, K.; Bellen, G.; Reybrouck, R.; Van Den Bosch, T.; Riphagen, I.; Van Lierde, S. Predictive Value for Preterm Birth of Abnormal Vaginal Flora, Bacterial Vaginosis and Aerobic Vaginitis during the First Trimester of Pregnancy. BJOG An Int. J. Obstet. Gynaecol. 2009, 116, 1315–1324.
- Donders, G.G.G.; Bellen, G.; Rezeberga, D. Aerobic Vaginitis in Pregnancy. BJOG An Int. J. Obstet. Gynaecol. 2011, 118, 1163–1170.
- Donders, G.G.; Van Calsteren, C.; Bellen, G.; Reybrouck, R.; Van den Bosch, T.; Riphagen, I.; Van Lierde, S. Association between Abnormal Vaginal Flora and Cervical Length as Risk Factors for Preterm Birth. Ultrasound Obstet. Gynecol. 2010.
- Mohamed, I.; Zakeer, S.; Azab, M.; Hanora, A. Changes in Vaginal Microbiome in Pregnant and Nonpregnant Women with Bacterial Vaginosis: Toward Microbiome Diagnostics? Omi. A J. Integr. Biol. 2020, 24, 602–614.
- Daskalakis, G.; Papapanagiotou, A.; Mesogitis, S.; Papantoniou, N.; Mavromatis, K.; Antsaklis, A. Bacterial Vaginosis and Group B Streptococcal Colonization and Preterm Delivery in a Low-Risk Population. Fetal Diagn. Ther. 2006, 21, 172–176.
- Rick, A.M.; Aguilar, A.; Cortes, R.; Gordillo, R.; Melgar, M.; Samayoa-Reyes, G.; Frank, D.N.; Asturias, E.J. Group B Streptococci Colonization in Pregnant Guatemalan Women: Prevalence, Risk Factors, and Vaginal Microbiome. Open Forum Infect. Dis. 2017, 4, ofx020.
- Kim, D.H.; Min, B.J.; Jung, E.J.; Byun, J.M.; Jeong, D.H.; Lee, K.B.; Sung, M.S.; Kim, K.T.; Kim, Y.N. Prevalence of Group B Streptococcus Colonization in Pregnant Women in a Tertiary Care Center in Korea. Obstet. Gynecol. Sci. 2018, 61, 575–583.
- Lee, Y.H.; Lee, Y.J.; Jung, S.Y.; Kim, S.Y.; Son, D.W.; Seo, I.H. Pregnancy and Neonatal Outcomes of Group B Streptococcus Infection in Preterm Births. Perinatology 2018, 29, 147.
More