Cobras (genus Naja) are widely distributed over Asia and Africa, and cobra envenomation is responsible for a large number of mortality and morbidity on these continents. Like other elapid venoms, cobra venoms are neurotoxic in nature; however, they also exhibit local cytotoxic effects at the envenomed site, and the extent of cytotoxicity may vary from species to species. Cobra venoms are predominated by the non-enzymatic three-finger toxin family which constitutes about 60-75% of the total venom. Cytotoxins (CTXs), an essential class of the non-enzymatic three-finger toxin family, are ubiquitously present in cobra venoms. These low-molecular-mass toxins, contributing to about 40 to 60% of the cobra venom proteome, play a significant role in cobra venom-induced toxicity, more prominently in dermonecrosis (local effects).
- membrane perturbations
- necrosis
- cobra venom
- antivenom neutralization
1. Epidemiology of Cobra Bites in the World
| Snake Species | Common Name | Medical Importance/ Category |
Region of Distribution | Number of Countries | Human Population (2020) in This Species’ Range |
|---|---|---|---|---|---|
| N. anchietae | Anchieta’s cobra | Highest, Secondary | Africa | 6 | 19,008,230 |
| N. annulata | Banded water cobra | Highest, Secondary | Africa | 10 | 114,642,902 |
| N. annulifera | Snouted cobra | Highest | Africa | 8 | 70,731,878 |
| N. ashei | Ashe’s spitting cobra | Highest | Africa | 6 | 32,513,269 |
| N. atra | Chinese cobra | Highest | Asia and Australasia | 5 | 570,266,425 |
| N. christyi | Christy’s water cobra | Secondary | Africa | 3 | 15,111,896 |
| N. guineensis | Black forest cobra | Highest, Secondary | Africa | 7 | 55,106,930 |
| N. haje | Egyptian cobra | Highest, Secondary | Africa | 21 | 443,884,351 |
| N. kaouthia | Monocled cobra, Thai cobra | Highest, Secondary | Asia | 11 | 976,884,863 |
| N. katiensis | Mali cobra, West Africa brown spitting cobra | Highest, Secondary | Africa | 12 | 123,542,818 |
| N. mandalayensis | Mandalay spitting cobra | Highest | Asia and Australasia | 1 | 14,774,047 |
| N. melanoleuca | Black and white cobra, Forest cobra | Highest, Secondary | Africa | 11 | 244,375,176 |
| N. mossambica | Mozambique spitting cobra | Highest, Secondary | Africa | 10 | 130,049,980 |
| N. naja | Indian cobra, Spectacled cobra | Highest, Secondary | Asia and Australasia | 5 | 1,656,817,409 |
| N. nigricincta | Western barred spitting cobra, Zebra cobra | Highest, Secondary | Africa | 4 | 11,381,021 |
| N. nigricollis | Black-necked spitting cobra | Highest, Secondary | Africa | 33 | 727,256,279 |
| N. nivea | Cape cobra | Highest | Africa | 4 | 17,651,152 |
| N. nubiae | Nubian spitting cobra | Secondary | Africa | 5 | 39,843,095 |
| N. oxiana | Central Asian cobra, Transcaspian cobra | Highest, Secondary | Asia and Australasia, Middle East | 8 | 242,127,307 |
| N. pallida | Red spitting cobra | Secondary | Africa | 6 | 59,847,176 |
| N. peroescobari | Sao Tome cobra | Highest | Africa | 1 | 189,185 |
| N. philippinesis | Northern Philippine cobra | Highest | Asia and Australasia | 1 | 592,982,107 |
| N. sagittifera | Andaman cobra | Secondary | Asia and Australasia | 1 | 373,959 |
| N. samarensis | Southern Philippine cobra, Visayan cobra | Highest | Asia and Australasia | 1 | 30,350,207 |
| N. savannula | West African banded cobra | Highest, Secondary | Africa | 16 | 151,894,138 |
| N. senegalensis | Senegalese cobra | Highest, Secondary | Africa | 13 | 84,781,768 |
| N. siamensis | Indochinese spitting cobra, Siamese spitting cobra | Highest, Secondary | Asia and Australasia | 5 | 119,240,121 |
| N. sputatrix | Southern Indonesian spitting cobra | Highest, Secondary | Asia and Australasia | 2 | 167,089,984 |
| N. subfulva | Brown forest cobra | Highest, Secondary | Africa | 22 | 377,545,129 |
| N. sumatrana | Equatorial spitting cobra | Highest, Secondary | Asia and Australasia | 6 | 124,654,470 |
2. Composition of Cobra Venom: A Summary
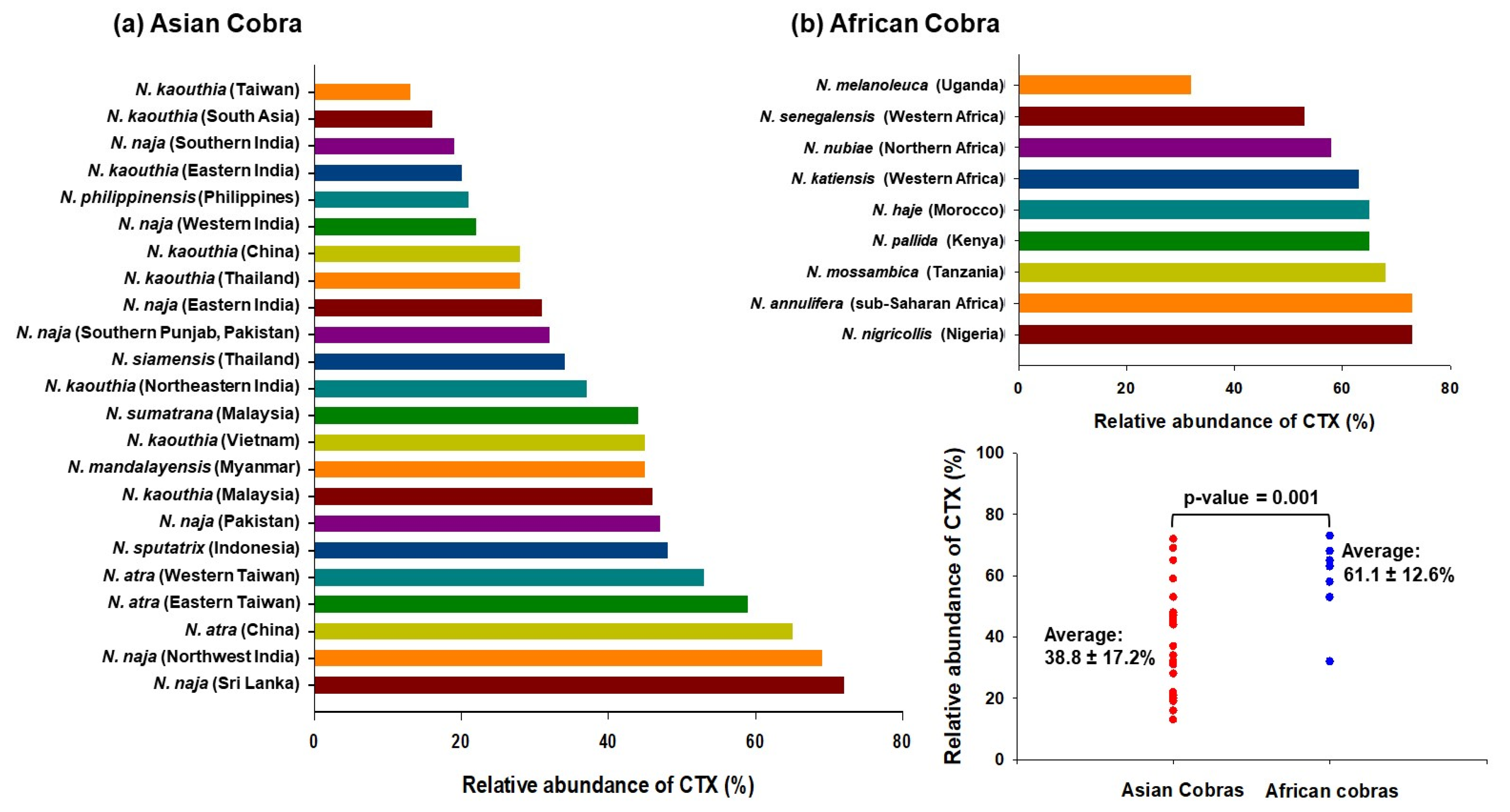
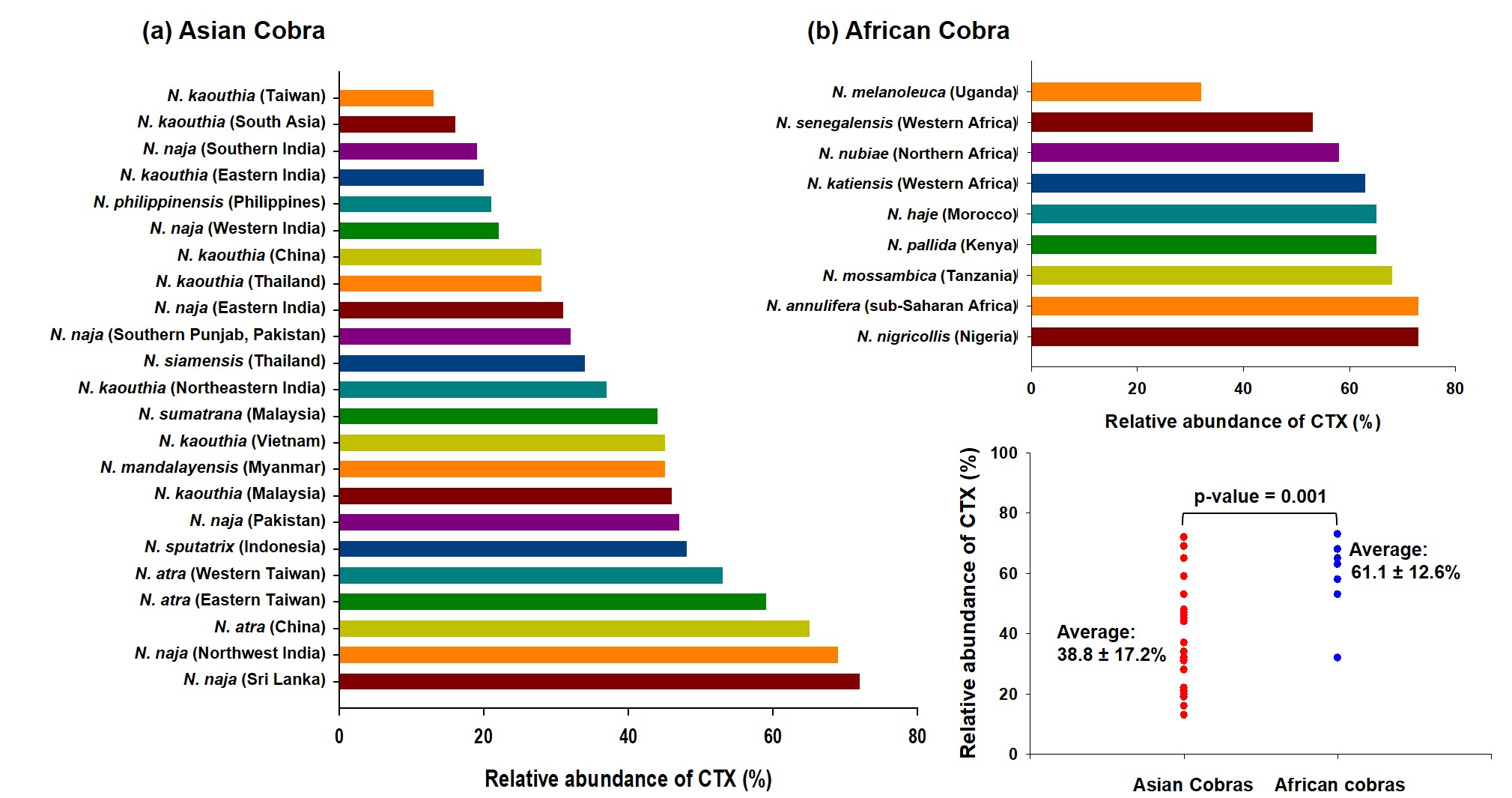
References
- Gutierrez, J.M.; Calvete, J.J.; Habib, A.G.; Harrison, R.A.; Williams, D.J.; Warrell, D.A. Snakebite envenoming. Nature Reviews. Disease Primers 2017, 3, 17079, doi:10.1038/nrdp.2017.79.
- Feola, A.; Marella, G.L.; Carfora, A.; Della Pietra, B.; Zangani, P.; Campobasso, C.P. Snakebite envenoming a challenging diagnosis for the forensic pathologist: A systematic review. Toxins 2020, 12, 699. doi:10.3390/toxins12110699.
- Kasturiratne, A.; Wickremasinghe, A.R.; de Silva, N.; Gunawardena, N.K.; Pathmeswaran, A.; Premaratna, R.; Savioli, L.; Lalloo, D.G.; de Silva, H.J. The global burden of snakebite: a literature analysis and modelling based on regional estimates of envenoming and deaths. PLoS Medicine 2008, 5, e218, doi:10.1371/journal.pmed.0050218.
- Tasoulis, T.; Isbister, G.K. A review and database of snake venom proteomes. Toxins 2017, 9, 290. doi:10.3390/toxins9090290.
- Organization, W.H. Snakebite envenoming: a strategy for prevention and control. 2019.
- Wallach, V.; Wuester, W.; Broadley, D.G. In praise of subgenera: taxonomic status of cobras of the genus Naja Laurenti (Serpentes: Elapidae). Zootaxa 2009, 2236, 26–36-26–36.
- Wuster, W. Taxonomic changes and toxinology: systematic revisions of the Asiatic cobras (Naja naja species complex). Toxicon 1996, 34, 399-406, doi:10.1016/0041-0101(95)00139-5.
- Warrell, D.A. Snake bite. Lancet 2010, 375, 77-88, doi:10.1016/S0140-6736(09)61754-2.
- Suchithra, N.; Pappachan, J.M.; Sujathan, P. Snakebite envenoming in Kerala, South India: clinical profile and factors involved in adverse outcomes. Emergency Medicine Journal 2008, 25, 200-204, doi:10.1136/emj.2007.051136.
- Tan, N.H.; Fung, S.Y.; Tan, K.Y.; Yap, M.K.; Gnanathasan, C.A.; Tan, C.H. Functional venomics of the Sri Lankan Russell's viper (Daboia russelii) and its toxinological correlations. Journal of Proteomics 2015, 128, 403-423, doi:10.1016/j.jprot.2015.08.017.
- Kalita, B.; Singh, S.; Patra, A.; Mukherjee, A.K. Quantitative proteomic analysis and antivenom study revealing that neurotoxic phospholipase A2 enzymes, the major toxin class of Russell's viper venom from southern India, shows the least immuno-recognition and neutralization by commercial polyvalent antivenom. International Journal of Biological Macromolecules 2018, 118, 375-385, doi:10.1016/j.ijbiomac.2018.06.083.
- Puzari, U.; Mukherjee, A.K. Recent developments in diagnostic tools and bioanalytical methods for analysis of snake venom: A critical review. Analytica Chimica Acta 2020, 1137, 208-224, doi:10.1016/j.aca.2020.07.054.
- Mukherjee, A.K.; Mackessy, S.P. Prevention and improvement of clinical management of snakebite in Southern Asian countries: A proposed road map. Toxicon 2021, 200, 140-152, doi:10.1016/j.toxicon.2021.07.008.
- Mao, Y.C.; Liu, P.Y.; Chiang, L.C.; Lai, C.S.; Lai, K.L.; Ho, C.H.; Wang, T.H.; Yang, C.C. Naja atra snakebite in Taiwan. Clinical Toxicology 2018, 56, 273-280, doi:10.1080/15563650.2017.1366502.
- Kularatne, S.A.; Budagoda, B.D.; Gawarammana, I.B.; Kularatne, W.K. Epidemiology, clinical profile and management issues of cobra (Naja naja) bites in Sri Lanka: first authenticated case series. Transactions of the Royal Society of Tropical Medicine and Hygiene 2009, 103, 924-930, doi:10.1016/j.trstmh.2009.04.002.
- Wang, W.; Chen, Q.F.; Yin, R.X.; Zhu, J.J.; Li, Q.B.; Chang, H.H.; Wu, Y.B.; Michelson, E. Clinical features and treatment experience: a review of 292 Chinese cobra snakebites. Environmental Toxicology and Pharmacology 2014, 37, 648-655, doi:10.1016/j.etap.2013.12.018.
- Bawaskar, H.S.; Bawaskar, P.H. Envenoming by the common krait (Bungarus caeruleus) and Asian cobra (Naja naja): clinical manifestations and their management in a rural setting. Wilderness & Environmental Medicine 2004, 15, 257-266.
- Faiz, M.A.; Ahsan, M.F.; Ghose, A.; Rahman, M.R.; Amin, R.; Hossain, M.; Tareq, M.N.U.; Jalil, M.A.; Kuch, U.; Theakston, R.D.G.; et al. Bites by the Monocled Cobra, Naja kaouthia, in Chittagong Division, Bangladesh: Epidemiology, clinical features of envenoming and management of 70 identified cases. The American Journal of Tropical Medicine and Hygiene 2017, 96, 876-884, doi:10.4269/ajtmh.16-0842.
- Utkin, Y.N. Last decade update for three-finger toxins: Newly emerging structures and biological activities. World Journal of Biological Chemistry 2019, 10, 17-27, doi:10.4331/wjbc.v10.i1.17.
- Utkin, Y.N. Three-finger toxins, a deadly weapon of elapid venom--milestones of discovery. Toxicon 2013, 62, 50-55, doi:10.1016/j.toxicon.2012.09.007.
- Dutta, S.; Chanda, A.; Kalita, B.; Islam, T.; Patra, A.; Mukherjee, A.K. Proteomic analysis to unravel the complex venom proteome of eastern India Naja naja: Correlation of venom composition with its biochemical and pharmacological properties. Journal of Proteomics 2017, 156, 29-39, doi:10.1016/j.jprot.2016.12.018.
- Chanda, A.; Kalita, B.; Patra, A.; Senevirathne, W.; Mukherjee, A.K. Proteomic analysis and antivenomics study of Western India Naja naja venom: correlation between venom composition and clinical manifestations of cobra bite in this region. Expert Review of Proteomics 2019, 16, 171-184, doi:10.1080/14789450.2019.1559735.
- Chanda, A.; Patra, A.; Kalita, B.; Mukherjee, A.K. Proteomics analysis to compare the venom composition between Naja naja and Naja kaouthia from the same geographical location of eastern India: Correlation with pathophysiology of envenomation and immunological cross-reactivity towards commercial polyantivenom. Expert Review of Proteomics 2018, 15, 949-961, doi:10.1080/14789450.2018.1538799.
- Chanda, A.; Mukherjee, A.K. Quantitative proteomics to reveal the composition of Southern India spectacled cobra (Naja naja) venom and its immunological cross-reactivity towards commercial antivenom. International Journal of Biological Macromolecules 2020, 160, 224-232, doi:10.1016/j.ijbiomac.2020.05.106.
- Petras, D.; Sanz, L.; Segura, A.; Herrera, M.; Villalta, M.; Solano, D.; Vargas, M.; Leon, G.; Warrell, D.A.; Theakston, R.D.; et al. Snake venomics of African spitting cobras: toxin composition and assessment of congeneric cross-reactivity of the pan-African EchiTAb-Plus-ICP antivenom by antivenomics and neutralization approaches. Journal of Proteome Research 2011, 10, 1266-1280, doi:10.1021/pr101040f.
- Kakati, H.; Patra, A.; Kalita, B.; Chanda, A.; Rapole, S.; Mukherjee, A.K. A comparison of two different analytical workflows to determine the venom proteome composition of Naja kaouthia from North-East India and immunological profiling of venom against commercial antivenoms. International Journal of Biological Macromolecules 2022, 208, 275-287, doi:10.1016/j.ijbiomac.2022.03.095.
- Tan, C.H.; Wong, K.Y.; Chong, H.P.; Tan, N.H.; Tan, K.Y. Proteomic insights into short neurotoxin-driven, highly neurotoxic venom of Philippine cobra (Naja philippinensis) and toxicity correlation of cobra envenomation in Asia. Journal of Proteomics 2019, 206, 103418.
- Mukherjee, A.K.; Maity, C.R. The composition of Naja naja venom samples from three districts of West Bengal, India. Comparative Biochemistry and Physiology. Part A, Molecular & Integrative Physiology 1998, 119, 621-627, doi:10.1016/s1095-6433(97)00475-3.
- Shashidharamurthy, R.; Jagadeesha, D.K.; Girish, K.S.; Kemparaju, K. Variations in biochemical and pharmacological properties of Indian cobra (Naja naja naja) venom due to geographical distribution. Molecular and Cellular Biochemistry 2002, 229, 93-101, doi:10.1023/a:1017972511272.
- Tasoulis, T.; Pukala, T.L.; Isbister, G.K. Investigating toxin diversity and abundance in snake venom proteomes. Frontiers in Pharmacology 2021, 12, 768015, doi:10.3389/fphar.2021.768015.
- Chanda, A.; Mukherjee, A.K. Mass spectrometric analysis to unravel the venom proteome composition of Indian snakes: opening new avenues in clinical research. Expert Review of Proteomics 2020, 17, 411-423, doi:10.1080/14789450.2020.1778471.
- Panagides, N.; Jackson, T.N.; Ikonomopoulou, M.P.; Arbuckle, K.; Pretzler, R.; Yang, D.C.; Ali, S.A.; Koludarov, I.; Dobson, J.; Sanker, B.; et al. How the cobra got its flesh-eating venom: Cytotoxicity as a defensive innovation and its co-evolution with hooding, aposematic marking, and spitting. Toxins 2017, 9, 103, doi:10.3390/toxins9030103.
- Kalita, B., Utkin, Y.N. and Mukherjee, A.K. Current Insights in the Mechanisms of Cobra Venom Cytotoxins and Their Complexes in Inducing Toxicity: Implications in Antivenom Therapy. Toxins 2022, 14(12), 839, doi:10.3390/toxins14120839
