Much effort has been devoted to developing Pdots with emission bands located in the second near-infrared (NIR-II, 1000–1700 nm) region, which hold great advantages of higher spatial resolution, better signal-to-background ratios (SBR), and deeper tissue penetration for solid-tumor imaging in comparison with the visible region (400–680 nm) and the first near-infrared (NIR-I, 680–900 nm) window, by virtue of the reduced tissue autofluorescence, minimal photon scattering, and low photon absorption.
Herein, we will focus on the latest reported NIR-II Pdots for in vivo tumor imaging, and in particular on the molecular engineering to optimize the fluorescence quantum yields and surface functionalization to promote the ability of active tumor targeting. Some of the NIR-II theranostic Pdots used for integrated FI diagnosis and phototherapy are also included. Finally, we will discuss the future directions and challenges in this field.
1. Introduction
In vivo biomedical imaging is a technique that generates internal images of the body by non-invasive means and is commonly used in clinical analysis and medical interventions. Various biological imaging modalities have been validated through previous research and clinical practice, such as photoacoustic imaging (PAI)
[1][2][3][1,2,3], fluorescence imaging (FI)
[4][5][4,5], computed tomography (CT)
[6], positron emission tomography (PET)
[7][8][7,8], and single-photon emission computed tomography (SPECT)
[9]. In comparison to these clinical imaging techniques, fluorescence imaging (FI) has the advantages of real-time imaging, high sensitivity, rapid feedback, good spatial and temporal resolution, and good biocompatibility
[10][11][10,11]. Despite the many advantages of FI imaging, in vivo FI is not optimal in the visible (400–680 nm) and the first near-infrared (NIR-I, 680–900 nm) regions due to photon attenuation caused by biological tissues and autofluorescence
[12][13][12,13]. In recent years, as shown in
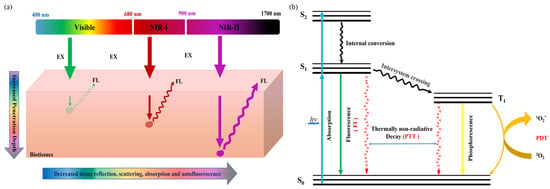
Figure 1a, the second near-infrared (NIR-II, 1000–1700 nm) region has attracted much attention for its advantages of greatly improved penetration depth (5–20 mm), spatial and temporal resolution (25 mm and 20 ms), high signal-to-background ratio (SBR), low tissue absorption, and minimal autofluorescence interference
[14][15][14,15]. When NIR-II light passes through tissues, the interaction between photons and tissues is significantly reduced, and the photons can penetrate into deeper tissues, so as to achieve better surgical guided anatomy (
Figure 1b). For this purpose, various NIR-II emissive fluorescent probes, such as metal nanoparticles
[16][17][18][16,17,18], inorganic semiconductor nanoparticles
[19][20][19,20], small molecular-based nanoparticles
[20][21][20,21], and aggregation-induced emission (AIE) nanoparticles
[22][23][22,23], have been established.
Figure 1. (a) Schematic illustration of the fluorescence signals with different wavelengths in biological tissues and (b) the mechanism of the fluorescence-generation process and photothermal/photodynamic therapy.
Very recently, semiconducting polymer dots (Pdots) have attracted extensive attention for in vivo fluorescence imaging applications
[24]. Pdots are types of organic polymer-based nanoparticles, mainly composed of π-conjugated hydrophobic polymer as light absorber and fluorescent emitter
[25], with additional non-conjugated polyethylene glycol (PEG)-based amphiphilic polymers as a surfactant to stabilize the nanoparticles in aqueous solution and to avoid non-specific binding of Pdots to biological species
[26]. Pdots can be prepared by nanoprecipitation, mini-emulsion polymerization, self-assembly of amphiphilic block copolymers, or a microfluidic approach, which are detailed in previous reviews
[24][27][28][24,27,28]. Usually, the mass or volume fraction of the conjugated polymer in a single Pdot must be greater than 50% and with a diameter less than 40 nm
[29]. Compared with nanoparticles prepared by traditional materials such as single carbon nanotubes (SWCNTs)
[30], lanthanide metals
[31], and small organic molecules
[14][32][14,32], Pdots have demonstrated significant fluorescence properties in biological studies, such as good biocompatibility
[33][34][33,34] and photostability
[35][36][35,36], fast radiation decay rate
[37][38][37,38], large absorption coefficient
[39], ultrahigh single-particle brightness
[40]. Combined with the adjustable structures and easy surface functionalization properties, Pdots have become a promising diagnostic and therapeutic platform for biosensors
[41][42][41,42], cell labeling
[43][44][43,44], tissue imaging
[45][46][45,46], phototherapy applications (
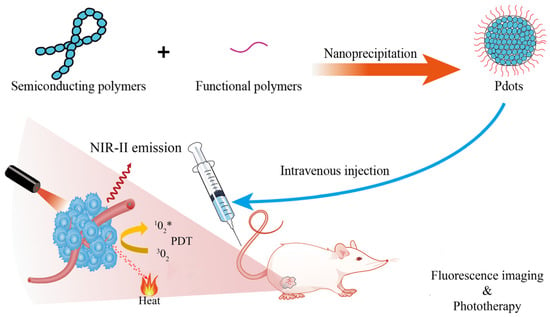
Figure 2)
[47], and the nanocarriers for drug delivery, which have been well-summarized by recent reviews
[48][49][50][51][48,49,50,51]. Despite the above-mentioned advantages and the great progress achieved, Pdots still have some problems to be solved for better biologic applications. For example, it is difficult to prepare Pdots with uniform nano-size by a simple nanoprecipitation method. Second, it is difficult to obtain small enough Pdots (≤10 nm, with a high content of conjugated polymer) to enhance their tumor targeting/permeability ability, and little work has been reported on their metabolism and long-term biosafety in vivo. Thirdly, the fluorescence quantum efficiency (
Φf) of the Pdot probe is usually lower than comparable small-molecular probes or inorganic metallic quantum dots (Qdots), especially for NIR-II emissive Pdots.
Figure 2. Illustration the nanoformulation of Pdots and their applications for tumor fluorescence imaging and phototherapy.
Many NIR-II emissive Pdots have been presented for deep-tissue FI with high spatial temporal resolution, such as blood vessels, bones, lymph node, solid tumors, through-skull imaging, and cell tracking, which are referenced in recent reviews
[52][53][52,53].
Herein, reswearchers will focus on the latest reported NIR-II Pdots for in vivo tumor imaging, and in particular on the molecular engineering to optimize the fluorescence quantum yields and surface functionalization to promote the ability of active tumor targeting. Some of the NIR-II theranostic Pdots used for integrated FI diagnosis and phototherapy are also included. The properties and their applications of the NIR-II Pdots summarized in this review are listed in Table 1. Finally, researchwers will discuss the future directions and challenges in this field
2. Molecular Engineering of Efficient NIR-II Pdots for In Vivo Tumor FI
As mentioned above, light with wavelengths in the NIR-II region has a high development potential for in vivo imaging of tumors due to its small absorption and scattering in animal tissues, better tissue penetration and higher spatial resolution. However, NIR-II emissive Pdots usually exhibit low
Φf due to an intrinsic small energy bandgap between the lowest unoccupied molecular orbital (LUMO) and the highest occupied molecular orbital (HOMO) of conjugated polymers, as well as the severe aggregation-caused quenching (ACQ) effect in the Pdot state, both of which facilitate non-radiative decay, resulting in low
Φf of NIR-II Pdots
[49][54][55][49,62,63]. Therefore, one important challenge is to design NIR-II Pdots with high
Φf, to realize high brightness in vivo and hence better SBR of fluorescence imaging.
To develop NIR-II Pdot probes with high
Φf, Liu et al., proposed a fluorination strategy to design semiconducting polymers to optimize the
Φf of corresponding Pdot probes
[56][54]. Using benzodithiophene (BDT) as electron donor (D) and triazole [4,5-g]-quinoxaline (TQ) derivatives as electron acceptor (A), the team synthesized two sets of fluorine-substituted D-A type semiconducting polymers based on the two fluorine substitution modes, which are named as m-PBTQ, m-PBTQ2F, m-PBTQ4F, m-PBTQ4F, with alkoxy chains anchored at the meta site of benzene and p-PBTQ, p-PBTQ2F, and p-PBTQ4F, with alkoxy chains anchored to the para position of benzene. The semiconducting polymers and amphiphilic polystyrene polymer (PS-PEG-COOH) were prepared into Pdots by the classic nano-precipitation method. The
Φf of obtained m-series Pdots (1.0%, 2.2%, and 3.2%) were consistently higher than those of p-series Pdots (0.6%, 0.9%, and 1.5%), mainly because the different alkoxy positions on TQ affected the hydrophobicity and steric hindrance of molecules. Additionally, the
Φf of Pdots was increased with more fluorine atoms on the TQ acceptor. As a result, the tetrafluorinated m-PBTQ4F Pdots yielded the highest
Φf of 3.2%, which was three times higher than that of the non-fluoridated counterpart and six times higher than that of IR26. Liu et al. attributed the fluorescence enhancement in the fluorinated Pdots to the nanoscale fluorous effect that increases the planarity of the conjugated backbone and minimizes the structure distortion between the excited-state and ground-state, thus decreasing the nonradiative decay rates. The fluorescence intensity of m-PBTQ4F Pdots remained at 80% of the initial state under 120 min laser irradiation, indicating good photostability. The authors suggest that fluorination of PBTQ polymers can effectively modulate the optical properties of the resulting Pdot in several ways, including energy-level reduction and fluorescence enhancement. Quantitative cranial and scalp-puncture imaging of brain tumor vasculature in vivo demonstrated that m-PBTQ4F Pdot images showed better imaging performance than non-fluoridated m-PBTQ images, and could distinguish normal, uniform, and orderly brain blood vessels from the images, and also clearly differentiate uneven and chaotic distribution. These results indicate that fluorinated Pdots have good photostability and high brightness, and have great development potential in the diagnosis and detection of brain tumors.
To solve the low
Φf problem of NIR-II probes arising from the ACQ effect, Li et al., proposed two strategies to develop efficient NIR-II Pdots, by incorporating an anti-ACQ unit or an aggregation-induced emission (AIE) segment into the polymer backbone
[57][55]. The authors first designed a conjugated polymer skeleton using [1,2,5]thiadiazolo [3,4-g]quinoxaline (TQ) as a strong A unit and alkylthio-thiophene-substituted benzodithiophene as the D unit. A phenothiazines with Pttc (anti-ACQ), TPA (AIE), or TPE (AIE) unit was then added to the semiconducting polymer backbone. The amphiphilic lipids were subsequently made into Pdots by a nanoprecipitate with semiconducting polymers. The results showed that IR-PTTC Pdots had the lowest
Φf of 4.9% among the three Pdots, while IR-TPA and IR-TPE Pdots had
Φf of 6.7% and 14%, respectively. T
o the
best of our knowledge, the IR-TPE Pdot is, to date, the most efficient NIR-II emissive Pdot. At the same time, IT-TPE has a strong absorption at 700 nm, and the maximum emission peak is 1010 nm. Due to the high
Φf of IR-TPE Pdots, the authors used these Pdots for subsequent bioimaging. First, the authors functionalized the surface of IR-TPE Pdots with folic acid so that specific cancer cells with folate receptors could internalize them. Subsequently, Pdots were injected intravenously into mice through the tail vein, and the SBR reached 2.42 when equipped with a 1400 nm long-pass filter. At the same time, their fluorescence intensity remained more than 80% of their original intensity after 20 min of continuous UV irradiation. These results indicate that Pdots are very light resistant and suitable for long-term fluorescence imaging or tracking. The authors performed in vivo tumor imaging using IR-TPE Pdots and ICG in live mice bearing 4T1 tumors and compared the performance of these two probes. At the same time, 3D tumor mapping was performed in vivo in tumor-bearing mice 6 h after injection. The images in the film were reconstructed from a series of images with different rotation angles from −45° to 45°, from which
rwe
searchers could easily identify the location of the tumor.