Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Catherine Yang and Version 1 by Bingzhe Xu.
Brain tumors can be classified into two major classes: primary brain tumors that start in the brain and secondary brain tumors that are generated by the cancer cells that migrated from other parts of the body. To reduce the damage of nanomedicines to normal brain function and immune system, the targeting of brain nanodrug delivery systems to brain tumor regions should be enhanced. Currently, brain tumor targeting mainly relies on active targeting strategies, which enhance the accumulation of drugs at tumor sites through passive targeting strategies. Several emerging strategies have recently emerged, such as magnetically targeted nano-drug delivery systems, tumor microenvironment-triggered drug delivery systems, etc.
- glioma
- brain tumor
- blood–brain barrier
- nano-drug delivery systems
1. Passive Targeting
Due to the highly leaky vasculature environment around the tumor, passive targeting systems have been applied to the design of brain cancer-targeting nanomedicines [18][1]. Passive targeting of brain tumors mainly relies on enhanced permeability and retention (EPR) effects. In the pathological state of brain tumors, the BBB may be disturbed to some extent by edema, swelling, and increased pressure in the brain [6][2]. Nanoparticles of a certain size or smaller can enter the tumor through the gap between endothelial cells, which is the EPR effect caused by the collapse of blood vessels caused by the formation of solid tumors [5][3], resulting in a higher accumulation of nanoparticles in brain tumors. The success of the ERP effect prompted researchers to develop nanoparticles with different physicochemical properties, including size, surface charge, surface hydrophilicity, and geometry, in order to enhance the aggregation of drugs [44][4]. Relevant studies have shown that the nanoparticle size range for ideal EPR effect is 10–200 nm [5,18][1][3]. When larger than 200 nm, the particles cannot fully penetrate the tumor vasculature and interstitial space, while when smaller than 10 nm, the kidneys clear them, resulting in the inability of particles to accumulate at the tumor site [5,101][3][5]. In addition, the lipophilicity and stability of nanoparticles is also an important factor affecting the ERP effect. Liposomes and polymer nanoparticles are often used to encapsulate drugs to enhance the ERP effect. PEGylation modification is widely used to modify the drug itself or drug carrier to increase the systemic circulation time of nanoparticles, so as to enhance the EPR effect [102][6]. The biomimetic nano drug loading system also has an enhanced EPR effect because of its long body circulation. While the EPR effect may be in effect for administered nanoparticles, the majority (>95%) of administered nanoparticles are known to accumulate in other organs, in particular the liver, spleen, and lungs [102][6]. Nano drug delivery systems do not rely on passive targeting strategy alone but are combined with active targeting strategy.
2. Active Targeting
Active targeting usually refers to targeting by ligand-receptor-specific interactions between a drug or drug carrier and the target cell [102][6]. Compared with passive targeting, active targeting is selective for brain tumors and can effectively deliver therapeutic drugs or diagnostic reagents to the lesion site and reduce cytotoxicity [18][1]. Some receptors are closely related to tumor growth. They are highly expressed in blood vessels near brain tumors or in brain tumor cells, but not expressed or expressed low in other tissues. Therefore, by modifying the corresponding ligands on the surface of drugs or drug carriers, the receptors can guide them to aggregate at brain tumors or enter tumor cells to exert therapeutic effects. In the introduction of brain targeting strategy, this paper also mentioned the receptor-mediated targeting strategy, but these receptors are expressed in the BBB and brain tumors, which increases the brain targeting of drugs; however, they lack the targeting of brain tumors in the brain [103][7], which may lead to severe neurotoxicity.
In order to solve this problem, receptor-mediated endocytosis with high expression in tumor sites and low expression in the BBB are selected to enter brain tumors, such as NRP-1 receptor [14[8][9],22], integrin receptor [59][10], interleukin receptor, and so on [104][11]. At present, the development of dual targeted drug delivery system provides a more safe and effective method for brain tumor drug delivery. Dual-targeted drug delivery systems are two ligands that simultaneously modify the surface of the drug delivery system: one modifies the BBB and the other modifies the brain tumor [103][7] (Figure 41a). Therefore, the dual-targeted drug delivery system can improve drug efficacy and reduce toxicity and side effects to the body. TGN peptide (TGNYKALHPHNG) is selected from the 12-peptide library through phage display in vivo, which has a good brain targeting effect, while RGD peptide has been proved by many studies to have good tissue cell penetration and tumor targeting [22,24,105,106,107][9][12][13][14][15]. Shi et al. developed a glioma drug delivery system with iRGD/TGN double modified poly (amidoamine) dendrimer (PAMAM) encapsulated ATO. The experimental results show that iRGD/TGN-PEG-PAMAM-ATO has enhanced BBB penetration and GBM targeting, which effectively improves the efficacy of ATO, prolongs the medium survival time of mice, and reduces the systemic toxicity. This suggests that the dual targeting system is a promising technology in the treatment of glioma.
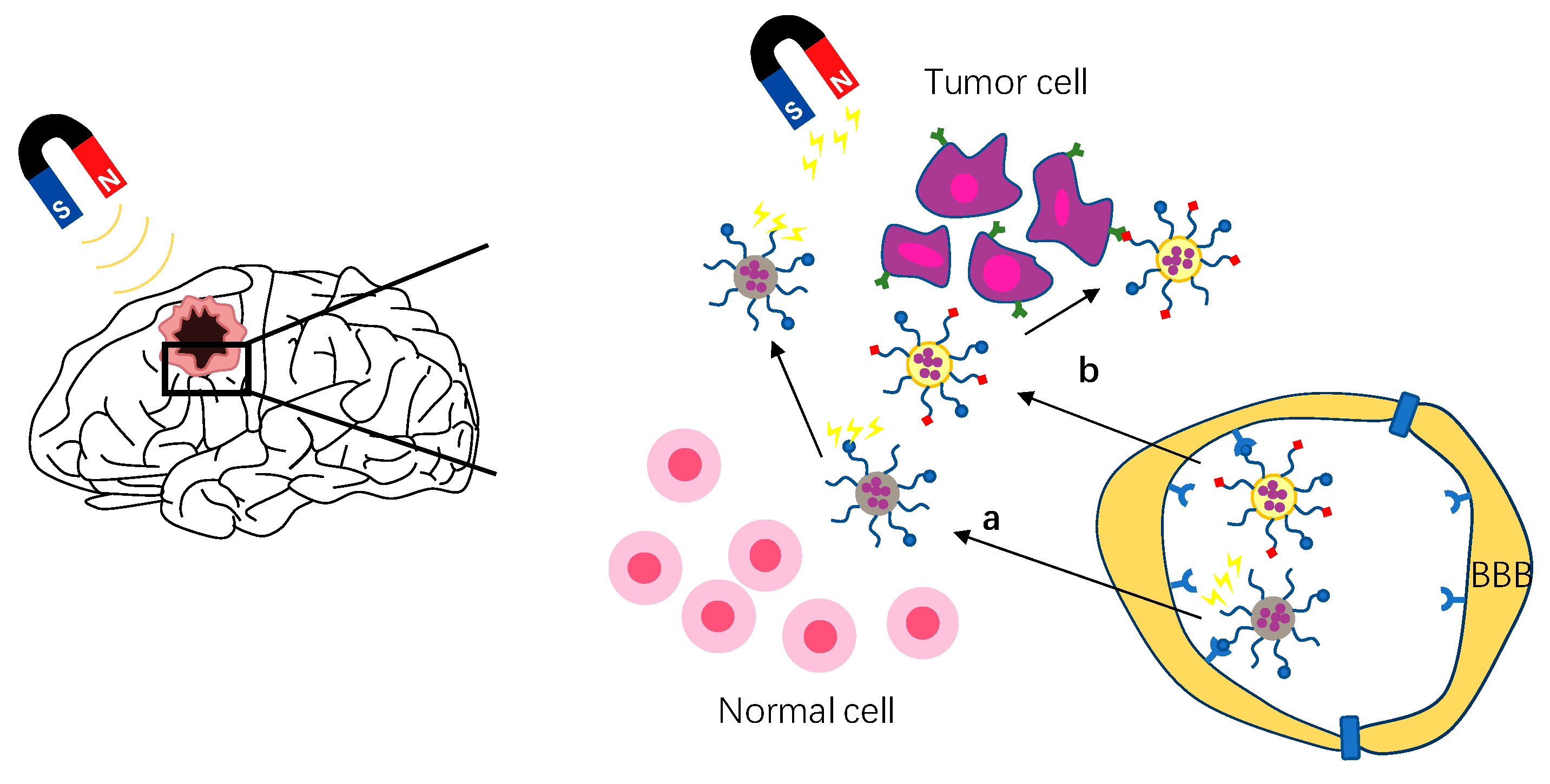
Figure 41. Schematic of strategies to enhance brain tumor targeting. (a) Schematic of magnetic targeting brain tumor strategy. (b) Schematic of active targeting strategy, shown here as double targeting strategy.
3. Magnetic Targeting
Magnetic targeted drug delivery is a method of using external magnetic field to manipulate magnetic drug carriers in vivo to reach tumor targets [108][16]. As magnetic targeting depends on the magnetic force between the external magnetic field and nanoparticles, the nano carrier used must be magnetic–that is, magnetic nanoparticles. Magnetic nanoparticles such as iron oxide nanoparticles (IONPs), superparamagnetic iron oxide nanoparticles (SPIONs), and fluorescent magnetic nanoparticles (MNPs) are widely used as diagnostic imaging agents and therapeutic carriers [109][17]. By coupling magnetic nanoparticles with specific ligands and guiding them by a magnetic targeting system, drugs can be targeted to brain tumor therapy. The magnetic targeting strategy is to expose MNPs to the external magnetic field by applying an external magnetic field to the head tumor after intravenous administration, which increases the movement of MNPs in the systemic circulation to the brain tumor [109,110][17][18] (Figure 41b). In recent years, many nano drug delivery systems using magnetic targeting strategy have been developed to treat brain tumors. Cui et al. developed a dual-targeting strategy by a combination of magnetic guidance and transferrin receptor-binding peptide T7-mediated active targeting delivery [111][19]. Compared with non-targeted NPs, this strategy increased cell uptake by more than 10 times and brain transmission by more than five times. The experimental results also showed that under the action of a magnetic field, the system improved the drug delivery efficiency, reduced adverse reactions, and improved the survival rate of mice with glioma in situ. Although the external magnetic field can gather the drug carrier near the brain tumor, it is difficult to accurately identify the brain tumor only by magnetic field guidance due to the lack of selectivity. Therefore, Lu et al. developed a dual thermal sensitive magnetic liposome (TML) with a thermal response and magnetic response to recognize the overexpressed epidermal growth factor receptor on the surface of cancer cells by conjugating with cetuximab (CET) [100][20]. Many studies have shown that the magnetic targeting strategy has the advantages of improving the curative effect, reducing drug dosage, and reducing side effects [100,110,111,112][18][19][20][21].
References
- Kang, Y.J.; Cutler, E.G.; Cho, H. Therapeutic nanoplatforms and delivery strategies for neurological disorders. Nano Converg. 2018, 5, 35.
- Sun, C.; Ding, Y.; Zhou, L.; Shi, D.; Sun, L.; Webster, T.J.; Shen, Y. Noninvasive nanoparticle strategies for brain tumor targeting. Nanomedicine 2017, 13, 2605–2621.
- Hwang, H.H.; Lee, D.Y. Protein-Based Drug Delivery in Brain Tumor Therapy. Adv. Exp. Med. Biol. 2020, 1249, 203–221.
- Gao, W.; Zhang, L. Coating nanoparticles with cell membranes for targeted drug delivery. J. Drug Target. 2015, 23, 619–626.
- Kang, H.; Rho, S.; Stiles, W.R.; Hu, S.; Baek, Y.; Hwang, D.W.; Kashiwagi, S.; Kim, M.S.; Choi, H.S. Size-Dependent EPR Effect of Polymeric Nanoparticles on Tumor Targeting. Adv. Healthc. Mater. 2019, 9, e1901223.
- Bae, Y.H.; Park, K. Targeted drug delivery to tumors: Myths, reality and possibility. J. Control Release 2011, 153, 198–205.
- Gao, H. Perspectives on Dual Targeting Delivery Systems for Brain Tumors. J. Neuroimmune Pharmacol. 2017, 12, 6–16.
- Ag Seleci, D.; Seleci, M.; Stahl, F.; Scheper, T. Tumor homing and penetrating peptide-conjugated niosomes as multi-drug carriers for tumor-targeted drug delivery. RSC Adv. 2017, 7, 33378–33384.
- Kuang, J.; Song, W.; Yin, J.; Zeng, X.; Han, S.; Zhao, Y.-P.; Tao, J.; Liu, C.-J.; He, X.-H.; Zhang, X.-Z. iRGD Modified Chemo-immunotherapeutic Nanoparticles for Enhanced Immunotherapy against Glioblastoma. Adv. Funct. Mater. 2018, 28, 1800025.
- Zhu, Q.; Ling, X.; Yang, Y.; Zhang, J.; Li, Q.; Niu, X.; Hu, G.; Chen, B.; Li, H.; Wang, Y.; et al. Embryonic Stem Cells-Derived Exosomes Endowed with Targeting Properties as Chemotherapeutics Delivery Vehicles for Glioblastoma Therapy. Adv. Sci. 2019, 6, 1801899.
- Wang, B.; Lv, L.; Wang, Z.; Zhao, Y.; Wu, L.; Fang, X.; Xu, Q.; Xin, H. Nanoparticles functionalized with Pep-1 as potential glioma targeting delivery system via interleukin 13 receptor alpha2-mediated endocytosis. Biomaterials 2014, 35, 5897–5907.
- Meenu Vasudevan, S.; Ashwanikumar, N.; Vinod Kumar, G.S. Peptide decorated glycolipid nanomicelles for drug delivery across the blood-brain barrier (BBB). Biomater. Sci. 2019, 7, 4017–4021.
- Zhong, Y.; Su, T.; Shi, Q.; Feng, Y.; Tao, Z.; Huang, Q.; Li, L.; Hu, L.; Li, S.; Tan, H.; et al. Co-Administration Of iRGD Enhances Tumor-Targeted Delivery And Anti-Tumor Effects Of Paclitaxel-Loaded PLGA Nanoparticles For Colorectal Cancer Treatment. Int. J. Nanomed. 2019, 14, 8543–8560.
- Yang, J.; Zhang, Q.; Liu, Y.; Zhang, X.; Shan, W.; Ye, S.; Zhou, X.; Ge, Y.; Wang, X.; Ren, L. Nanoparticle-based co-delivery of siRNA and paclitaxel for dual-targeting of glioblastoma. Nanomedicine 2020, 15, 1391–1409.
- Shi, X.; Ma, R.; Lu, Y.; Cheng, Y.; Fan, X.; Zou, J.; Zheng, H.; Li, F.; Piao, J.G. iRGD and TGN co-modified PAMAM for multi-targeted delivery of ATO to gliomas. Biochem. Biophys. Res. Commun. 2020, 527, 117–123.
- Liu, Y.L.; Chen, D.; Shang, P.; Yin, D.C. A review of magnet systems for targeted drug delivery. J. Control Release 2019, 302, 90–104.
- Gandhi, H.; Sharma, A.K.; Mahant, S.; Kapoor, D.N. Recent advancements in brain tumor targeting using magnetic nanoparticles. Ther. Deliv. 2020, 11, 97–112.
- Lee, K.; David, A.E.; Zhang, J.; Shin, M.C.; Yang, V.C. Enhanced accumulation of theranostic nanoparticles in brain tumor by external magnetic field mediated in situ clustering of magnetic nanoparticles. J. Ind. Eng. Chem. 2017, 54, 389–397.
- Cui, Y.; Zhang, M.; Zeng, F.; Jin, H.; Xu, Q.; Huang, Y. Dual-Targeting Magnetic PLGA Nanoparticles for Codelivery of Paclitaxel and Curcumin for Brain Tumor Therapy. ACS Appl. Mater. Interfaces 2016, 8, 32159–32169.
- Lu, Y.-J.; Chuang, E.-Y.; Cheng, Y.-H.; Anilkumar, T.S.; Chen, H.-A.; Chen, J.-P. Thermosensitive magnetic liposomes for alternating magnetic field-inducible drug delivery in dual targeted brain tumor chemotherapy. J. Chem. Eng. 2019, 373, 720–733.
- Lu, Y.J.; Lin, P.Y.; Huang, P.H.; Kuo, C.Y.; Shalumon, K.T.; Chen, M.Y.; Chen, J.P. Magnetic Graphene Oxide for Dual Targeted Delivery of Doxorubicin and Photothermal Therapy. Nanomaterials 2018, 8, 193.
More
