Inspired by natural photosynthesis, the photocatalytic CO2 reduction reaction (CO2RR) stands as a viable strategy to produce solar fuels and mitigate the high dependence on highly polluting fossil fuels, as well as to decrease the CO2 concentration in the atmosphere. The design of efficient photocatalytic materials is crucial to ensure the long-term application of the CO2RR process. So far, perovskite materials have shown high efficiencies in CO2RR to generate different solar fuels, specially lead halide perovskites (LHP), which exhibit valuable features for the obtention of high production yields (e.g., narrow band gaps, adequate potentials for CO2RR, good charge transport properties, etc.). Nonetheless, the presence of lead involves an important environmental impact that cannot be negligible in the design of industrial-scale photocatalytic processes. Hence, the search for efficient Lead-free Halide Perovskites (LFHP) remains a high-priority task in the researchtudy of functional materials for CO2RR, since LFHPs could maintain the properties of LHPs, while keeping low environmental impacts and accessible costs of production. As an alternative, bismuth-based LFHPs have gained much attention due to their higher absorption coefficients, their more efficient charge transfer (compared to oxide perovskites), and their required thermodynamic potential for CO2RR. However, despite all the remarkable advantages of bismuth halide perovskites, their use has been limited, owing to instability concerns. TIn this article, the performance of bismuth-based LFHPs are discussed, as well as stability strategies from intrinsic and extrinsic standpoints.
- bismuth halide perovskites
- CO2RR
- fuels
1. Introduction
2. Metal Halide Perovskites for CO2RR
(i) Ease of synthesis. These materials are distinguished by their low enthalpy of formation, which often grants them facile synthesis processes at normal temperatures (~25 °C) and pressures (~1 atm). Also, it allows for the formation of different morphologies with a high number of active sites [41][42].
(ii) Structure modulation. It has been demonstrated that metal halide perovskites can modulate their crystalline structures and energy bands by incorporating anions or cations from different groups [43][44][45][46]. In addition, the polarization refinement structure can induce an intensive internal electric field, which facilitates charge migration to the surface, improving the efficiency of CO2RR [47]. On the other hand, the structure modulation can be carried out by variation of the morphology (e.g., bulk, nanoplates, nanorods, quantum dots, etc.) [48][49][50].
(iii) Light harvesting. The modulation of the composition of a halide perovskite can significantly alter its band gap, which can make it an excellent light harvester compared with other semiconductors. At an atomic level, the most common strategies for light-harvesting enhancement include extending the absorption range via band gap engineering, applying the plasmonic resonance enhancement effect, and the arrangement of light absorption using tandem absorber devices [51]. Recently, Jian et al. have proposed the formation of CsPbBr3 nanocrystals with O-defective WO3 composites as a solution for stable and highly efficient materials that can harvest the broadest solar spectrum possible [52]. This strategy increases CO2RR efficiency up to 7-fold compared to pristine materials.
(iv) Exciton generation. The efficient generation of electron–hole pairs under excitation can enhance the photoconversion efficiency in CO2RR and other applications, such as photovoltaics and photon detection [53]. This phenomenon is mainly seen in narrow-band perovskites, which can promote multiple exciton generation (MEG). Perovskite materials can exhibit a variety of exciton species under different excitation conditions, including biexciton and triple exciton [54]. The surface defects of perovskites are the main source of the production of charged excitons. Under weak light excitation, this is mostly generated by single exciton recombination; meanwhile, at medium excitation intensity, biexciton recombination is favored. On the other hand, by using strong light excitation, Auger recombination dominates the generation of charged excitons, and perovskite materials are mainly characterized by their recombination of these excitons. Lin et al. demonstrated that the decay time of single excitons and biexcitons in Mn–CsPbBr3 perovskites can be modified by applying an external magnetic field (300 mT), suppressing the recombination of the photogenerated charges and subsequently improving the efficiency of CO and CH4 generation from CO2RR [55].
(v) Long carrier diffusion lengths. Long carrier lifetime makes halide perovskite materials high-performance photovoltaic materials. Chen et al. proposed that the band-edge carrier lifetime increases when the system transitions from a lower rotational entropy to another phase with higher entropy [56]. These results suggest that the recombination of the photogenerated pair is inhibited by screening, leading to the formation of polarons and thereby extending their lifetime.
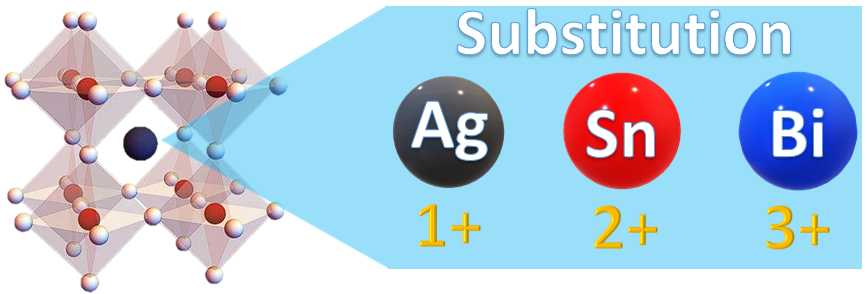
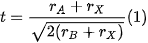
3. Bismuth Halide Perovskites
In the case of LFHPs, bismuth has a similar ionic radius to lead, as well as comparable properties to substitute this atom. Thus, the tolerance factor rule is satisfied, and the stability of the Bi-based perovskite materials can theoretically be enhanced when this substitution takes place. Bismuth provides an attractive option due to its nontoxic nature and chemical stability [74][98]. Its trivalent (3+) oxidation state causes materials to form an A3Bi2X9 structure, where A can be K+, Rb+, Cs+, or methylammonium MA+, and X can be I, Br, or Cl. Moreover, it was found that Bi-based perovskite materials possess a higher absorption coefficient, which renders them an efficient light-absorbing material for solar cell applications and, recently, for photocatalytic applications [73][75][76][77][78][79][92,93,94,95,96,97]. There are also reports on the use of Cs3Bi2I6Br3 films as solar cells, for which the highest power conversion efficiency (PCE) reported so far was 1.15% [75][93]. In addition, bismuth halides have been demonstrated to be good candidates for oxidizing a variety of organic compounds, such as thiamazole [76][94], vanillyl alcohol [77][95], rhodamine B [78][96], phenol [79][97], etc.
So far, most of the reports related to CO2RR using Bismuth Halide Perovskites employ Cs+ as the A-site cation in the A3Bi2X9 (X= I, Cl, Br, F) configuration. The best result for CO generation for these perovskites was obtained via the design of a Cs3Bi2I9–CeO2 heterostructure (135 μmol g−1 [80][139]), whereas the implementation of three stability strategies such as encapsulation, structure reconfiguration, and heterostructure design allowed for the highest efficiency to produce CH4 (151 μmol g−1 [81][127]). Both efficiencies (higher than other traditional and LHP perovskites) demonstrated the feasibility of using CO2RR to generate clean and renewable solar fuels, and these results were often attributed to a more efficient charge transfer with longer lifetimes, which grants more free charges for CO2RR.
Even with these remarkable findings, it is well-known that the low stability of Bismuth Halide Perovskites in ambient conditions hinders their commercialization and upscaling to the industrial level. Hence the lack of evidence regarding the recycling of these materials in consecutive cycles of evaluation, only reporting 5 h to 21 days of stability in gas–solid reactions. Some of the most common reasons for low stability in perovskites are shown in Figure 2.
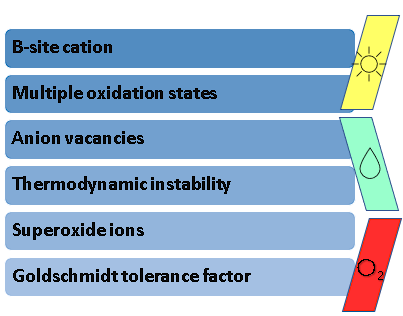
Figure 2. Factors that hinder perovskite stability.
In the researchtudy of stable perovskite photocatalysts, there are concerns regarding several factors that lead to instability, such as: (i) the B-site cation in the perovskite A3B2X9 configuration, as it must possess a good compatibility with this structure; (ii) the oxidation states of the ions that conform said structure, which determine the spontaneity with which these can undergo reduction or oxidation processes and lead to instability; (iii) anion vacancies, as these are inherent defects present in the perovskite structure that cause its degradation; (iv) thermodynamic instability, since the energetic feasibility that allows perovskites to be synthesized at room temperatures can also signify that these materials are prone to dissociate with the same ease; (v) the formation of superoxide ions, which are derived from the excitation of irradiated perovskites in the presence of atmospheric oxygen; and (vi) the aforementioned Goldschmidt tolerance factor, as it is an accurate indicator of the stability of a theoretical perovskite structure. Considering these aspects, several authors have proposed different strategies to increase the stability of halide perovskites.
4. Stability of Bismuth Halide Perovskites
The strategies with which the stability of photocatalysts can be improved are classified into two main approaches: intrinsic and extrinsic, depending on the nature of the employed methodology.
4.1 Intrinsic approach
This approach is widely used to improve the stability of metal halide perovskites through structural modification, which involves the alteration of the composition of cations and anions that are inherent to the perovskite structure [8273][8374]. The formation of solid solutions and double perovskites are examples of this approach. Likewise, the obtention of low-dimensional-networked perovskites promotes the formation of more stable crystal structures by selecting appropriate manufacturing techniques. Stoichiometry modification then settles the network in a quasi-zero-dimensional (0D) configuration, where the metal halide octahedra are almost isolated [8475]. Another successfully intrinsic strategy is passivation [8576]. This strategy allows the removal of dangling bonds, which act as recombination centers associated with defects, promoting better stability and optical properties. Antisolvent engineering is another strategy that speeds up nucleation during perovskite synthesis through solvent extraction [8677][8778][8879]. This strategy allows the obtention of dense, high-quality films of bismuth halide perovskites with enhanced stability.
4.2 Extrinsic approach
This approach is characterized by the modification of the perovskite’s external properties without altering its internal composition. Among these strategies, encapsulation is one of the most used. It consists of the coverage of the perovskite to prevent exposure to moisture, oxygen, UV light, and temperature, thus protecting the structure. The encapsulation can be done by glass–glass covering of the edges of the substrate with an adhesive, as well as the deposition of a polymer coating. The adhesives can be ethylene methacrylate (EMA), ethylene vinyl acetate (EVA), polyisobutylene (PIB), and epoxy resins activated with UV light [8980]. Alternatively, encapsulation can be done by depositing polymer coatings, e.g., polyethylene oxide, polyvinyl pyridine. The construction of heterostructures or Z-schemes between perovskites and other semiconductors has contributed to the design of promising stabilities for photocatalytic reactions since electrons are not available to react with the adsorbed oxygen. This avoids the degradation of the perovskite structure [9081][9182][9283]. Another option is the dispersion of the perovskite nanoparticles in porous support for enhanced electron and hole separation, more accessible active sites, and close contact with the reaction species [42]. At the same time, adding adequate reactants to the medium can promote good stability of the perovskite structure. For example, it has been proved that the addition of HI during the evaluation of MA3Bi2I9 promotes excellent phase stability and enhanced photocatalytic activity for H2 evolution [9384].
Some of these strategies have been implemented in bismuth halide perovskites to achieve better stabilities and efficiencies in photocatalytic CO2RR, mainly for Cs3Bi2X9 perovskites. So far, the stability of the Cs3Bi2X9 perovskite has been boosted by implementing two intrinsic strategies: solid solutions [9485] and double perovskites [9586]; as well as two extrinsic methodologies: the formation of heterojunctions with other semiconductors [9687] and the encapsulation of the perovskites in porous supports [9788]. The strategies implemented for the stable performance of bismuth halide perovskite photocatalysts are summarized in Figure 3.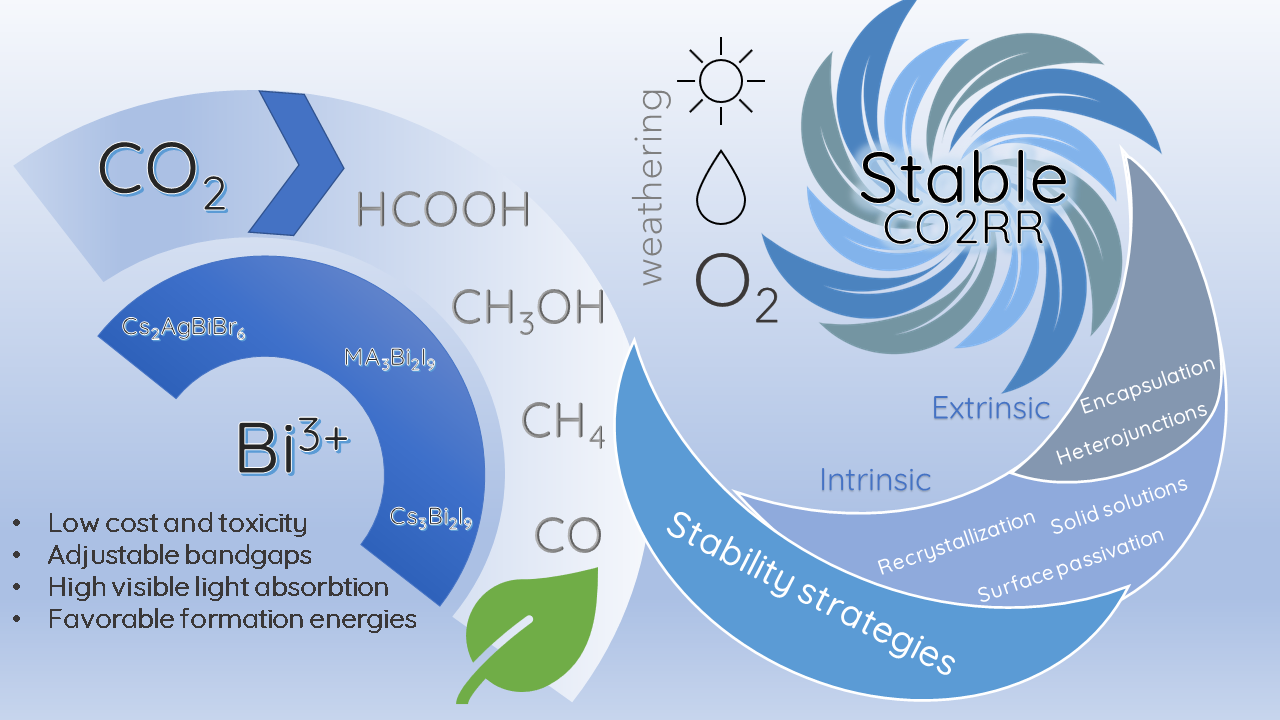
Figure 3. Strategies in bismuth halide perovskite for a stable CO2RR.
References
- IEA. Global Energy Review: CO2 Emissions in 2021—Analysis. 2021. Available online: https://www.iea.org/reports/global-energy-review-co2-emissions-in-2021-2 (accessed on 1 September 2022).
- Energy Agency, I. Global Energy Review: CO2 Emissions in 2021 Global Emissions Rebound Sharply to Highest Ever Level. 2021. Available online: www.iea.org/t&c/ (accessed on 1 September 2022).
- Ponce, P.; Khan, S.A.R. A causal link between renewable energy, energy efficiency, property rights, and CO2 emissions in developed countries: A road map for environmental sustainability. Environ. Sci. Pollut. Res. 2021, 28, 37804–37817.
- Answer, M.K.; Iqbal, W.; Ahmad, U.S.; Fatima, A.; Chaudhry, I.S. Environmental efficiency and the role of energy innovation in emissions reduction. Environ. Sci. Pollut. Res. 2020, 27, 29451–29463.
- Salehizadeh, H.; Yan, N.; Farnood, R. Recent advances in microbial CO2 fixation and conversion to value-added products. Chem. Eng. J. 2020, 390, 124584.
- Meunier, N.; Chauvy, R.; Mouhoubi, S.; Thomas, D.; De Weireld, G. Alternative production of methanol from industrial CO2. Renew. Energy 2020, 146, 1192–1203.
- Kumaravel, V.; Bartlett, J.; Pillai, S.C. Photoelectrochemical Conversion of Carbon Dioxide (CO2) into Fuels and Value-Added Products. ACS Energy Lett. 2020, 5, 486–519.
- Garba, M.D.; Usman, M.; Khan, S.; Shehzad, F.; Galadima, A.; Ehsan, M.F.; Ghanem, A.S.; Humayun, M. CO2 towards fuels: A review of catalytic conversion of carbon dioxide to hydrocarbons. J. Environ. Chem. Eng. 2021, 9, 104756.
- Mustafa, A.; Lougou, B.G.; Shuai, Y.; Wang, Z.; Tan, H. Current technology development for CO2 utilization into solar fuels and chemicals: A review. J. Energy Chem. 2020, 9, 96–123.
- Gong, E.; Ali, S.; Hiragond, C.B.; Kim, H.S.; Powar, N.S.; Kim, D.; Kim, H.; In, S.-I. Solar fuels: Research and development strategies to accelerate photocatalytic CO2 conversion into hydrocarbon fuels. Energy Environ. Sci. 2022, 15, 880–893.
- Patial, S.; Kumar, R.; Raizada, P.; Singh, P.; Le, Q.V.; Lichtfouse, E.; Nguyen, D.L.T.; Nguyen, V.-H. Boosting light-driven CO2 reduction into solar fuels: Mainstream avenues for engineering ZnO-based photocatalysts. Environ. Res. 2021, 197, 111134.
- Omr, H.A.E.; Horn, M.W.; Lee, H. Low-Dimensional Nanostructured Photocatalysts for Efficient CO2 Conversion into Solar Fuels. Catalysts 2021, 11, 418.
- Shen, Y.; Han, Q.; Hu, J.; Gao, W.; Wang, L.; Yang, L.; Gao, C.; Shen, Q.; Wu, C.; Wang, X.; et al. Artificial Trees for Artificial Photosynthesis: Construction of Dendrite-Structured α-Fe2O3/g-C3N4 Z-Scheme System for Efficient CO2 Reduction into Solar Fuels. ACS Appl. Energy Mater. 2020, 3, 6561–6572.
- Burke, R.; Bren, K.L.; Krauss, T.D. Semiconductor nanocrystal photocatalysis for the production of solar fuels. J. Chem. Phys. 2021, 154, 030901.
- Xia, Y.; Zhang, L.; Hu, B.; Yu, J.; Al-Ghamdi, A.A.; Wageh, S. Design of highly-active photocatalytic materials for solar fuel production. Chem. Eng. J. 2021, 421, 127732.
- Ma, Z.; Wang, Y.; Lu, Y.; Ning, H.; Zhang, J. Tackling Challenges in Perovskite-Type Metal Oxide Photocatalysts. Energy Technol. 2021, 9, 2001019.
- Inoue, T.; Fujishima, A.; Konishi, S.; Honda, K. Photoelectrocatalytic reduction of carbon dioxide in aqueous suspensions of semiconductor powders. Nature 1979, 277, 637–638.
- Kamal, K.M.; Narayan, R.; Chandran, N.; Popović, S.; Nazrulla, M.A.; Kovač, J.; Vrtovec, N.; Bele, M.; Hodnik, N.; Likozar, M.M.K.B. Synergistic enhancement of photocatalytic CO2 reduction by plasmonic Au nanoparticles on TiO2 decorated N-graphene heterostructure catalyst for high selectivity methane production. Appl. Catal. B Environ. 2022, 307, 121181.
- Tahir, B.; Tahir, M.; Ghazali, M.; Nawawi, M. Highly stable 3D/2D WO3/g-C3N4 Z-scheme heterojunction for stimulating photocatalytic CO2 reduction by H2O/H2 to CO and CH4 under visible light. J. CO2 Util. 2020, 41, 101270.
- Deng, H.; Xu, F.; Cheng, B.; Yu, J.; Ho, W. Photocatalytic CO2 reduction of C/ZnO nanofibers enhanced by an Ni-NiS cocatalyst. Nanoscale 2020, 12, 7206–7213.
- Wang, S.; Wang, X. Photocatalytic CO2 reduction by CdS promoted with a zeolitic imidazolate framework. Appl. Catal. B Environ. 2015, 162, 494–500.
- Xiao, S.; Guan, Y.; Shang, H.; Li, H.; Tian, Z.; Liu, S.; Chen, W.; Yang, J. An S-scheme NH2-UiO-66/SiC photocatalyst via microwave synthesis with improved CO2 reduction activity. J. CO2 Util. 2022, 55, 101806.
- Galli, F.; Compagnoni, M.; Vitali, D.; Pirola, C.; Bianchi, C.L.; Villa, A.; Prati, L.; Rossetti, I. CO2 photoreduction at high pressure to both gas and liquid products over titanium dioxide, Appl. Catal. B Environ. 2017, 200, 386–391.
- Luévano-Hipólito, E.; Torres-Martínez, L.M. Ink-jet printing films of molybdates of alkaline earth metals with scheelite structure applied in the photocatalytic CO2 reduction. J. Photochem. Photobiol. A Chem. 2019, 368, 15–22.
- Aguirre-Astrain, A.; Luévano-Hipólito, E.; Torres-Martínez, L.M. Integration of 2D printing technologies for AV2O6 (A=Ca, Sr, Ba)-MO (M=Cu, Ni, Zn) photocatalyst manufacturing to solar fuels production using seawater. Int. J. Hydrog. Energy 2021, 46, 37294–37310.
- Gusain, R.; Kumar, P.; Sharma, O.P.; Jain, S.L.; Khatri, O.P. Reduced graphene oxide–CuO nanocomposites for photocatalytic conversion of CO2 into methanol under visible light irradiation. Appl. Catal. B Environ. 2016, 181, 352–362.
- Wang, S.; Cabrero-Antonino, M.; Navalón, S.; Cao, C.-c.; Tissot, A.; Dovgaliuk, I.; Marrot, J.; Martineau-Corcos, C.; Yu, L.; Wang, H.; et al. A Robust Titanium Isophthalate Metal-Organic Framework for Visible-Light Photocatalytic CO2 Methanation. Chem 2020, 6, 3409–3427.
- Mateo, D.; Morlanes, N.; Maity, P.; Shterk, G.; Mohammed, O.F.; Gascon, J. Efficient Visible-Light Driven Photothermal Conversion of CO2 to Methane by Nickel Nanoparticles. Adv. Funct. Mater. 2021, 31, 2008244.
- Wang, X.; Ng, D.; Du, H.; Hornung, C.H.; Polyzos, A.; Seeber, A.; Li, H.; Huo, Y.; Xie, Z. Copper decorated indium oxide rods for photocatalytic CO2 conversion under simulated sun light. J. CO2 Util. 2022, 58, 101909.
- Luévano-Hipólito, E.; Torres-Martínez, L.M. CO2 photoreduction with H2O to C1 and C2 products over perovskite films of alkaline niobates ANbO3 (A = Li, Na, K). Fuel 2022, 320, 123934.
- Teramura, K.; Okuoka, S.; Tsuneoka, H.; Shishido, T.; Tanaka, T. Photocatalytic reduction of CO2 using H2 as reductant over ATaO3 photocatalysts (A = Li, Na, K). Appl. Catal. B. 2010, 96, 565–568.
- Luo, C.; Zhao, J.; Li, Y.; Zhao, W.; Zeng, Y.; Wang, C. Photocatalytic CO2 reduction over SrTiO3: Correlation between surface structure and activity. Appl. Surf. Sci. 2018, 447, 627–635.
- Qin, J.; Lin, L.; Wang, X. A perovskite oxide LaCoO3 cocatalyst for efficient photocatalytic reduction of CO2 with visible light. ChemComm 2018, 54, 2272–2275.
- Raza, M.A.; Li, F.; Que, M.; Zhu, L.; Chen, X. Photocatalytic reduction of CO2 by halide perovskites: Recent advances and future perspectives. Mater. Adv. 2021, 2, 7187–7209.
- Manikandan, A.; Slimani, Y.; Dinesh, A.; Khan, A.; Thanrasu, K.; Baykal, A.; Jaganathan, S.K.; Dzudzevic-Cancari, H.; Asirid, A.M. Perovskite’s potential functionality in a composite structure. In Hybrid Perovskite Composite Materials: Design to Applications; Woodhead Publishing: Cambridge, UK, 2021; Volume 1, pp. 181–202.
- Brittman, S.; Adhyaksa, G.W.P.; Garnett, E.C. The expanding world of hybrid perovskites: Materials properties and emerging applications. MRS Commun. 2015, 5, 7–26.
- Gao, P.; Grätzel, M.; Nazeeruddin, M.K. Organohalide Lead Perovskites for Photovoltaic Applications. Energy Environ. Sci. 2014, 7, 2448–2463.
- Shahrokhi, S.; Gao, W.; Wang, Y.; Anandan, P.R.; Rahaman, M.Z.; Singh, S.; Wang, D.; Cazorla, C.; Yuan, G.; Liu, J.-M.; et al. Emergence of ferroelectricity in halide perovskites. Small Methods 2020, 4, 2000149.
- Burger, S.; Grover, S.; Butler, K.T.; Boström, H.L.; Grau-Crespo, R.; Kieslich, G. Tilt and shift polymorphism in molecular perovskites. Mater Horizon. 2021, 8, 2444–2450.
- Dong, Y.; Zhao, Y.; Zhang, S.; Dai, Y.; Liu, L.; Li, Y.; Chen, Q. Recent advances toward practical use of halide perovskite nanocrystals. J. Mater. Chem. A 2018, 6, 21729–21746.
- Sanders, S.; Stümmler, D.; Pfeiffer, P.; Ackermann, N.; Schimkat, F.; Simkus, G.; Heuken, M.; Baumann, P.K.; Vescan, A.; Kalisch, H. Morphology Control of Organic–Inorganic Bismuth-Based Perovskites for Solar Cell Application. Phys. Status. Solidi A 2018, 215, 1800409.
- Dai, Y.; Poidevin, C.; Ochoa-Hernández, C.; Auer, A.A.; Tüysüz, H. A Supported Bismuth Halide Perovskite Photocatalyst for Selective Aliphatic and Aromatic C–H Bond Activation. Angew. Chem. 2020, 59, 5788–5796.
- Jin, S. Can We Find the Perfect A-Cations for Halide Perovskites? ACS Energy Lett. 2021, 6, 3386–3389.
- Tao, J.; Liu, X.; Shen, J.; Han, S.; Guan, L.; Fu, G.; Kuang, D.-B.; Yang, S. F-Type Pseudo-Halide Anions for High-Efficiency and Stable Wide-Band-Gap Inverted Perovskite Solar Cells with Fill Factor Exceeding 84%. ACS Nano 2022, 16, 10798–10810.
- Sun, X.; Ji, L.Y.; Chen, W.W.; Guo, X.; Wang, H.H.; Lei, M.; Wang, Q.; Li, Y.F. Halide anion–fullerene π noncovalent interactions: N-doping and a halide anion migration mechanism in p–i–n perovskite solar cells. J. Mater. Chem. 2017, 5, 20720–20728.
- Wang, Y.; Zhang, X.; Wang, D.; Li, X.; Meng, J.; You, J.; Yin, Z.; Wu, J. Compositional Engineering of Mixed-Cation Lead Mixed-Halide Perovskites for High-Performance Photodetectors. ACS Appl. Mater. Interfaces 2019, 11, 28005–28012.
- Yu, Z.; Yang, K.; Yu, C.; Lu, K.; Huang, W.; Xu, L.; Zou, L.; Wang, S.; Chen, Z.; Hu, J.; et al. Steering Unit Cell Dipole and Internal Electric Field by Highly Dispersed Er atoms Embedded into NiO for Efficient CO2 Photoreduction. Adv. Funct. Mater. 2022, 32, 2111999.
- Liu, X.; Zhang, Z.; Lin, F.; Cheng, Y. Structural modulation and assembling of metal halide perovskites for solar cells and light-emitting diodes. InfoMat 2021, 3, 1218–1250.
- Guan, X.; Lei, Z.; Yu, X.; Lin, C.-H.; Huang, J.-K.; Huang, C.-Y.; Feng, L.; Ajayan Vinu, L.; Yi, J.; Wu, T. Low-Dimensional Metal-Halide Perovskites as High-Performance Materials for Memory Applications. Small 2022, 18, 2203311.
- Ling, X.; Yuan, J.; Ma, W. The Rise of Colloidal Lead Halide Perovskite Quantum Dot Solar Cells. Acc. Mater. Res. 2022, 8, 866–878.
- Levchuk, I.; Osvet, A.; Tang, X.; Brandl, M.; Perea, J.D.; Hoegl, F.; Matt, G.J.; Hock, R.; Batentschuk, M.; Brabec, C.J. Brightly luminescent and color-tunable formamidinium lead halide perovskite FAPbX (X = Cl, Br, I) colloidal nanocrystals. Nano Lett. 2017, 17, 2765–2770.
- Jiang, X.; Ding, Y.; Zheng, S.; Ye, Y.; Li, Z.; Xu, L.; Wang, J.; Li, Z.; Loh, X.J.; Ye, E.; et al. In-Situ Generated CsPbBr3 Nanocrystals on O-Defective WO3 for Photocatalytic CO2 Reduction. ChemSusChem 2021, 15, e202102295.
- Chen, Y.; Yin, J.; Wei, Q.; Wang, C.; Wang, X.; Ren, H.; Yu, S.F.; Bakr, O.M.; Mohammed, O.F.; Li, M. Multiple exciton generation in tin–lead halide perovskite nanocrystals for photocurrent quantum efficiency enhancement. Nat. Photon. 2022, 16, 485–490.
- Gao, W.; Ding, J.; Bai, Z.; Qi, Y.; Wang, Y.; Lv, Z. Multiple excitons dynamics of lead halide perovskite. Nanophotonics 2021, 10, 3945–3955.
- Lin, C.-C.; Liu, T.-R.; Lin, S.-R.; Boopathi, K.M.; Chiang, C.-H.; Tzeng, W.-Y.; Chien, W.-H.C.; Hsu, H.-S.; Luo, C.-W.; Tsai, H.-Y.; et al. Spin-Polarized Photocatalytic CO2 Reduction of Mn-Doped Perovskite Nanoplates. J. Am. Chem. Soc. 2022, 144, 15718–15726.
- Chen, T.; Chen, W.-L.; Foley, B.J.; Lee, S.-H. Origin of long lifetime of band-edge charge carriers in organic–inorganic lead iodide perovskites. Appl. Phys. Sci. 2017, 114, 7519–7524.
- Meyer, E.; Mutukwa, D.; Zingwe, N.; Taziwa, R. Lead-free halide double perovskites: A review of the structural, optical, and stability properties as well as their viability to replace lead halide perovskites. Metals 2018, 8, 667.
- Han, C.; Zhu, X.; San Martin, J.; Lin, Y.; Spears, S.; Yan, Y. Recent Progress in Engineering Metal Halide Perovskites for Efficient Visible-Light-Driven Photocatalysis. ChemSusChem 2020, 13, 4005–4025.
- Wang, J.; Wang, J.; Li, N.; Du, X.; Ma, J.; He, C.; Li, Z. Direct Z scheme 0D/2D heterojunction of CsPbBr3 quantum dots/Bi2WO6 nanosheets for efficient photocatalytic CO2 reduction. ACS Appl. Mater. Interfaces 2020, 12, 31477–31485.
- Jin, Z.; Zhang, Z.; Xiu, J.; Song, H.; Gatti, T.; He, Z. A critical review on bismuth and antimony halide based perovskites and their derivatives for photovoltaic applications: Recent advances and challenges. J. Mater. Chem. A 2020, 8, 16166–16188.
- Liao, J.-F.; Cai, Y.-T.; Li, J.-Y.; Jiang, Y.; Wang, X.-D.; Chen, H.-Y.; Kuang, D.-B. Plasmonic CsPbBr3–Au nanocomposite for excitation wavelength dependent photocatalytic CO2 reduction. J. Energy Chem. 2021, 53, 309–315.
- Fan, Q.; Biesold-McGee, G.V.; Ma, J.; Xu, Q.; Pan, S.; Peng, J.; Lin, Z. Lead-free halide perovskite nanocrystals: Crystal structures, synthesis, stabilities, and optical properties. Angew Chem. Int. Ed. 2020, 59, 1030–1046.
- Chen, Z.; Hu, Y.; Wang, J.; Shen, Q.; Zhang, Y.; Ding, C.; Bai, Y.; Jiang, G.; Li, Z.; Gaponik, N. Boosting photocatalytic CO2 reduction on CsPbBr3 perovskite nanocrystals by immobilizing metal complexes. Chem. Mater. 2020, 32, 1517–1525.
- Ou, M.; Tu, W.; Yin, S.; Xing, W.; Wu, S.; Wang, H.; Wan, S.; Zhong, Q.; Xu, R. Amino-assisted anchoring of CsPbBr3 perovskite quantum dots on porous g-C3N4 for enhanced photocatalytic CO2 reduction. Angew. Chem. 2018, 57, 13570–13574.
- Wang, X.; He, J.; Li, J.; Lu, G.; Dong, F.; Majima, T.; Zhu, M. Immobilizing perovskite CsPbBr3 nanocrystals on Black phosphorus nanosheets for boosting charge separation and photocatalytic CO2 reduction. Appl. Catal. B. 2020, 277, 119230.
- Connor, B.A.; Leppert, L.; Smith, M.D.; Neaton, J.B.; Karunadasa, H.I. Layered halide double perovskites: Dimensional reduction of Cs2AgBiBr6. J. Am. Chem. Soc. 2018, 140, 5235–5240.
- Brandt, R.E.; Stevanović, V.; Ginley, D.S.; Buonassisi, T. Identifying defect-tolerant semiconductors with high minority-carrier lifetimes: Beyond hybrid lead halide perovskites. MRS Commun. 2015, 5, 265–275.
- Huang, S.; Shan, H.; Xuan, W.; Xu, W.; Hu, D.; Zhu, L.; Huang, C.; Sui, W.; Xiao, C.; Zhao, Y.; et al. High-Performance Humidity Sensor Based on CsPdBr3 Nanocrystals for Noncontact Sensing of Hydromechanical Characteristics of Unsaturated Soil. Phys. Status Solidi–Rapid Res. Lett. 2022, 16, 2200017.
- Guo, J.; Hu, Q.; Lu, M.; Li, A.; Zhang, X.; Sheng, R.; Chen, P.; Zhang, Y.; Wu, J.; Fu, Y.; et al. Pb2+ doped CsCdBr3 perovskite nanorods for pure-blue light-emitting diodes. Chem. Eng. J. 2022, 427, 131010.
- Chaiyawat, K.; Laosiritaworn, Y.; Jaroenjittichai, A.P. First-principles study on structural stability and reaction with H2O and O2 of vacancy-ordered double perovskite halides: Cs2 (Ti, Zr, Hf) X6. Results Phys. 2021, 25, 104225.
- Gundiah, G.; Brennan, K.; Yan, Z.; Samulon, E.C.; Wu, G.; Bizarri, G.A.; Derenzo, S.E.; Bourret-Courchesne, E.D. Structure and scintillation properties of Ce3+-activated Cs2NaLaCl6, Cs3LaCl6, Cs2NaLaBr6, Cs3LaBr6, Cs2NaLaI6 and Cs3LaI6. J. Lumin. 2014, 149, 374–384.
- Krishnamoorthy, T.; Ding, H.; Yan, C.; Leong, W.L.; Baikie, T.; Zhang, Z.; Sherburne, M.; Li, S.; Asta, M.; Mathews, N.; et al. Lead-free germanium iodide perovskite materials for photovoltaic applications. J. Mater. Chem. A 2015, 3, 23829.
- Ahmad, K. Bismuth Halide Perovskites for Photovoltaic Applications. In Bismuth—Fundamentals and Optoelectronic Applications; IntechOpen: London, UK, 2020.
- Bhosale, S.S.; Kharade, A.K.; Jokar, E.; Fathi, A.; Chang, S.-m.; Diau, E.W.-G. Mechanism of Photocatalytic CO2 Reduction by Bismuth-Based Perovskite Nanocrystals at the Gas−Solid Interface. J. Am. Chem. Soc. 2019, 141, 20434–20442. Song, W.; Zhang, X.; Lammar, S.; Qiu, W.; Kuang, Y.; Ruttens, B.; D’Haen, J.; Vaesen, I.; Conard, T.; Abdulraheem, Y.; et al. Critical Role of Perovskite Film Stoichiometry in Determining Solar Cell Operational Stability: A Study on the Effects of Volatile A-Cation Additives. ACS Appl. Mater. Interfaces 2022, 14, 27922–27931.
- Yu, B.-B.; Liao, M.; Yang, J.; Chen, W.; Zhu, Y.; Zhang, X.; Duan, T.; Yao, W.; Wei, S.-H.; He, Z. Alloy-induced phase transition and enhanced photovoltaic performance: The case of Cs3Bi2I9−xBrx perovskite solar cells. J. Mater. Sci. 2019, 7, 8818–8825. Fabian, D.M.; Ganose, A.M.; Ziller, J.W.; Scanlon, D.O.; Beard, M.C.; Ardo, S. Influence of One Specific Carbon–Carbon Bond on the Quality, Stability, and Photovoltaic Performance of Hybrid Organic–Inorganic Bismuth Iodide Materials. ACS Appl. Energy Mater. 2019, 2, 1579–1587.
- Cui, Z.; Wu, Y.; Zhang, S.; Fu, H.; Chen, G.; Lou, Z.; Liu, X.; Zhang, Q.; Wang, Z.; Zheng, Z.; et al. Insight into a strategy to improve charge carrier migration in lead-free bismuth-based halide perovskite for efficient selective oxidation of thioanisole under visible light. Chem. Eng. J. 2023, 451, 138927. Trifiletti, V.; Luong, S.; Tseberlidis, G.; Riva, S.; Galindez, E.S.S.; Gillin, W.P.; Binetti, S.; Fenwick, O. Two-Step Synthesis of Bismuth-Based Hybrid Halide Perovskite Thin-Films. Materials 2021, 14, 7827.
- Estrada-Pomares, J.; Ramos-Terrón, S.; Lasarte-Aragonés, G.; Lucena, R.; Cárdenas, S.; Rodríguez-Padrón, D.; Luque, R.; de Miguel, G. Mechanochemically designed bismuth-based halide perovskites for efficient photocatalytic oxidation of vanillyl alcohol. J. Mater. Chem. A 2022, 10, 11298–11305. Leng, M.; Yang, Y.; Chen, Z.; Gao, W.; Zhang, J.; Niu, G.; Li, D.; Song, H.; Zhang, J.; Jin, S.; et al. Surface Passivation of Bismuth-Based Perovskite Variant Quantum Dots to Achieve Efficient Blue Emission. Nano Lett. 2018, 18, 6076–6083.
- Bresolin, B.-M.; Günnemann, C.; Bahnemann, D.W.; Sillanpää, M. Pb-Free Cs3Bi2I9 Perovskite as a Visible-Light-Active Photocatalyst for Organic Pollutant Degradation. Nanomaterials 2020, 10, 763. Mali, S.S.; Kim, H.; Kim, D.-H.; Hong, C.K. Anti-Solvent Assisted Crystallization Processed Methylammonium Bismuth Iodide Cuboids towards Highly Stable Lead-Free Perovskite Solar Cells. Energy Environ. Sci. 2017, 2, 1578–1585.
- Miodyńska, M.; Mikolajczyk, A.; Mazierski, P.; Klimczuk, T.; Lisowski, W.; Trykowski, G.; Zaleska-Medynska, A. Lead-free bismuth-based perovskites coupled with g–C3N4: A machine learning based novel approach for visible light induced degradation of pollutants. Appl. Surf. Sci. 2022, 588, 152921. Liu, Z.; Chen, M.; Wan, L.; Liu, Y.; Wang, Y.; Gan, Y.; Guo, Z.; Eder, D.; Wang, S. Anti-solvent spin-coating for improving morphology of lead-free (CH3NH3)3Bi2I9 perovskite films. SN Appl. Sci. 2019, 1, 706.
- Feng, Y.-X.; Dong, G.-X.; Su, K.; Liu, Z.-L.; Zhang, W.; Zhang, M.; Lu, T.-B. Self-template-oriented synthesis of lead-free perovskite Cs3Bi2I9 nanosheets for boosting photocatalysis of CO2 reduction over Z-scheme heterojunction Cs3Bi2I9/CeO2. J. Energy Chem. 2022, 69, 348–355. Konstantakou, M.; Perganti, D.; Falaras, P.; Stergiopoulos, T. Anti-Solvent Crystallization Strategies for Highly Efficient Perovskite Solar Cells. Crystals 2017, 7, 291.
- Sun, Q.-M.; Xu, J.-J.; Tao, F.-F.; Ye, W.; Zhou, C.; He, J.-H.; Lu, J.-M. Boosted Inner Surface Charge Transfer in Perovskite Titania Frameworks for Efficient and Selective Photocatalytic CO2 Reduction to Methane. Angew. Chem. 2022, 134, e202200872. Corsini, F.; Griffini, G. Recent progress in encapsulation strategies to enhance the stability of organometal halide perovskite solar cells. JPhys Energy 2020, 2, 031002.
- Song, W.; Zhang, X.; Lammar, S.; Qiu, W.; Kuang, Y.; Ruttens, B.; D’Haen, J.; Vaesen, I.; Conard, T.; Abdulraheem, Y.; et al. Critical Role of Perovskite Film Stoichiometry in Determining Solar Cell Operational Stability: A Study on the Effects of Volatile A-Cation Additives. ACS Appl. Mater. Interfaces 2022, 14, 27922–27931. Li, Q.; Song, T.; Zhang, Y.; Wang, Q.; Yang, Y. Boosting Photocatalytic Activity and Stability of Lead-Free Cs3Bi2Br9 Perovskite Nanocrystals via In Situ Growth on Monolayer 2D Ti3C2Tx MXene for C–H Bond Oxidation. ACS Appl. Mater. Interfaces 2021, 13, 27323–27333.
- Fabian, D.M.; Ganose, A.M.; Ziller, J.W.; Scanlon, D.O.; Beard, M.C.; Ardo, S. Influence of One Specific Carbon–Carbon Bond on the Quality, Stability, and Photovoltaic Performance of Hybrid Organic–Inorganic Bismuth Iodide Materials. ACS Appl. Energy Mater. 2019, 2, 1579–1587. Sun, Q.; Ye, W.; Wei, J.; Li, L.; Wang, J.; He, J.-H.; Lu, J.-M. Lead-free perovskite Cs3Bi2Br9 heterojunctions for highly efficient and selective photocatalysis under mild conditions. J. Alloys Compd. 2022, 893, 162326.
- Trifiletti, V.; Luong, S.; Tseberlidis, G.; Riva, S.; Galindez, E.S.S.; Gillin, W.P.; Binetti, S.; Fenwick, O. Two-Step Synthesis of Bismuth-Based Hybrid Halide Perovskite Thin-Films. Materials 2021, 14, 7827. Bresolin, B.-M.; Sgarbossa, P.; Bahnemann, D.W.; Sillanpää, M. Cs3Bi2I9/g-C3N4 as a new binary photocatalyst for efficient visible-light photocatalytic processes. Sep. Purif. Technol. 2020, 251, 117320.
- Leng, M.; Yang, Y.; Chen, Z.; Gao, W.; Zhang, J.; Niu, G.; Li, D.; Song, H.; Zhang, J.; Jin, S.; et al. Surface Passivation of Bismuth-Based Perovskite Variant Quantum Dots to Achieve Efficient Blue Emission. Nano Lett. 2018, 18, 6076–6083. Guo, Y.; Liu, G.; Li, Z.; Lou, Y.; Chen, J.; Zhao, Y. Stable Lead-Free (CH3NH3)3Bi2I9 Perovskite for Photocatalytic Hydrogen Generation. ACS Sustain. Chem. Eng. 2019, 7, 15080–15085.
- Mali, S.S.; Kim, H.; Kim, D.-H.; Hong, C.K. Anti-Solvent Assisted Crystallization Processed Methylammonium Bismuth Iodide Cuboids towards Highly Stable Lead-Free Perovskite Solar Cells. Energy Environ. Sci. 2017, 2, 1578–1585. Wu, D.; Zhao, X.; Huang, Y.; Lai, J.; Yang, J.; Tian, C.; Hea, P.; Huang, Q.; Tang, X. Synthesis and CO2 Photoreduction of Lead-Free Cesium Bismuth Halide Perovskite Nanocrystals. J. Phys. Chem. C 2021, 25, 18328–18333.
- Liu, Z.; Chen, M.; Wan, L.; Liu, Y.; Wang, Y.; Gan, Y.; Guo, Z.; Eder, D.; Wang, S. Anti-solvent spin-coating for improving morphology of lead-free (CH3NH3)3Bi2I9 perovskite films. SN Appl. Sci. 2019, 1, 706. Zhou, L.; Xu, Y.-F.; Chen, B.-X.; Kuang, D.-B.; Su, C.-Y. Synthesis and Photocatalytic Application of Stable Lead-Free Cs2AgBiBr6 Perovskite Nanocrystals. Small 2018, 14, 1703762.
- Konstantakou, M.; Perganti, D.; Falaras, P.; Stergiopoulos, T. Anti-Solvent Crystallization Strategies for Highly Efficient Perovskite Solar Cells. Crystals 2017, 7, 291. Liu, Z.-L.; Liu, R.-R.; Mu, Y.-F.; Feng, Y.-X.; Dong, G.-X.; Zhang, M.; Lu, T.-B. In Situ Construction of Lead-Free Perovskite Direct Z-Scheme Heterojunction Cs3Bi2I9/Bi2WO6 for Efficient Photocatalysis of CO2 Reduction. Sol. RRL 2021, 5, 2000691.
- Corsini, F.; Griffini, G. Recent progress in encapsulation strategies to enhance the stability of organometal halide perovskite solar cells. JPhys Energy 2020, 2, 031002. Otero-Martínez, C.; Fiuza-Maneiro, N.; Polavarapu, L. Enhancing the Intrinsic and Extrinsic Stability of Halide Perovskite Nanocrystals for Efficient and Durable Optoelectronics. ACS Appl. Mater. Interfaces 2022, 14, 34291–34302.
- Li, Q.; Song, T.; Zhang, Y.; Wang, Q.; Yang, Y. Boosting Photocatalytic Activity and Stability of Lead-Free Cs3Bi2Br9 Perovskite Nanocrystals via In Situ Growth on Monolayer 2D Ti3C2Tx MXene for C–H Bond Oxidation. ACS Appl. Mater. Interfaces 2021, 13, 27323–27333.
- Sun, Q.; Ye, W.; Wei, J.; Li, L.; Wang, J.; He, J.-H.; Lu, J.-M. Lead-free perovskite Cs3Bi2Br9 heterojunctions for highly efficient and selective photocatalysis under mild conditions. J. Alloys Compd. 2022, 893, 162326.
- Bresolin, B.-M.; Sgarbossa, P.; Bahnemann, D.W.; Sillanpää, M. Cs3Bi2I9/g-C3N4 as a new binary photocatalyst for efficient visible-light photocatalytic processes. Sep. Purif. Technol. 2020, 251, 117320.
- Guo, Y.; Liu, G.; Li, Z.; Lou, Y.; Chen, J.; Zhao, Y. Stable Lead-Free (CH3NH3)3Bi2I9 Perovskite for Photocatalytic Hydrogen Generation. ACS Sustain. Chem. Eng. 2019, 7, 15080–15085.
- Wu, D.; Zhao, X.; Huang, Y.; Lai, J.; Yang, J.; Tian, C.; Hea, P.; Huang, Q.; Tang, X. Synthesis and CO2 Photoreduction of Lead-Free Cesium Bismuth Halide Perovskite Nanocrystals. J. Phys. Chem. C 2021, 25, 18328–18333.
- Zhou, L.; Xu, Y.-F.; Chen, B.-X.; Kuang, D.-B.; Su, C.-Y. Synthesis and Photocatalytic Application of Stable Lead-Free Cs2AgBiBr6 Perovskite Nanocrystals. Small 2018, 14, 1703762.
- Liu, Z.-L.; Liu, R.-R.; Mu, Y.-F.; Feng, Y.-X.; Dong, G.-X.; Zhang, M.; Lu, T.-B. In Situ Construction of Lead-Free Perovskite Direct Z-Scheme Heterojunction Cs3Bi2I9/Bi2WO6 for Efficient Photocatalysis of CO2 Reduction. Sol. RRL 2021, 5, 2000691.
- Otero-Martínez, C.; Fiuza-Maneiro, N.; Polavarapu, L. Enhancing the Intrinsic and Extrinsic Stability of Halide Perovskite Nanocrystals for Efficient and Durable Optoelectronics. ACS Appl. Mater. Interfaces 2022, 14, 34291–34302.
