Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 3 by Sirius Huang and Version 2 by Sirius Huang.
Laryngeal dystonia (LD), or spasmodic dysphonia (SD), is a chronic, task-specific, focal movement disorder affecting the larynx. It interferes primarily with the essential functions of phonation and speech. LD affects patients’ ability to communicate effectively and significantly diminishes their quality of life. Botulinum neurotoxin was first used as a therapeutic agent in the treatment of LD four decades ago and remains the standard of care for the treatment of LD.
- botulinum neurotoxin
- laryngeal dystonia
- spasmodic dysphonia
- injection
- electromyography
1. Introduction
Various botulinum toxin preparations are currently available on the commercial market. These include onabotulinium toxin Type A (Botox®, Allergan, Irvine, CA, USA), abobotulinum toxin Type A (Dysport®, Ipsen, Slough, UK) and incobotulinum toxin Type A (Xeomin®, Merz Pharma, Frankfurt am Main, Germany). Whilst most of the LD literature relates to treatment with onabotulinumtoxinA, there is evidence for the successful use of incobotulinumtoxinA [1] and abobutulinumtoxinA [2] in the treatment of LD. Additionally, preparation of botulinum toxin Type B, rimabotulinumtoxinB (Myobloc®, Solstice Neurosciences LLC, Rockville, MD, USA) has been used as a safe and effective treatment option for laryngeal dystonia [3][4][5]. Each of these preparations has a unique formulation and varying manufacturing processes, giving rise to different pharmacological properties and requiring different dosing regimens. For consistency and ease of use, all BoNT-A dosing regimens outlined in this articleerein refer to units of onabotulinum toxin Type A (Botox®) based on the researchers’ clinical experience and the current literature.
To minimise variability associated with volume diffusion of botulinum toxin beyond the intrinsic laryngeal muscles, the researchers recommend the use of a consistent volume of 0.1 mL in each vocal fold for ADLD whilst the concentration is adjusted so that the desired units of BoNT-A is delivered. This is achieved by making up a standard stock of 100 units of Botox® with 4 mL of sterile 0.9% NaCl (2.5 units of Botox® per 0.1 mL). The desired concentration of BoNT-A is achieved by further diluting this standard stock (2.5 units per 0.1 mL) with sterile 0.9% NaCl. A 0.1 mL aliquot containing the required units of BoNT-A is then drawn up into a 1-millilitre luer-slip syringe for injection. If higher concentrations of BoNT-A are required, i.e., greater than 2.5 units per 0.1 mL, then a ‘double-strength’ stock can be made with 100 units of Botox® in 2 mL of sterile 0.2% NaCl achieving a concentration of 5 U per 0.1 mL. This can be further diluted to the desire concentration as described above.
2. Adductor Spasmodic Dysphonia (ADLD)
Patients with ADLD typically present with a strained, tight, and strangled voice, with limited variation in pitch. Harsh, intermittent adductor pitch breaks are most evident in voice-weighted (vowel) sentences. Vocal fatigue and difficulties with both volume and projection are typical complaints among this patient group [6][7].
2.1. Cricothyroid Membrane Approach to Adductor Muscle Complex
The patient can be positioned upright or semi-reclined in a treatment chair or supine on an examination couch with the neck slightly extended. The cricothyroid membrane is identified using the superior border of the cricoid cartilage and the inferior border of the thyroid cartilage as landmarks (Figure 1). The needle is inserted via the cricothyroid space approximately 3 mm lateral to the midline and directed 30–45 degrees superolateral (Figure 2). The patient is advised to refrain from coughing, swallowing or phonation unless instructed to do so. The EMG machine is activated, and the needle is advanced into the paraglottic space below the inferior lip of the thyroid cartilage. Sharp, crisp EMG potentials indicate that the motor endplates of the target TA muscle are near the tip of the injection needle. The patient is asked to phonate gently in modal voice for EMG confirmation of needle position before delivery of BoNT. In cases where the LCA muscle is targeted, a similar approach is deployed with the needle angled more posterolateral.
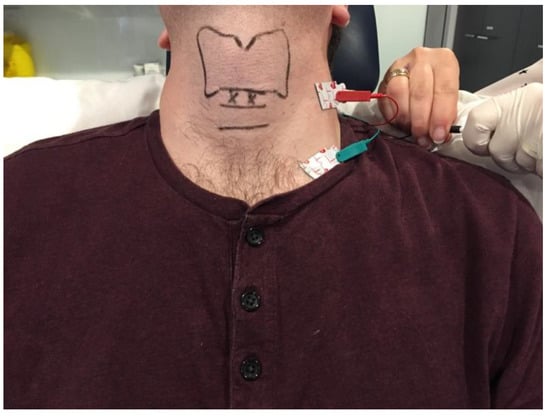
Figure 1. Clinical photograph depicting the ground and negative transcutaneous leads required for laryngeal EMG setup and the landmarks for the cricothyroid membrane approach to the adductor muscle complex. The surface landmarks for the thyroid and cricoid cartilages are delineated in black. The entry points for the injection needle are marked by ‘X’.
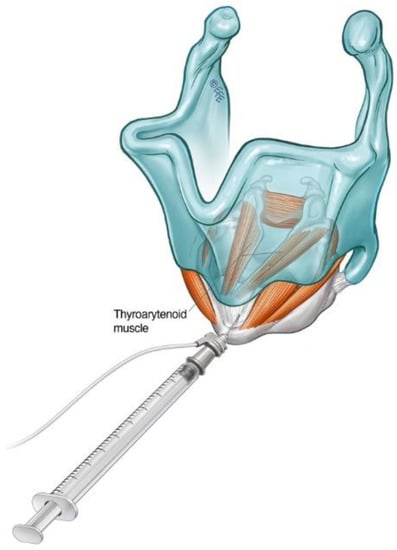
Figure 2. Schematic diagram illustrating the cricothyroid membrane injection approach to TA muscle in the treatment of ADLD.
2.2. Transthyrohyoid Approaches to the Larynx
Where EMG is not available, the adductor complex can alternatively be accessed via a transthyrohyoid approach using flexible transnasal endoscopic guidance according to the method described by Amin [8], where the needle enters the airway and is directed towards the paraglottic space under endoscopic visualisation (Figure 3).
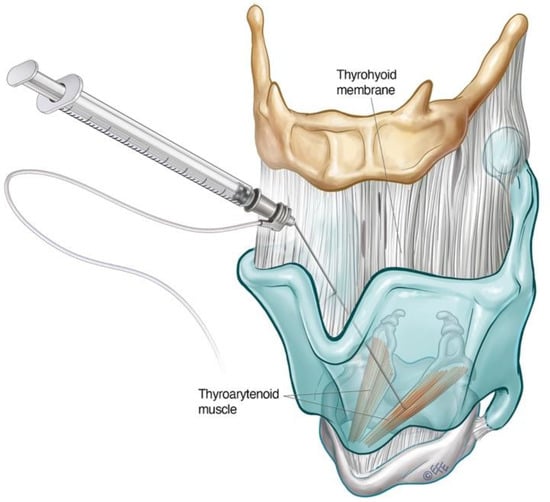
Figure 3. Schematic diagram illustrating transthyrohyoid injection approach to the TA. The supraglottic structures can also be approached in this way.
Schönweiler et al. first described a transoral approach for injection of BoNT-A into the supraglottis for treatment of ADLD based on the observation that some ADLD patients exhibited hyperfunction of the supraglottic musculature in addition to the typical glottic ‘squeeze’ [9]. Young and Blitzer subsequently reported good symptom control delivering BoNT-A into the supraglottis via a trans thyrohyoid technique in this subgroup of ADLD patients, who derived limited functional benefit treatment of the TA alone. [10]. Transient mild to moderate dysphagia was experienced in 50% of their small cohort of patients treated in this way [10].
The trans thyrohyoid approach to the supraglottic structures requires endoscopic guidance to ensure accurate toxin delivery. The patient is positioned upright with the head in extension. The thyrohyoid membrane is identified using the superior thyroid notch as a landmark. The needle is inserted through the thyrohyoid space at the midline just above the superior thyroid notch, with a 45-degrees bend at the hub directed inferiorly. Once over the notch, the needle is slowly advanced downwards at an acute angle and rotated slightly towards the side being injected. The position of the needle is confirmed when tenting of the supraglottic mucosa is seen on endoscopy. The toxin can then be delivered submucosally under visualisation
The change of primary injection site from the TA to the supraglottis may offer the advantage of more gradual onset with less severe side-effects of breathiness [11]. In a longitudinal functional study of ADLD patients, 7.5 units (average dose) were delivered into the submucosal space of each false vocal fold. Of the patients, 76% treated in this way reported no decline in the percentage of normal vocal function, whilst 24% experienced only a small transient post-injection decline [11]. In view of these findings, the researchers have advocated this approach in the treatment of professional voice users and patients who experience excessive breathiness with TA/LCA injections to minimise vocal downtime.
2.3. Interarytenoid BoNT-A Injections for ADLD
Involvement of the interarytenoid (IA) muscle has been demonstrated in fine-wire EMG studies in patients with ADLD [12]. In patients who have not achieved a good therapeutic response with BoNT injections to the TA/LCA muscle complex alone, additional injections to the IA (mean BoNT-A dose 2.0 units) have achieved success in symptom control [12][13]. The needle enters the CT membrane in the midline, directed 30 degrees supero-posteriorly. It is then advanced until the air microphonic on EMG ceases, and mild resistance is re-encountered. The patient is asked to phonate on an ‘eee’ at fundamental frequency until EMG confirmatory signal is heard. Encountering firm resistance without a positive EMG signal indicates the needle has hit the posterior plate of the cricoid cartilage. The needle should be drawn back into the airway and inched superiorly incrementally until the IA muscle is encountered beyond the superior border of the cricoid cartilage.
2.4. Dosing and Laterality Considerations in ADLD
The aim of laryngeal BoNT-A treatment for ADLD should be to achieve a fluent voice with improved vocal function for as long as possible whilst minimising the side-effects throughout the treatment cycle [14]. In a 10-year follow-up of over 200 patients, Lerner et al. reported a difference in BoNT-A dose based on gender [15]. The average doses of BoNT-A received by male and female ADLD patients were 0.6 units and 1.3 units on each side, respectively. In contrast, no significant difference in dosage based on age or gender was observed in another large series of 155 patients [16].
In the researchers’ practice, 1.0 unit to each vocal fold bilaterally is typically offered at the initial treatment session, as recommended by Blitzer et al. [17]. This may be reduced to 0.6–0.8 units per vocal fold if the vocal function is critical to the patient in the coming weeks. The patient is then reviewed at 2–4 weeks to assess response. Symmetrical vocal fold movement on laryngoscopy with ongoing adductor pitch breaks may indicate under-dosing. A ‘top-up’ dose can be administered bilaterally at this stage.
The average duration of benefit from one treatment cycle is between 11–15 weeks [17][18]. Transient breathiness of the voice is common after ADLD treatment and has been proposed as a marker for successful injection [19][20][21]. Novakovic et al. found that 28.5% of patients experience an initial deterioration in vocal function associated with a weak and breathy voice lasting an average of 20 days (median 14 days) [14]. This initial side effect may be unacceptable to a proportion of patients. Staggered bilateral dosing can be considered to mitigate this [17][22]. One vocal fold is injected, with the patient returning 2–3 weeks after for BoNT-A injection to the contralateral vocal fold. The researchers find that some patients prefer to present at regular, closely spaced intervals, on average 6 weeks apart, to receive smaller alternating doses to minimise breathiness and vocal downtime. Unilateral botulinum toxin injections have also been reported as an effective primary treatment for ADLD. Lee et al. [23] have observed that both alternating unilateral and bilateral injection regimes demonstrated comparable levels of efficacy, durability, and stability for the treatment of ADLD. The group concluded that alternating unilateral injections could be routinely performed at shorter intervals, with fewer side-effects compared to bilateral injections. These findings are in contrast to another cohort study [24], which found bilateral BoNT-A injections to be more effective in producing optimal therapeutic effects to side-effect profiles.
3. Abductor Spasmodic Dysphonia (ABLD)
Patients with abductor spasmodic dysphonia present with a weak and breathy voice with characteristic abductor pitch breaks on connected speech [25][26][27]. These typically manifest as sudden aphonic whispered moments of speech, indicating inappropriate PCA muscle activity [25][26] which are best assessed on voiceless weighted sentences. Treatment of ABLD is more challenging than ADLD. The average self-reported best voice achieved is 70% of normal function in this group of patients [17][22]. The maximal dose effect may be limited by the potential for airway compromise. Additional treatment options, including BoNT-A injection to the cricothyroid muscle and vocal fold medialisation [17][28], may be considered when the limit of airway compromise has been reached.
Access to the PCA muscle is more challenging than to the adductor complex. It lies posterior to the larynx, emerging as a broad fan from the posterior surface of the cricoid lamina, coursing obliquely upwards in a lateral direction before inserting into the muscular process of the arytenoid.
3.1. Lateral Rotation Approach to PCA Muscle
The lateral rotation injection approach to the PCA is a commonly used technique for ABLD. It allows transcutaneous access to the PCA muscle without breach of the airway [29]. The cricoid cartilage and lateral posterior border of the thyroid cartilage serve as landmarks. The larynx is rotated between the thumb and forefingers such that the thyroid notch is displaced away from the side being injected, thereby opening the posterior face of the cricoid of the injected side for access [25][29] (Figure 4). The needle is inserted along the lower half of and posterior to the thyroid cartilage with care until the posterior plate of the cricoid is encountered. The needle is withdrawn slightly, and the patient is asked to sniff in through the nose to activate the PCA muscle. This is deployed as a confirmatory manoeuvre on LEMG before the toxin is injected. The contralateral side can be treated in a similar way, but one must be aware of the potential for airway compromise in synchronous injections [25][30].
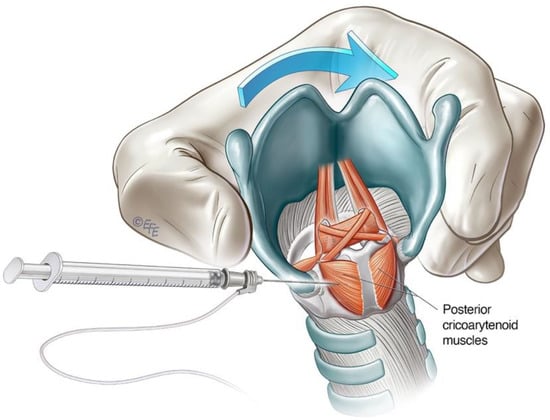
Figure 4. Schematic diagram illustrating lateral rotational injection approach to the PCA.
3.2. Anterior Trans-Airway Approach to PCA Muscle
Alternative transmucosal access to the PCA muscle via an anterior approach was reported by Rontal et al. [31]. This approach relied on the diffusion of high doses of BoNT-A by injection superior to the cricoid lamina above the PCA muscle under endoscopic guidance. The technique has been modified over time, evolving into a trans-cricoid approach as described by Meleca et al. [32], allowing for more accurate PCA muscle localisation with the aid of EMG.
The airway is anaesthetised with a transmucosal injection of 2 mL of lidocaine 2% delivered through the cricothyroid membrane using a 25-gauge needle. This will trigger airway reflexes. The patient is encouraged to cough to distribute the anaesthetic throughout the laryngeal region. The cricoid cartilage and inferior anterior border of the thyroid cartilage are identified as landmarks. The needle is inserted via the cricothyroid space in the midline and directed posteriorly, approximately 30 degrees laterally, towards the target PCA muscle (Figure 5). This approach transverses the cricoid plate. A larger 26-gauge needle may be helpful, especially if calcification of the cartilage has occurred. The patient should refrain from coughing, swallowing or phonate whilst the needle is inserted. The EMG machine is activated as the needle is advanced through the cricothyroid membrane towards the posterior cricoid plate. The injector looks and listens for the airway microphonic (a loud buzzing sound) and distortion of the EMG signal, indicating that the needle has entered the airway. The needle is advanced until resistance is met, inferring that the posterior plate of the cricoid has been reached. The needle is then further advanced slowly and firmly in a controlled manner through the cartilage, taking care not to breach the mucosa posterior to the PCA muscle. A gentle twisting motion may assist the needle through the cartilage. The return of crisp, sharp EMG potentials indicates the tip of the needle is near the motor end plates of the PCA muscle. The patient is asked to sniff in to confirm the needle position before the predetermined aliquot of BoNT-A is injected. 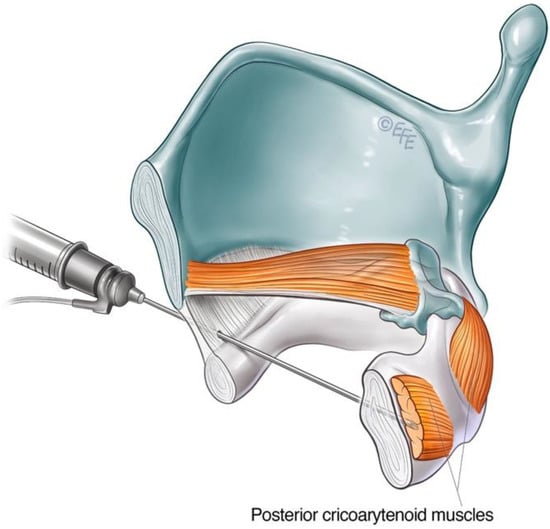


Figure 5. Schematic diagram illustrating anterior trans airway approach to the PCA muscle.
3.3. Cricothyroid (CT) Muscle Injection for ABLD
Spasmodic bursts of heightened activity in the CT muscle have been observed in some patients with ABLD, with inappropriate CT activity demonstrated on LEMG [33]. This contrasts with other types of laryngeal dystonia, where abnormal CT activity was not found on fine-wire EMG [34][35]. Some 60% of patients in this subgroup reported voice improvement following selective treatment of the CT muscle with BoNT-A [36]. CT muscle injections may therefore be considered in the treatment of ABLD in those with ongoing voice symptoms despite adequate treatment of the PCA or in the presence of a narrow airway precluding further PCA injections. The CT is primarily a tensioner and lengthening of the vocal fold via the cricothyroid joint but also exhibits weak adductor action. The CT muscle is large and readily accessible via an anterior transcutaneous approach (Figure 6).
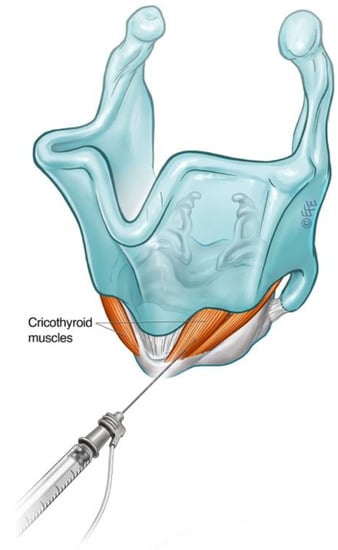
Figure 6. Schematic diagram illustrating technique for injection into the cricothyroid muscle.
3.4. Dosing and Laterality Considerations for ABLD
BoNT-A dosage for the treatment of ABLD varies widely from 3.75 units to 10 units [25][37][38]. Both bilateral and unilateral injections have been described [25][37]. The researchers’ preference is to offer 3.75 units into the more active PCA muscle at initial treatment, followed by a clinical review at 2 weeks post-injection to assess response. The desired dose and concentration of BoNT-A is prepared using a standard stock (2.5 units of Botox® in 0.1 mL of 0.9% NaCl) either directly or diluted with normal saline. For doses greater than 5.0 units, double-strength stock (5.0 units of Botox® in 0.1 mL of 0.9% NaCl) can be prepared to reduce the risk of volume diffusion to the contralateral side or to the inferior constrictor muscle.
Patients with ABLD generally have poorer self-reported voice outcomes than the ADLD group [22]. ABLD can be challenging to manage, and a careful and graduated approach is therefore recommended with a regular clinical assessment to evaluate treatment efficacy.
The technique for BoNT-A treatment delivery to the PCA will depend on the clinician's experience and comfort with each of the described approaches. Patient anatomy is also an important factor, as it can be more difficult to accurately localise the PCA using the lateral rotational approach when the patient has a thick neck [29]. Approximately 25% of patients will respond adequately to the unilateral treatment of PCA [25]. The primary goal is control of breathy voice breaks or complete immobilisation of unilateral PCA function during each treatment cycle [37]. Larger doses can be deployed to achieve this. BoNT-A dose should be adjusted individually for each patient based on an endoscopic assessment of PCA activity and the length of which the treatment cycle lasts. In the researchers’ experience, the required dosage of BoNT-A typically varies between 3.75 to 10.0 units for unilateral PCA immobilisation [25][37][38]. The decision of whether to treat the same side at each treatment cycle or whether to alternate sides is reached in discussion with the patient.
Up to 80% of patients with ABLD require treatment beyond the unilateral PCA muscle [25]. The researchers recommend unilateral dosing of the more active PCA muscle, as described above. Follow-up laryngoscopy at 2–4 weeks helps discern between under-dosing or technical failure of the treated side and ongoing contralateral abductor pitch breaks. In order to minimise the risk of airway complications, smaller doses of 0.625 units to 2.5 units may be used to weaken but not immobilise the contralateral side [25]. The use of higher staged doses, such as 5.0 units on each side, has also been described [37][38], but the researchers recommend proceeding with caution.
Bilateral synchronous treatment of the PCA muscle for ABLD has been reported as safe and effective. The use of a lower dosage regime (1.25 to 1.70 units on one side and 0.9 units on the other side) did not precipitate any breathing difficulties in the patients treated [32]. Stong et al. found a 5% incidence of significant dyspnoea with synchronous BoNT-A injections into PCA muscles with 2.0 to 2.5 units on each side [39]. Improvement in voice with only mild shortness of breath was reported by 89% of patients receiving bilateral synchronous BoNT-A injections to the PCA [40]. Treatment decisions regarding staged or synchronous PCA injections should be informed by clinician experience and extensive discussion with the patient.
4. Mixed Laryngeal Dystonia (Mixed LD)
Mixed LD is a rare form of laryngeal dystonia presenting with features of both adductor and abductor LD, thought to comprise 1–5% of all presentations of LD [17][41]. Affected individuals experience both abductor and adductor vocal spasms during speech. Diagnosis of this condition is challenging as voice presentation is atypical and may not fall into the usually recognised patterns. In addition, the researchers have encountered compensatory functional overlay in their clinical experience. Clinical findings of supraglottic hyperfunction and inhalational speech are common among this group of patients [17]. In comparison to ADLD and ABLD, patients affected by mixed LD struggle with both voice-weighted and voiceless-weighted sentences. Typical features in the clinical history including a positive response to alcohol and non-responsiveness to speech therapy [42][43]. Furthermore, mixed LD patients will have often failed a trial of laryngeal botulinum toxin injection targeted for either ADLD or ABLD. Therefore, a high index of suspicion must be exercised when a patient with suspected LD reports no or limited improvement with adductor or abductor-targeted chemodenervation. Routine evaluation of patients commencing BoNT treatment for LD is recommended by the researchers 2–4 weeks after treatment to check treatment responses based on the patient’s self-reported outcome measures in conjunction with endoscopic evaluation and perceptual voice analysis [14]. Patients with mixed LD may present with predominantly adductor or abductor voice pitch breaks, towards which initial treatment should be directed. Perceptual voice strain and tightness after BoNT-A treatment to PCA for a presumed case of ABLD should raise the possibility of mixed dystonia. Breathiness after BoNT-A treatment to the adductor complex for ADLD may be more difficult to evaluate. This symptom itself is an expected initial side-effect following BoNT-A treatment for ADLD. In these cases, voiceless-weighted phrases can help to unmask adductor pitch breaks both perceptually and endoscopically for the astute clinician.
Mixed LD is challenging to treatment [44]. Patients should be counselled for close follow-up over a period of time and may require an individualised dosing regimen optimised for their condition. Researchers find that baseline and serial voice recording after each treatment, along with longitudinal self-reported voice outcome measures, are useful in guiding treatment [14].
Both the adductor and abductor muscles serve as targets for BoNT chemodenervation. The options for the technical approach to each muscle group have been described in previous sections of this article. Unilateral treatment of the PCA and TA complex is initially recommended. As a starting point, a dose of 3.75 units in 0.15 mL for the PCA [25] and 1.0 units in 0.1 mL for the TA complex may be employed [17] (Table 1). The patient may be followed up at 2 weeks for the perceptual and endoscopic assessment to help guide subsequent dosing and injections. Treatment can be prescribed to the contralateral TA complex in the presence of ongoing adductor pitch breaks. In the event of persistent breathiness following treatment, the researchers find laryngoscopy with stroboscopy useful in differentiating the cause between adductor paralysis or contralateral abductor muscle hyperfunction [45]. The latter should prompt treatment of the contralateral PCA muscle.
Table 1. Suggested initial onabotulinum toxin A (Botox®) dosing regimen for laryngeal dystonia [10][17][25][36][37][46][47][48].
| Laryngeal Dystonia Type | Target Muscle | Suggested Botox® Dosing (Each Side)—Bilateral Treatment. In Units | Suggested Botox® Dosing—Unilateral Treatment. In Units (U) |
|---|---|---|---|
| Adductor laryngeal dystonia | TA/LCA | 0.6–1.3 | 2.5–3.75 |
| Supraglottis | 7.5 | - | |
| IA | - | 2 | |
| Abductor laryngeal dystonia | PCA | 1.25–2.5 | 3.75–10 |
| CT | 3.75–5 | - | |
| Adductor breathing laryngeal dystonia | TA/LCA | 0.625–3.75 | 2.5–5 |
| Singer’s dystonia | TA | 0.25–0.5 | 0.5–1.0 |
5. Other Types of Laryngeal Dystonia
5.1. Adductor Laryngeal Breathing Dystonia (ALBD)
In addition to affecting the voice, adductor muscle spasms during inspiration can be the predominate feature in a subtype of laryngeal dystonia, producing paradoxical vocal fold motion and stridor. This unusual subtype of LD, in which the primary abnormality is associated with respiration rather than phonation, was observed in the early 1990s [7]. This condition is now widely recognised as adductor laryngeal breathing dystonia (ALBD), sometimes known as respiratory laryngeal dystonia [48][49]. In ALBD, the voice is usually normal, with an absence of the characteristic adductor pitch breaks seen in ADLD. The main clinical features comprise persistent stridor, which may vary from moderate to severe. Affected patients may also complain of a dystonic cough, paroxysmal sneezing or hiccups [47][49]. The respiratory symptoms are often exacerbated by physical exertion. Interestingly, oxygen desaturation is not typically observed despite significant stridor [47][48]. On laryngoscopic examination, paradoxical vocal fold movement can be seen on inspiration, which adductor muscle spasms being triggered by a normal level of respiratory effort, resulting in significant narrowing at the glottic level, leading to stridor. The injection of BoNT-A into TA muscles is effective in treating ALBD. Nine patients with ALBD received 0.625–3.75 units of BoNT-A into each TA muscle in a retrospective case series, depending on the severity of symptoms [47] (Table 1). A statistically significant improvement in function of 55% (range 30–90%) was demonstrated [47]. The adverse effect of transient breathy voice and mild choking on liquids in 5 patients did not persist beyond 2 weeks [47].
A more recent prospective case series conducted by Tierney et al. deployed a wider range of management options. Of 16 patients, 100% underwent respiratory retraining therapy, 68.8% received laryngeal BoNT-A injections, and 31.3% required a tracheostomy for symptomatic relief [48]. The group concluded that although benzodiazepines, anticholinergics, dopamine blockers, neurogenic modulators, tricyclic antidepressants, and anti-reflux medication have been tried in patients with ALBD, all of these have failed to incite an improvement. To date, the BoNT-A injections into the adductor muscle complex remain the most effective treatment. ALBD is a rare but severely disabling condition for which there are limited treatment options, making it very challenging to manage.
5.2. Singer’s Dystonia
It is traditionally thought that laryngeal dystonia only affected the task of speaking. LD patients are usually able to sing, laugh and express other emotions vocally without dystonic spasms. A 7-year experience described by Chitkara et al. [46] identified a subgroup of patients with laryngeal dystonia of the singing voice without affected conversational speech at initial presentation. Of the 5 patients in this case series, 80% were females, 80% exhibited adductor pitch breaks when singing, and the mean age of onset was 35.8 years. Of these patients, 60% had received classical training in singing. All genres, including opera, folk, pop, and musical theatre, were involved, with all pitch ranges (top, middle or low) affected. The mainstay of effective treatment was voice therapy used in conjunction with BoNT-A injections into the TA muscle. It was observed that patients with singer’s dystonia exhibited narrower margins of tolerance to the undesirable side-effects of BoNT-A chemodenervation, which included a reduction in volume, decreasing vibrato and truncated pitch range in their singing voice. As such, a smaller average dose of 0.25 units was advocated for use in each TA muscle (Table 1). This rare clinical presentation has since been expanded upon by Halstead et al. [50], who observed that singing pitch breaks were reproducible at specific pitches that are unrelated to the passagio or occurred while performing specific tasks such as singing voiceless consonants. Singer’s dystonia is often misdiagnosed, with the patient’s singing difficulties commonly but incorrectly attributed to problems with technique, including increased muscle tension, register transition or wobble. Nevertheless, it is an important diagnosis to make due to its detrimental ramifications on an individual’s career and psychological ability relative to their ability to perform.
References
- Kohli, N.; Lerner, M.; Rashty, J.; Kirke, D.; Stewart, T.; Blitzer, A. IncobotulinimtoxinA (Xeomin) for the treatment of adductor laryngeal dystonia: A prospective, open-label clinical trial. Am. J. Otolaryngol. 2022, 43, 103613.
- Elmiyeh, B.; Prasad, V.M.; Upile, T.; Saunders, N.; Youl, B.D.; Epstein, R.; Rubin, J.S. A single-centre retrospective review of unilateral and bilateral Dysport injections in adductor spasmodic dysphonia. Logoped. Phoniatr. Vocol. 2010, 35, 39–44.
- Adler, C.H.; Bansberg, S.F.; Krein-Jones, K.; Hentz, J.G. Safety and efficacy of botulinum toxin type B (Myobloc) in adductor spasmodic dysphonia. Mov. Disord. 2004, 19, 1075–1079.
- Sataloff, R.T.; Heman-Ackah, Y.D.; Simpson, L.L.; Park, J.B.; Zwislewski, A.; Sokolow, C.; Mandel, S. Botulinum toxin type B for treatment of spasmodic dysphonia: A case report. J. Voice 2002, 16, 422–424.
- Blitzer, A. Botulinum toxin A and B: A comparative dosing study for spasmodic dysphonia. Otolaryngol.-Head Neck. Surg. 2005, 133, 836–838.
- Blitzer, A.; Brin, M.F.; Fahn, S.; Lovelace, R.E. Clinical and laboratory characteristics of focal laryngeal dystonia: Study of 110 cases. Laryngoscope 1988, 98, 636–640.
- Blitzer, A.; Brin, M.F. Laryngeal Dystonia: A Series with Botulinum Toxin Therapy. Ann. Otol. Rhinol. Laryngol. 1991, 100, 85–89.
- Amin, M.R. Thyrohyoid approach for vocal fold augmentation. Ann. Otol. Rhinol. Laryngol. 2006, 115, 699–702.
- Schönweiler, R.; Wohlfarth, K.; Dengler, R.; Ptok, M. Supraglottal injection of botulinum toxin type A in adductor type spasmodic dysphonia with both intrinsic and extrinsic hyperfunction. Laryngoscope 1998, 108, 55–63.
- Young, N.; Blitzer, A. Management of supraglottic squeeze in adductor spasmodic dysphonia: A new technique. Laryngoscope 2007, 117, 2082–2084.
- Simpson, C.B.; Lee, C.T.; Hatcher, J.L.; Michalek, J. Botulinum toxin treatment of false vocal folds in adductor spasmodic dysphonia: Functional outcomes. Laryngoscope 2016, 126, 118–121.
- Hillel, A.D.; Maronian, N.C.; Waugh, P.F.; Robinson, L.; Klotz, D.A. Treatment of the interarytenoid muscle with botulinum toxin for laryngeal dystonia. Ann. Otol. Rhinol. Laryngol. 2004, 113, 341–348.
- Kendall, K.A.; Leonard, R.J. Interarytenoid muscle botox injection for treatment of adductor spasmodic dysphonia with vocal tremor. J. Voice 2011, 25, 114–119.
- Novakovic, D.; Waters, H.H.; D'Elia, J.B.; Blitzer, A. Botulinum toxin treatment of adductor spasmodic dysphonia: Longitudinal functional outcomes. Laryngoscope 2011, 121, 606–612.
- Lerner, M.Z.; Lerner, B.A.; Patel, A.A.; Blitzer, A. Gender differences in onabotulinum toxin A dosing for adductor spasmodic dysphonia. Laryngoscope 2017, 127, 1131–1134.
- Vasconcelos, S.; Birkent, H.; Sardesai, M.G.; Merati, A.L.; Hillel, A.D. Influence of age and gender on dose and effectiveness of botulinum toxin for laryngeal dystonia. Laryngoscope 2009, 119, 2004–2007.
- Blitzer, A.; Brin, M.F.; Stewart, C.F. Botulinum toxin management of spasmodic dysphonia (laryngeal dystonia): A 12-year experience in more than 900 patients. Laryngoscope 1998, 108, 1435–1441.
- Shoffel-Havakuk, H.; Rosow, D.E.; Lava, C.X.; Hapner, E.R.; Johns, M.M., 3rd. Common practices in botulinum toxin injection for spasmodic dysphonia treatment: A national survey. Laryngoscope 2019, 129, 1650–1656.
- Cannito, M.P.; Woodson, G.E.; Murry, T.; Bender, B. Perceptual analyses of spasmodic dysphonia before and after treatment. Arch. Otolaryngol.-Head Neck Surg. 2004, 130, 1393–1399.
- Watts, C.C.; Whurr, R.; Nye, C. Botulinum toxin injections for the treatment of spasmodic dysphonia. Cochrane Database Syst. Rev. 2004, 2010, CD004327.
- Hirose, K.; Asano, K.; Sakaguchi, M.; Nagao, A.; Nakahira, M.; Doi, N.; Kobayashi, T.; Hyodo, M. Post-treatment clinical course following botulinum toxin injection therapy for adductor spasmodic dysphonia: Analysis of data from a placebo-controlled, randomized, double-blinded clinical trial in Japan. Laryngoscope Investig. Otolaryngol. 2021, 6, 1088–1095.
- Blitzer, A. Spasmodic dysphonia and botulinum toxin: Experience from the largest treatment series. Eur. J. Neurol. 2010, 17, 28–30.
- Lee, S.J.; Kang, M.S.; Choi, H.S.; Lim, J.Y. Alternating Unilateral Versus Bilateral Injections of Botulinum Toxin for the Treatment of Adductor Spasmodic Dysphonia. Otolaryngol. Head Neck Surg. 2021, 164, 815–820.
- Dharia, I.; Bielamowicz, S. Unilateral versus bilateral botulinum toxin injections in adductor spasmodic dysphonia in a large cohort. Laryngoscope 2020, 130, 2659–2662.
- Blitzer, A.; Brin, M.F.; Stewart, C.; Aviv, J.E.; Fahn, S. Abductor laryngeal dystonia: A series treated with botulinum toxin. Laryngoscope 1992, 102, 163–167.
- Edgar, J.D.; Sapienza, C.M.; Bidus, K.; Ludlow, C.L. Acoustic measures of symptoms in abductor spasmodic dysphonia. J. Voice 2001, 15, 362–372.
- Ludlow, C.L.; Domangue, R.; Sharma, D.; Jinnah, H.A.; Perlmutter, J.S.; Berke, G.; Sapienza, C.; Smith, M.E.; Blumin, J.H.; Kalata, C.E.; et al. Consensus-Based Attributes for Identifying Patients With Spasmodic Dysphonia and Other Voice Disorders. JAMA Otolaryngol Head Neck Surg. 2018, 144, 657–665.
- Dewan, K.; Berke, G.S. Bilateral Vocal Fold Medialization: A Treatment for Abductor Spasmodic Dysphonia. J. Voice 2019, 33, 45–48.
- Kaye, R.; Blitzer, A. Chemodenervation of the Larynx. Toxins 2017, 9, 356.
- Venkatesan, N.N.; Johns, M.M.; Hapner, E.R.; DelGaudio, J.M. Abductor paralysis after botox injection for adductor spasmodic dysphonia. Laryngoscope 2010, 120, 1177–1180.
- Rontal, M.; Rontal, E.; Rolnick, M.; Merson, R.; Silverman, B.; Truong, D.D. A method for the treatment of abductor spasmodic dysphonia with botulinum toxin injections: A preliminary report. Laryngoscope 1991, 101, 911–914.
- Meleca, R.J.; Hogikyan, N.D.; Bastian, R.W. A comparison of methods of botulinum toxin injection for abductory spasmodic dysphonia. Otolaryngol. Head Neck Surg. 1997, 117, 487–492.
- Rodriquez, A.A.; Ford, C.N.; Bless, D.M.; Harmon, R.L. Electromyographic assessment of spasmodic dysphonia patients prior to botulinum toxin injection. Electromyogr. Clin. Neurophysiol. 1994, 34, 403–407.
- Hillel, A.D. The study of laryngeal muscle activity in normal human subjects and in patients with laryngeal dystonia using multiple fine-wire electromyography. Laryngoscope 2001, 111, 1–47.
- Klotz, D.A.; Maronian, N.C.; Waugh, P.F.; Shahinfar, A.; Robinson, L.; Hillel, A.D. Findings of multiple muscle involvement in a study of 214 patients with laryngeal dystonia using fine-wire electromyography. Ann. Otol. Rhinol. Laryngol. 2004, 113, 602–612.
- Ludlow, C.L.; Naunton, R.F.; Terada, S.; Anderson, B.J. Successful treatment of selected cases of abductor spasmodic dysphonia using botulinum toxin injection. Otolaryngol. Head Neck Surg. 1991, 104, 849–855.
- Woodson, G.; Hochstetler, H.; Murry, T. Botulinum toxin therapy for abductor spasmodic dysphonia. J. Voice 2006, 20, 137–143.
- Bielamowicz, S.; Squire, S.; Bidus, K.; Ludlow, C.L. Assessment of posterior cricoarytenoid botulinum toxin injections in patients with abductor spasmodic dysphonia. Ann. Otol. Rhinol. Laryngol. 2001, 110, 406–412.
- Stong, B.C.; DelGaudio, J.M.; Hapner, E.R.; Johns, M.M., 3rd. Safety of simultaneous bilateral botulinum toxin injections for abductor spasmodic dysphonia. Arch. Otolaryngol. Head Neck Surg. 2005, 131, 793–795.
- Klein, A.M.; Stong, B.C.; Wise, J.; DelGaudio, J.M.; Hapner, E.R.; Johns, M.M., 3rd. Vocal outcome measures after bilateral posterior cricoarytenoid muscle botulinum toxin injections for abductor spasmodic dysphonia. Otolaryngol. Head Neck Surg. 2008, 139, 421–423.
- Tisch, S.H.; Brake, H.M.; Law, M.; Cole, I.E.; Darveniza, P. Spasmodic dysphonia: Clinical features and effects of botulinum toxin therapy in 169 patients-an Australian experience. J. Clin. Neurosci. 2003, 10, 434–438.
- Simonyan, K.; Barkmeier-Kraemer, J.; Blitzer, A.; Hallett, M.; Houde, J.F.; Jacobson Kimberley, T.; Ozelius, L.J.; Pitman, M.J.; Richardson, R.M.; Sharma, N.; et al. Laryngeal Dystonia. Multidiscip. Update Terminol. Pathophysiol. Res. Priorities 2021, 96, 989–1001.
- Kirke, D.N.; Frucht, S.J.; Simonyan, K. Alcohol responsiveness in laryngeal dystonia: A survey study. J. Neurol. 2015, 262, 1548–1556.
- Novakovic, D. Chapter 32: Atypical Spasmodic Dysphonia with Tremor. In Laryngology: A Case-Based Approach; Allen, J.E., Nouraei, S.A., Sandhu, G., Eds.; Plural Publishing Inc.: San Diego, CA, USA, 2020; Volume 1, pp. 327–338.
- Daraei, P.; Villari, C.R.; Rubin, A.D.; Hillel, A.T.; Hapner, E.R.; Klein, A.M.; Johns, M.M., 3rd. The role of laryngoscopy in the diagnosis of spasmodic dysphonia. JAMA Otolaryngol. Head Neck Surg. 2014, 140, 228–232.
- Chitkara, A.; Meyer, T.; Keidar, A.; Blitzer, A. Singer's dystonia: First report of a variant of spasmodic dysphonia. Ann. Otol. Rhinol. Laryngol. 2006, 115, 89–92.
- Grillone, G.A.; Blitzer, A.; Brin, M.F.; Annino, D.J., Jr.; Saint-Hilaire, M.H. Treatment of adductor laryngeal breathing dystonia with botulinum toxin type A. Laryngoscope 1994, 104, 30–32.
- Tierney, W.S.; Bryson, P.C.; Nelson, R.; Kaplan, S.E.; Benninger, M.S.; Milstein, C.F. Respiratory Laryngeal Dystonia: Characterization and Diagnosis of a Rare Neurogenic Disorder. Laryngoscope 2020, 130, 2843–2846.
- Payne, S.; Tisch, S.; Cole, I.; Brake, H.; Rough, J.; Darveniza, P. The clinical spectrum of laryngeal dystonia includes dystonic cough: Observations of a large series. Mov. Disord. 2014, 29, 729–735.
- Halstead, L.A.; McBroom, D.M.; Bonilha, H.S. Task-specific singing dystonia: Vocal instability that technique cannot fix. J. Voice 2015, 29, 71–78.
More
