Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 3 by Conner Chen and Version 2 by Conner Chen.
There are many studies on the different synthesis methods of carbon dots. Each process aims to improve the synthesis strategy and optimize the reaction conditions so that the carbon dots are not only more cost-effective and eco-friendly, but also provide more excellent performance.
- carbon dots
- composite materials
- synthesis
- bioapplication
1. Introduction
Carbon is the most basic element in organic matter and exists in the form of different compounds. Carbon dots (CDs) are a new carbon based zero-dimensional nanomaterial [1]. During the process of separation and purification of single-walled carbon nanotubes (SWCNTs) by gel electrophoresis, Sun et al. used laser ablation to make a series of surface treatments on the obtained carbon nanoparticles and named them carbon dots [2]. CDs are a large number of different, quasi spherical carbon atom aggregation regions with a size of less than 10 nm. CDs are usually composed of sp2 and sp3 hybrid carbon nuclei and rich active functional groups (such as hydroxyl, carboxyl, amino, etc.), which makes them have excellent water solubility. Through a series of chemical reactions, small molecules, organic polymers, or biomolecules can be adsorbed on the surface of CDs for surface passivation or functionalization, but the quantum yield of CDs is low, which greatly limits their practical application in the development of biological imaging and therapeutic diagnostics. In addition, because there is no specific analyte recognition group on the surface of CDs, the detection process of CDs in biosensors is often affected by potential disruptors. Most importantly, the interaction between CDs and biological systems is usually poor and lacks specificity, which greatly limits their potential clinical application. Generally, surface passivation can enhance the luminescence properties of CDs, and surface functionalization can change the physicochemical properties of CDs [3]. The preparation method and application classification of carbon dots and carbon dot composites are shown in Figure 1 [4][5][6][7][8][9][10]. Carbon dot matrix composites have excellent luminescence properties and good biocompatibility, and they have attracted extensive attention in the fields of biological imaging [11][12][13], sensing [14][15][16], detection, and biochemical analysis.
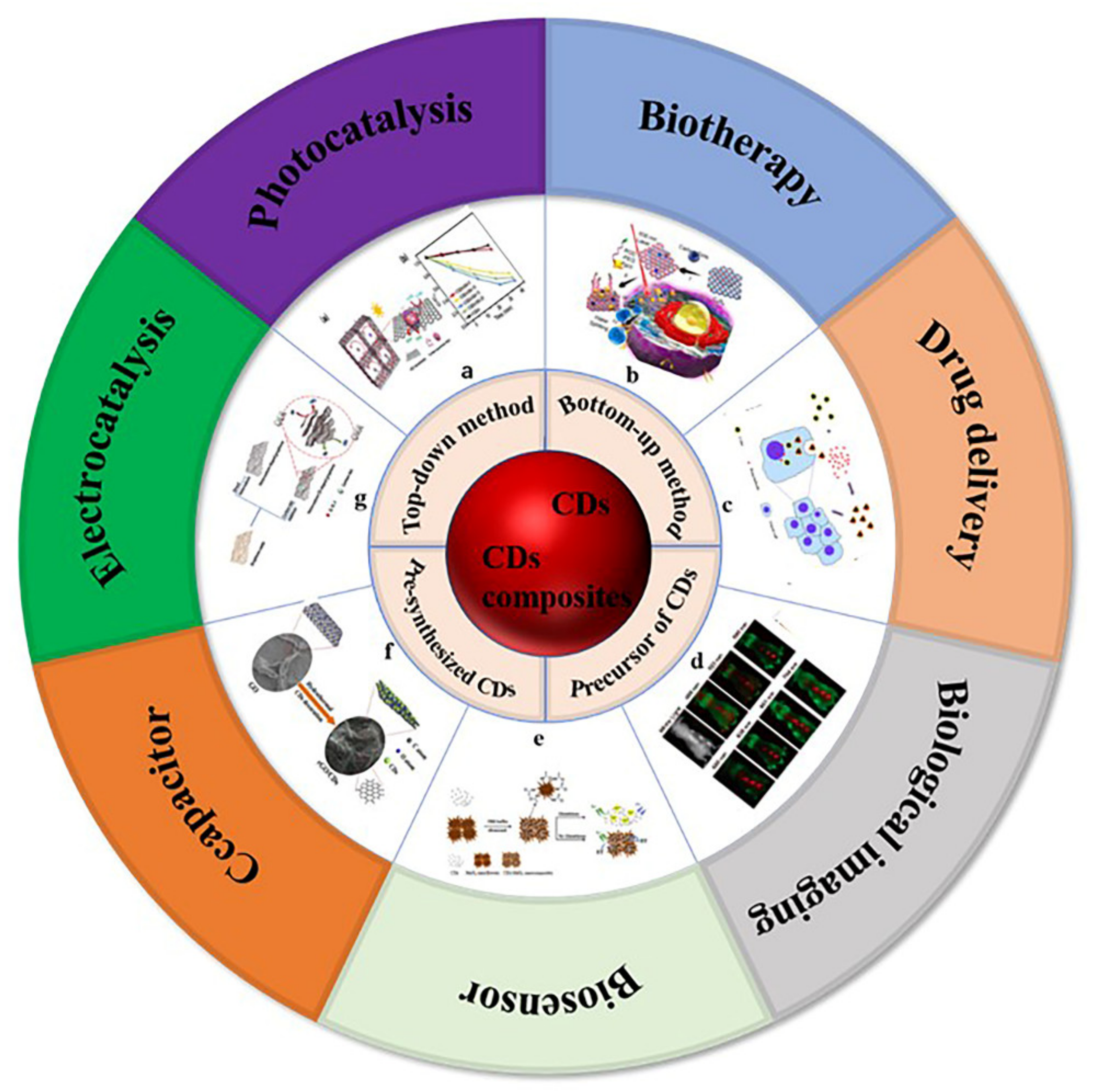
Figure 1. Preparation methods and application classification of carbon dots and carbon dots composites. (a) Photocatalysis: Photocatalytic mechanism of 3D colloidal quantum dots (CQDs)/GA composites, photocatalytic reduction of Cr (VI) with different proportions of CQDs/GA and original CQD [4]. (b) Biotherapy: Structure of PCCN and schematic diagram of 630 nm light-driven water splitting enhanced PDT [5]. (c) Drug delivery: Image of fluorescent carbon nanoparticles in medical cancer treatment [6]. (d) Biological imaging: In vivo imaging and biodistribution of the carboxylated Graphene Quantum Dots [7]. (e) Biosensor: Schematic diagram of GSH detection of CD-MnO2 nanocomposites [8]. (f) Capacitor: Schematic diagram of synthesis process of three-dimensional interconnected CD decorative reduced graphene oxide nanosheets (rGO/CDs) [9]. (g) Electrocatalysis: Schematic preparation process of NS-CD@gf [10].
2. Synthesis of Carbon Dots
There are many studies on the different synthesis methods of carbon dots. Each process aims to improve the synthesis strategy and optimize the reaction conditions so that the carbon dots are not only more cost-effective and eco-friendly, but also provide more excellent performance. According to the carbon source and the used process, the synthesis methods are mainly divided into the “top-down” method and “bottom-up” method (as shown in Figure 2).
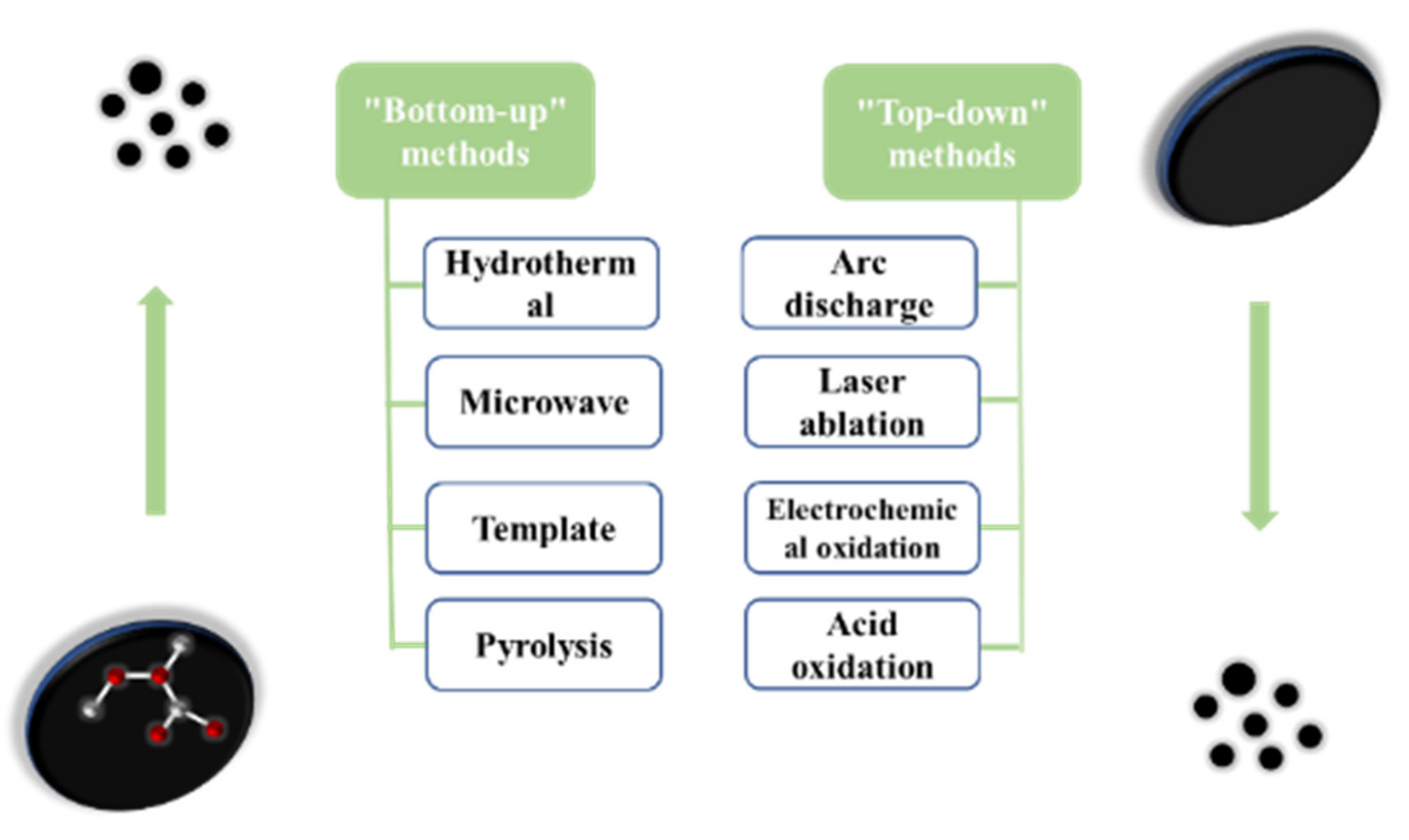
Figure 2. The two preparation methods of carbon dots.
2.1. “Top-Down” Method
The “top-down” method is a method of peeling or breaking large carbon materials to form small carbon nanoparticles, and then modifying their surfaces to improve their luminous efficiency, mainly including arc discharge method [17], laser ablation [18], electrochemical oxidation [19], and acid oxidation.
2.1.1. Arc Discharge Method
The earliest method to prepare carbon dots is the arc discharge method, which means that under a certain voltage condition, gaseous charged particles are used as conductors, and the reaction is accelerated by generating a strong current and high temperature. The advantage is that the prepared carbon dots have a small particle size and high oxygen content; the disadvantage is that the components of arc discharge are complex and there are many impurities, so the prepared carbon dots are difficult to separate and purify, the yield is very low, and the fluorescence quantum efficiency is also very low.
Research evolution: Xu et al. prepared single wall carbon nanotubes by the arc discharge method and separated the mixed products by electrophoresis. In this process, carbon dots were found [17]. In 2006, Bottini et al. [20] removed the fluorescent nanoparticles from the original carbon nanotubes and the fluorescence emission wavelength of the fluorescence nanoparticles gradually redshifted with the increase of the molecular weight of the nanoparticles, and the fluorescence emission from blue to yellow-green carbon points were obtained.
2.1.2. Laser Ablation
Laser ablation refers to the rapid laser passivation of carbon nanoparticles in organic solvents. The advantage is that the prepared carbon dots have bright, tunable, and stable photoluminescence properties; the disadvantage is that the carbon dots prepared by laser ablation usually need irradiation, oxidation, and passivation processes. The preparation method is complex, the output of carbon dots is low, the particle size distribution is uneven, and the purity is low.
Research evolution: Sun et al. prepared fluorescent carbon dots with good luminescence properties by laser ablation with graphite powder as the carbon source [21]. Firstly, graphite powder and cement were dried and solidified to make the graphite target, which was then annealed in a high-purity Ar atmosphere. Then, the graphite target was bombarded with Nd:YAG solid-state laser at 900 °C and 75 kPa, and then refluxed with concentrated nitric acid for 12 h. Finally, the surface of the previously prepared carbon nanoparticles was passivated using some simple organic substances to obtain fluorescent carbon dots. The fluorescence properties of the carbon dots prepared by this method depend on the surface post modified groups, and the emission wavelength is excitation dependent. By adjusting the excitation wavelength, the full band emission from red to blue light can be realized. Hu et al. reported an effective method to synthesize carbon dots by laser irradiation of carbon materials suspended in organic solution [22]. Firstly, a certain amount of graphite powder is dispersed in polyethylene glycol to form a black suspension, and then a Nd:YAG pulse laser with a wavelength of 1.064 mm is used. Finally, the suspension after laser irradiation was further separated and purified to obtain carbon dots.
2.1.3. Electrochemical Oxidation
Electrochemical oxidation is a method of preparing carbon quantum dots by electrolyzing some carbon materials, such as carbon nanotubes and graphite. The advantage is that the size and luminescence properties of the carbon dots can be adjusted by changing the current intensity, the preparation cost is low, and the yield is high; The disadvantage is that the fluorescence quantum efficiency of the product is low.
Research evolution: Zhou et al. prepared carbon dots by electrochemical synthesis for the first time. Firstly, the composite of multi walled carbon nanotubes and carbon film was prepared as the working electrode, Ag/AgClO4 as the reference electrode, and Pt wire as the counter electrode to form a three-electrode system [23]. An appropriate amount of tetrabutylammonium perchlorate was dissolved in an acetonitrile solution and used as an electrolyte after complete dissolution. The appropriate test conditions for a cyclic voltammetry test of the whole system are selected. In this process, the acetonitrile solution of tetrabutylammonium perchlorate changes from colorless to yellowish brown. After irradiation by UV lamp, it can emit blue fluorescence. Finally, it is separated and purified to obtain carbon dots with uniform size. Bao et al. proposed a new strategy to controllably prepare luminescent carbon dots by etching carbon fibers using the electrochemical method [24].
In this study, the target carbon dots could be prepared controllably only by adjusting the applied voltage.
2.1.4. Acid Oxidation
The acid oxidation method consists mainly of acid treatment of the carbon source to oxidize the functional groups on the surface of carbon source to generate fluorescence. The advantages are the simple preparation method and low requirements for the experimental equipment; The disadvantage is that the yield of preparing carbon dots is generally low, the separation is difficult, and the carbon dots generally require post-treatment passivation, poor control of particle size, a violent reaction process, harsh conditions, and many steps. In 2018, the N-CQDs, S-CQD, and Se-CQDs obtained by Iannazzo et al. showed tunable photoluminescence performance, higher quantum yield (QY), and longer fluorescence lifetime than pure CQDs [25]. The experimental results show that heavily doped heteroatoms will affect the photoluminescence characteristics, which is positively correlated with the electronegativity of N, S, and Se. The active heteroatoms on the surface of the CQDs will adjust the electronic structure of the corresponding CQDs. Therefore, when used as an electrocatalyst, it will have good electrocatalytic activity. On the other hand, the heavily doped CQDs have the ability to coordinate with transition metal ions. N-CQDs, S-CQD, and Se-CQDs may also have the potential to absorb other metal ions, such as Fe3+, CO2+, and Ni2+, to form so-called monatomic catalysts.2.2. “Bottom-Up” Method
The “bottom-up” method is used mainly to carbonize and assemble the molecular precursors (such as sugars, organic compounds, ethylenediamine, etc.) to synthesize carbon dots through combustion or heat treatment, and the methods used are mainly the hydrothermal method [26], microwave method [27], template method, etc.2.2.1. Water/Solvothermal Method
The hydrothermal method is used to prepare carbon dots by mixing carbon source and solvent and heating in autoclave. It is worth mentioning that the hydrothermal method is the most commonly used method to prepare carbon dots at present. Reaction temperature and reaction time are the two most important parameters, which directly affect the optical properties and quantum yield of CDs. The advantages are that the source of raw materials is very wide, the reaction equipment is simple, and the reaction conditions are easy to control. Because the reaction is carried out in a closed reactor, the influence of impurities in the air is avoided, the prepared carbon dots are relatively uniform, and most of the carbon dots synthesized by the hydrothermal method have good water solubility. The disadvantage is that due to the limited volume of the hydrothermal kettle, the amount of carbon dots prepared at one time is limited, and the prepared carbon dots will produce more impurities, which makes it difficult to separate and purify the carbon dots; it is therefore not suitable for large-scale industrial production, and the size of the prepared CDs is difficult to control, which also limits the use of this method to a certain extent. In addition, due to the use of organic solvents, the solvothermal method is more dangerous than the hydrothermal method at high temperature, and the post-treatment of excessive organic solvents after reaction also needs special consideration. Research Evolution: Peng et al. first reported the hydrothermal synthesis of fluorescent carbon dots in 2009 [28]. In this study, they first dehydrated carbohydrates with concentrated sulfuric acid to produce carbonaceous substances, then treated carbonaceous substances with nitric acid to oxidize them, and finally modified them with nitrogen-containing groups into carbon dots emitting blue fluorescence. However, the quantum yield of carbon dots synthesized by this method is not high, and the size distribution is uneven. Subsequently, the researchers found that in the process of hydrothermal synthesis of carbon dots, the size, surface chemical state, and luminescence wavelength of carbon dots can be achieved by adjusting the reaction conditions such as the type of carbon source and solvent. In 2013, Zhu et al. synthesized high fluorescence carbon dots using the hydrothermal method, and the proposed citric acid and ethylenediamine system is very classic in the field of carbon dots [29]. Citric acid and ethylenediamine undergo condensation reaction under the action of aqueous solvent to form polymer-like carbon dots, which are further carbonized to form carbon dots with an average size of 2–6 nm. The carbon dots prepared by this method have uniform size distribution, and their luminous effect is comparable to that of fluorescent dyes. In 2015, Jiang et al. used o-diphenylamine, p-Diphenylamine, and m-diphenylamine as carbon sources to synthesize carbon dots emitting red, green, and blue fluorescence in the ethanol solvent by the solvothermal method [30]. In this study, the isomers of phenylenediamine were used as carbon sources to react with the ethanol solvent. The mixed products were separated by silica gel column to obtain carbon dots with different emission wavelengths. In 2017, Raji et al. synthesized carbon dots using monk fruit as the carbon source using the hydrothermal method. The monk fruit was sealed in an autoclave, heated at 180 °C for 6 h, and the resulting solution was cooled to room temperature. The solution changed from light yellow to dark brown. The monk fruit was dehydrated, polymerized, and carbonized to finally form carbon dots with blue fluorescence [26].2.2.2. Microwave Method
Microwave heating is a method that uses a microwave to raise the temperature rapidly in a short time to polymerize and carbonize the reactant monomers to form carbon dots. It provides a new and rapid method for the synthesis of carbon dots. Experiments show that constant high-pressure reaction vessel is the best method to control the particle size distribution and photoluminescence properties of CDs [31]. The advantages are that it is convenient and fast, the preparation conditions are simple, raw materials are easily available, etc. The disadvantage is that the reaction process is unstable, the reaction temperature is difficult to control, and the fluorescence quantum yield is low. Research evolution: In 2009, Zhu et al. synthesized carbon dots under microwave-assisted reaction conditions with polyethylene glycol and sugars as the carbon sources [32]. They put all the reactants in the microwave oven and set the power to 500 W and the reaction time to 2–10 min. It could be observed that the color of the reaction solution experienced a change of colorless to a yellow dark-brown. Finally, the blue fluorescent carbon dots were obtained through separation and purification. By changing the reaction time, the size, emission peak position and quantum yield of carbon dots can effectively be controlled. In 2012, Tang et al. synthesized carbon dots emitting deep UV fluorescence using glucose as the carbon source by microwave-assisted hydrothermal synthesis [33]. In 2015, Pan et al. used formamide and citric acid as the carbon sources and microwave-heated them at 160 °C for 60 min. After separation and purification, they obtained fluorescent carbon dots with full-spectrum emission [30]. In 2020, Li et al. used guanine and ethylenediamine as the carbon sources and deionized water as the solvent, heated it in a household microwave oven with a set power of 700 W for 10 min, and removed large impurities with a dialysis bag to obtain an aqueous solution of carbon dots [26]. The carbon dots prepared by this method have excellent water solubility, excellent stability at different pH, and extremely high ionic strength, and have been successfully used as fluorescent probes to detect Ag+.2.2.3. Template Method
The advantage of template synthesis of carbon dots is that the size of the carbon dots can be controlled by the size of template, and the aggregation of the carbon dots can be reduced in the process of synthesis. The prepared carbon dots have a uniform size and high fluorescence quantum yield; The disadvantage is that some templates are difficult to separate from the carbon dots, and the fluorescence performance of the carbon dots may be affected in the process of acid-base etching or heating to remove templates. The advantages are that the size of the carbon dots can be controlled by the size of the template, and the aggregation of the carbon dots can be reduced in the process of synthesis. The prepared carbon dots have a uniform size and high fluorescence quantum yield. The disadvantage is that some templates are difficult to separate from the carbon dots, and the fluorescence performance of the carbon dots may be affected in the process of acid-base etching or heating to remove the templates. Research evolution: In 2012, Kwon et al. first synthesized fluorescent carbon dots with a size of 1.403 ± 0.148 nm using glucose as the carbon source by using the micro lotion template method [34]. Firstly, the water in the oil microemulsion was formed by mixing 10% glucose aqueous solution with 1-octanol in oil phase. Then, cetylammonium was added for heating and carbonization. Finally, cetylammonium modified fluorescent carbon dots were obtained. In this process, the number of carbon sources in each micelle can be limited by adjusting the concentration of micro lotion, and the size of carbon dots can be further controlled. In 2013, Yang et al. first proposed the method of soft hard template, using copolymer P123 as the soft template and ordered mesoporous silica as the hard template to prepare fluorescent carbon dots [35].References
- Zheng, M.; Liu, K.M.; Su, Y. Carbon Dots for Biomedical Applications. Chin. J. Lumin. 2021, 42, 1233–1244.
- Zhang, Z.; Qu, D.; An, L.; Wang, X.Y.; Sun, Z.C. Preparation, Luminescence Mechanism and Application of Fluorescent Carbon Dots. Chin. J. Lumin. 2021, 42, 1125–1140.
- Cao, W.B.; Sun, Z.G.; Wu, Y.H.; Zhang, Y.H.; Zhan, Y. Progresses in preparation and application of organosilane functionalized carbon dots. Acta Mater. Compos. Sin. 2022, 39, 896–906.
- Wang, R.; Lu, K.-Q.; Zhang, F.; Tang, Z.-R.; Xu, Y.-J. 3D carbon quantum dots/graphene aerogel as a metal-free catalyst for enhanced photosensitization efficiency. Appl. Catal. B Environ. 2018, 233, 11–18.
- Permatasari, F.A.; Fukazawa, H.; Ogi, T.; Iskandar, F.; Okuyama, K. Design of pyrrolic-N-rich carbon dots with absorption in the first near-infrared window for photothermal therapy. ACS Appl. Nano Mater. 2018, 1, 2368–2375.
- Jaleel, J.A.; Pramod, K. Artful and multifaceted applications of carbon dot in biomedicine. J. Control. Release 2018, 269, 302–321.
- Tao, H.; Yang, K.; Ma, Z.; Wan, J.; Zhang, Y.; Kang, Z.; Liu, Z. In vivo NIR fluorescence imaging, biodistribution, and toxicology of photoluminescent carbon dots produced from carbon nanotubes and graphite. Small 2012, 8, 281–290.
- Wu, J.; Chen, G.; Jia, Y.; Ji, C.; Wang, Y.; Zhou, Y.; Leblanc, R.M.; Peng, Z. Carbon dot composites for bioapplications: A review. J. Mater. Chem. B 2022, 10, 843–869.
- Zhao, X.; Li, M.; Dong, H.; Liu, Y.; Hu, H.; Cai, Y.; Liang, Y.; Xiao, Y.; Zheng, M. Interconnected 3 D network of graphene-oxide nanosheets decorated with carbon dots for high-performance supercapacitors. ChemSusChem 2017, 10, 2626–2634.
- Shin, J.; Guo, J.; Zhao, T.; Guo, Z. Functionalized carbon dots on graphene as outstanding non-metal bifunctional oxygen electrocatalyst. Small 2019, 15, 1900296.
- Du, F.; Zhang, L.; Zhang, L.; Zhang, M.; Gong, A.; Tan, Y.; Miao, J.; Gong, Y.; Sun, M.; Ju, H. Engineered gadolinium-doped carbon dots for magnetic resonance imaging-guided radiotherapy of tumors. Biomaterials 2017, 121, 109–120.
- Liao, H.; Wang, Z.; Chen, S.; Wu, H.; Ma, X.; Tan, M. One-pot synthesis of gadolinium (III) doped carbon dots for fluorescence/magnetic resonance bimodal imaging. RSC Adv. 2015, 5, 66575–66581.
- Ren, X.; Yuan, X.; Wang, Y.; Liu, C.; Qin, Y.; Guo, L.; Liu, L. Facile preparation of Gd3+ doped carbon quantum dots: Photoluminescence materials with magnetic resonance response as magnetic resonance/fluorescence bimodal probes. Opt. Mater. 2016, 57, 56–62.
- Zhang, J.; Yu, S.-H. Carbon dots: Large-scale synthesis, sensing and bioimaging. Mater. Today 2016, 19, 382–393.
- Hou, Y.; Lu, Q.; Deng, J.; Li, H.; Zhang, Y. One-pot electrochemical synthesis of functionalized fluorescent carbon dots and their selective sensing for mercury ion. Anal. Chim. Acta 2015, 866, 69–74.
- Dong, Y.; Cai, J.; You, X.; Chi, Y. Sensing applications of luminescent carbon based dots. Analyst 2015, 140, 7468–7486.
- Xu, X.; Ray, R.; Gu, Y.; Ploehn, H.J.; Gearheart, L.; Raker, K.; Scrivens, W.A. Electrophoretic analysis and purification of fluorescent single-walled carbon nanotube fragments. J. Am. Chem. Soc. 2004, 126, 12736–12737.
- Hu, S.-L.; Niu, K.-Y.; Sun, J.; Yang, J.; Zhao, N.-Q.; Du, X.-W. One-step synthesis of fluorescent carbon nanoparticles by laser irradiation. J. Mater. Chem. 2009, 19, 484–488.
- Qiao, Z.-A.; Wang, Y.; Gao, Y.; Li, H.; Dai, T.; Liu, Y.; Huo, Q. Commercially activated carbon as the source for producing multicolor photoluminescent carbon dots by chemical oxidation. Chem. Commun. 2010, 46, 8812–8814.
- Bottini, M.; Balasubramanian, C.; Dawson, M.I.; Bergamaschi, A.; Bellucci, S.; Mustelin, T. Isolation and characterization of fluorescent nanoparticles from pristine and oxidized electric arc-produced single-walled carbon nanotubes. J. Phys. Chem. B 2006, 110, 831–836.
- Sun, Y.-P.; Zhou, B.; Lin, Y.; Wang, W.; Fernando, K.S.; Pathak, P.; Meziani, M.J.; Harruff, B.A.; Wang, X.; Wang, H. Quantum-sized carbon dots for bright and colorful photoluminescence. J. Am. Chem. Soc. 2006, 128, 7756–7757.
- Xu, D.; Lei, F.; Chen, H.; Yin, L.; Shi, Y.; Xie, J. One-step hydrothermal synthesis and optical properties of self-quenching-resistant carbon dots towards fluorescent ink and as nanosensors for Fe3+ detection. RSC Adv. 2019, 9, 8290–8299.
- Zhou, J.; Booker, C.; Li, R.; Zhou, X.; Sham, T.-K.; Sun, X.; Ding, Z. An electrochemical avenue to blue luminescent nanocrystals from multiwalled carbon nanotubes (MWCNTs). J. Am. Chem. Soc. 2007, 129, 744–745.
- Bao, L.; Zhang, Z.L.; Tian, Z.Q.; Zhang, L.; Liu, C.; Lin, Y.; Qi, B.; Pang, D.W. Electrochemical tuning of luminescent carbon nanodots: From preparation to luminescence mechanism. Adv. Mater. 2011, 23, 5801–5806.
- Iannazzo, D.; Pistone, A.; Ferro, S.; De Luca, L.; Monforte, A.M.; Romeo, R.; Buemi, M.R.; Pannecouque, C. Graphene quantum dots based systems as HIV inhibitors. Bioconjug. Chem. 2018, 29, 3084–3093.
- Atchudan, R.; Edison, T.N.J.I.; Chakradhar, D.; Perumal, S.; Shim, J.-J.; Lee, Y.R. Facile green synthesis of nitrogen-doped carbon dots using Chionanthus retusus fruit extract and investigation of their suitability for metal ion sensing and biological applications. Sens. Actuators B Chem. 2017, 246, 497–509.
- Wang, B.; Yu, J.; Sui, L.; Zhu, S.; Tang, Z.; Yang, B.; Lu, S. Rational Design of Multi-Color-Emissive Carbon Dots in a Single Reaction System by Hydrothermal. Adv. Sci. 2021, 8, 2001453.
- Peng, H.; Travas-Sejdic, J. Simple aqueous solution route to luminescent carbogenic dots from carbohydrates. Chem. Mater. 2009, 21, 5563–5565.
- Zhu, S.; Meng, Q.; Wang, L.; Zhang, J.; Song, Y.; Jin, H.; Zhang, K.; Sun, H.; Wang, H.; Yang, B. Highly photoluminescent carbon dots for multicolor patterning, sensors, and bioimaging. Angew. Chem. 2013, 125, 4045–4049.
- Jiang, K.; Sun, S.; Zhang, L.; Lu, Y.; Wu, A.; Cai, C.; Lin, H. Red, green, and blue luminescence by carbon dots: Full-color emission tuning and multicolor cellular imaging. Angew. Chem. Int. Ed. 2015, 54, 5360–5363.
- Strauss, V.; Margraf, J.T.; Dolle, C.; Butz, B.; Nacken, T.J.; Walter, J.; Bauer, W.; Peukert, W.; Spiecker, E.; Clark, T. Carbon nanodots: Toward a comprehensive understanding of their photoluminescence. J. Am. Chem. Soc. 2014, 136, 17308–17316.
- Zhu, H.; Wang, X.; Li, Y.; Wang, Z.; Yang, F.; Yang, X. Microwave synthesis of fluorescent carbon nanoparticles with electrochemiluminescence properties. Chem. Commun. 2009, 4, 5118–5120.
- Tang, L.; Ji, R.; Cao, X.; Lin, J.; Jiang, H.; Li, X.; Teng, K.S.; Luk, C.M.; Zeng, S.; Hao, J. Deep ultraviolet photoluminescence of water-soluble self-passivated graphene quantum dots. ACS Nano 2012, 6, 5102–5110.
- Kwon, W.; Do, S.; Rhee, S.W. Formation of highly luminescent nearly monodisperse carbon quantum dots via emulsion-templated carbonization of carbohydrates. RSC Adv. 2012, 2, 11223–11226.
- Yang, Y.; Wu, D.; Han, S.; Hu, P.; Liu, R. Bottom-up fabrication of photoluminescent carbon dots with uniform morphology via a soft–hard template approach. Chem. Commun. 2013, 49, 4920–4922.
More
