Mesenchymal stem cells (MSCs) have been reported as promising candidates for the treatment of various diseases, especially allergic diseases, as they have the capacity to differentiate into various cells. MSCs itself has several limitations such as risk of aneuploidy, difficulty in handling, immune rejection, tumorigenicity, so interest in the extracellular vesicles (EVs) released from MSCs is increasing and many studies have been reported.
Mesenchymal stem cells (MSCs) have been reported as promising candidates for the treatment of various diseases, especially allergic diseases, as they have the capacity to differentiate into various cells. However, MSCs itself has several limitations such as risk of aneuploidy, difficulty in handling, immune rejection, tumorigenicity, so interest in the extracellular vesicles (EVs) released from MSCs is increasing and many studies have been reported. Previous studies have shown that extracellular vesicles (EVs) produced by MSCs is as effective as the MSCs themselves in suppression of allergic airway inflammation through the suppression of Th2 cytokine production and induction of regulatory T cells (Treg) expansion. EVs is one of the substances secreted by paracrine induction from MSCs, and because it exerts its effect by delivering contents such as mRNA, microRNA, and protein to the receptor cell, it can reduce the problems or risks related to stem cell therapy.
Previous studies have shown that extracellular vesicles (EVs) produced by MSCs is as effective as the MSCs themselves in suppression of allergic airway inflammation through the suppression of Th2 cytokine production and induction of regulatory T cells (Treg) expansion. EVs is one of the substances secreted by paracrine induction from MSCs, and because it exerts its effect by delivering contents such as mRNA, microRNA, and protein to the receptor cell, it can reduce the problems or risks related to stem cell therapy.
This article reviews the immunomodulatory properties of MSCs-derived EVs and their therapeutic implications for allergic airway disease.
- mesenchymal stem cells
- extracellular vesicles
- asthma
- allergic rhinitis
1. Introduction
2. Immunomodulatory Effects of EVs for Allergic Airway Diseases
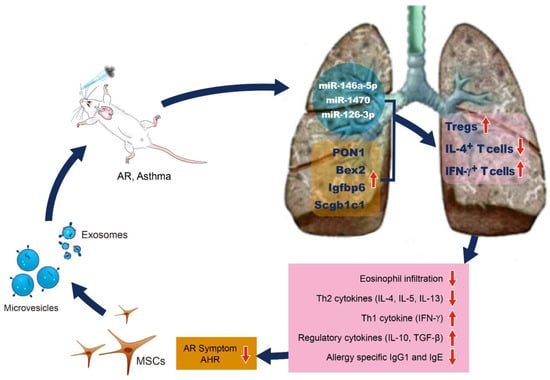
MSCs could improve the allergic airway disease by inhibiting the proliferation and function of dendritic cells which have an immunomodulatory effect, and which are differentiated into T cells and B cells [27][28][29][95,96,97]. The intravenous injection of MSCs significantly reduced eosinophil infiltration in the nasal mucosa and lung tissue of allergic mouse models and improved the degree of airway hypersensitivity and allergic symptoms [30][31][32][24,25,26]. The MSCs administrated intravenously decreased the number of Th2 cytokines such as IL-4, IL-5, IL-13, and IL-4-positive CD4+ T cells, but they increased the number of Th1 cytokine, IFN- γ and IFN- γ positive CD4+ T cells in the bronchoalveolar lavage (BAL) fluid and lung draining lymph nodes (LLNs) in an AR and asthma mouse model. The MSCs resulted in a significant decrease in the total and ovalbumin (OVA)-specific IgE and IgG1 levels. Tregs, which is characterized by the expression of transcription factor Foxp3, was significantly increased in the LLNs of asthmatic mice after the MSCs administration. Various soluble factors, including TGF-β and PGE2, are secreted by the MSCs that have migrated to the lungs by intravenous or nasal routes of administration, leading to the expansion of Tregs. Anti-inflammatory cytokines (IL-10 and TGF-β) are secreted by Tregs, which ultimately reduce the amount of pulmonary eosinophil infiltration as well as the production of allergy-specific Th2 cytokines and Ig. [30][31][32][24,25,26]. Additionally, in lung histology, eosinophil infiltration and inflammatory cell deposition in the peribronchial and perivascular areas were significantly decreased in the EV group compared to that of the asthma-inducing group [33][98]. Several studies have reported that the MSC secretomes and MSCs-derived EVs show the same immunomodulatory effects as the stem cells themselves do in allergic airway diseases [24][34][35][36][32,99,100,101]. Previous studies have shown that bone marrow, umbilical cord, and adipose tissue-derived MSCs and their EVs have the similar immunomodulatory effects in asthmatic mice. Therefore, the efficacy of MSCs-derived EVs does not depend on the MSC source tissue. Furthermore, the systemic and intranasal administration of MSC-derived EVs showed similar immunosuppressive effects in asthmatic mice [34][99]. De Castro et al. reported that intravenous stem cell culture media and EVs significantly reduced the degree of airway hypersensitivity and eosinophil infiltration of the lung tissues in a mouse model of asthma in the same manner as stem cells did, and the levels of IL-4, IL-5, and IL-6 were significantly reduced. Furthermore, the intravenous administration of human adipose tissue-derived MSCs and EVs reduced the total number of inflammatory cells and the ratio of eosinophils in BAL fluid, IL-5 levels in lung tissues and CD3+ CD4+ T cells in the thymus. However, the number of eosinophils in the lung tissues, the levels of IL-4, IL-13, eotaxin, and CD3+ CD4+ T cells in the BALF, and the pulmonary function showed inconsistent results [35][100]. Recently, intranasally administrated ASC-derived EVs significantly reduced the degree of allergic airway inflammation and improved AHR through induction of Tregs expansion in asthmatic mice. The intranasal administration of ASC-derived EVs to asthmatic mice resulted in a remarkable reduction of eosinophils and inflammatory cells in the BAL fluid, the serum total and the OVA-specific IgE levels, and the degree of eosinophilic lung inflammation. The level of IL-4 was significantly decreased in the BAL fluid and LLNs, whereas IFN-γ was significantly increased in the BAL fluid. Additionally, CD4+IL-4+ T cells were markedly decreased after an ASC-derived EV treatment, whereas the CD4+CD25+Foxp3+ T cells and CD4+IFN-γ+ T cells were notably increased in the LLNs of asthmatic mice [33][98]. In an in vitro study, the authors isolated EVs from culture supernatants of murine ASC, which were evaluated the immunomodulatory effects of EVs on Th2-mediated inflammation which was induced by Aspergillus protease antigens in mouse lung epithelial cells and primary lung epithelial cells. Cho et al. reported that ASC-derived EVs suppressed Th2-mediated inflammation through the upregulation of TGF-β and IL-10 and the downregulation of IL-25 and eotaxin which stimulate the release and recruitment of eosinophils to the sites of inflammation synergistically with IL-5. Furthermore, the ASC-derived EVs induced an anti-inflammatory state in Th2-mediated inflammation through polarization to the M1 and M2 macrophages and dendritic cell maturation for effector T cell induction [36][101]. The functional enhancement of gene analysis and the microRNA expression pattern analysis, which are methods used to identify a set of overexpressed genes or proteins, have been performed, and studies on the expression pattern and differential expression characteristics of specific genes have been reported. Kim et al. performed DNA microarray to identify the differentially expressed genes (DEGs) related to the suppression of allergic airway inflammation by ASCs-derived EVs. After the hierarchical clustering of DEGs and after the functional and pathway analysis of the potential DEGs, a total of 249 DEGs were identified, of which 21 were upregulated in the EVs group, resulting in more than 2-fold changes compared to that which was seen in the OVA group. These results suggest that PON1, Bex2, Igfbp6, and Scgb1c1 may be involved in the immunosuppressive mechanism mechanisms of MSCs-derived EVs in allergic airway diseases [37][102]. Paraoxonase1 (PON1) is a calcium-dependent aryldialkylphosphatase belonging to the paraoxonase (PON) family, and it has antioxidant, anti-adhesive, anti-inflammatory, anti-thrombotic, and anti-apoptotic effects. In addition to asthma, various diseases such as diabetes, rheumatism, arthritis, psoriasis, and systemic lupus erythematosus are also associated with PON1 [38][39][40][41][42][43][103,104,105,106,107,108]. The expression and activity of PON1 in asthmatic patients were significantly lower when they were compared to those of the healthy controls [39][44][45][104,109,110]. Furthermore, PON1 reduced the degree of airway inflammation and airway remodeling and inhibited the lipopolysaccharide (LPS)-induced inflammatory cytokine expression and lung fibroblast proliferation in asthmatic mice, thereby having significant potential effects in allergic airway disease [46][111]. Bex2 is a protein-coding gene known to be involved in carcinogenesis and it is a regulator of mitochondrial apoptosis and the G1 cell cycle, particularly in breast cancer [47][112]. Although few reports have been reported on allergic diseases, a recent study reported that Bex2 expression was suppressed by the increased DNA methylation of IL-13 which was induced in allergic airway inflammation [48][113]. Igfbp6 is a family of insulin-like growth factor (IGF) binding proteins related to the growth inhibitory protein regulating the availability of IGFs. The family of proteins binding to IGFs includes Igfbp1, Igfbp2, Igfbp3, Igfbp4, and Igfbp5 in addition to Igfbp6 [49][114]. The biological functions of Igfbp can be classified into IGF-independent and IGF-dependent actions. In particular, Igfbp has been reported as a biomarker and a therapeutic target acting on the pathogenesis of various autoimmune diseases, and Igfbp6 was associated with fibroblast proliferation and cell growth in asthmatic patients [50][51][115,116]. Fpr1 is a group of G protein-coupled cell surface receptors that have important roles in inflammation and host defenses. Since Fpr1 are expressed across a variety of cell types and interact with structurally diverse chemotactic agents, they either accelerate or inhibit the inflammatory processes upon binding to other ligands [52][117]. In allergic airway disease, Fpr1 has been reported to stimulate neutrophil chemotaxis and inflammatory cytokine production by phagocytes such as dendritic cells and macrophages [53][118]. Scgb1c1 is a member of the secretoglobin family of secreted proteins which are found in high concentrations in body fluids of the lungs, lacrimal glands, salivary glands, prostate, uterus, and in other tissues. In the human respiratory mucosa, Scgb1c1 is upregulated by IL-4 and IL-13, and it is downregulated by IFN-γ, and it plays an important role in recognizing and clearance of pathogenic microorganisms in the lung epithelial mucosa [54][55][56][119,120,121]. The important pulmonary genes associated with suppression of allergic airway inflammation by MSC-derived EVs are summarized in Table 1.| Genes | Description | General Characteristics | Potential Effects in Allergic Airway Disease | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PON1 | Family of PON related to calcium-dependent aryldialkylphosphatase | Antioxidant, anti-adhesive, anti-inflammatory, anti-thrombotic, and anti-apoptotic effects [38][39][40[43] | Antioxidant, anti-adhesive, anti-inflammatory, anti-thrombotic, and anti-apoptotic effects [103,104 | ][ | ,105 | 41][ | ,106 | 42] | ,107,108] | Reduced airway inflammation and airway remodeling and inhibited LPS-induced inflammatory cytokine expression and lung fibroblast proliferation in asthmatic mice [44][45][46] | Reduced airway inflammation and airway remodeling and inhibited LPS-induced inflammatory cytokine expression and lung fibroblast proliferation in asthmatic mice [109,110,111] |
| Bex2 | Family of brain expressed X-linked gene and protein-coding gene, highly expressed in brain, pancreas, and testis | Carcinogenesis, regulator of mitochondrial apoptosis and the G1 cell cycle in breast cancer [47] | Carcinogenesis, regulator of mitochondrial apoptosis and the G1 cell cycle in breast cancer [112] | Associated with inhibition of IL-13 induced in allergic airway inflammation [48] | Associated with inhibition of IL-13 induced in allergic airway inflammation [113] | ||||||
| Igfbp6 | Family of IGFBP related to growth inhibitory protein that regulate the availability of insulin-like growth factors | Biomarker and therapeutic target acting on the pathogenesis of various autoimmune diseases [51] | Biomarker and therapeutic target acting on the pathogenesis of various autoimmune diseases [116] | Associated with fibroblast proliferation and cell growth in asthma [50] | Associated with fibroblast proliferation and cell growth in asthma [115] | ||||||
| Fpr1 | Family of FPR, group of G protein-coupled cell surface receptors of mammalian phagocytic cells | Important roles in host defense as well as inflammatory responses including cell adhesion, directed migration, granule release, and superoxide production [52] | Important roles in host defense as well as inflammatory responses including cell adhesion, directed migration, granule release, and superoxide production [117] | Associated with stimulation of neutrophil chemotaxis and inflammatory cytokine production by phagocytes such as dendritic cells and macrophages [53] | Associated with stimulation of neutrophil chemotaxis and inflammatory cytokine production by phagocytes such as dendritic cells and macrophages [118] | ||||||
| Scgb1c1 | Family of secretoglobin secreted proteins found in high concentrations in body fluids of the lungs, lacrimal glands, salivary glands, prostate, uterus, and other tissues | Localized to Bowman’s glands in the olfactory mucosa [55] | Localized to Bowman’s glands in the olfactory mucosa [120] | Upregulated by IL-4, IL-13 and downregulated by IFN-γ, and it plays an important role in recognizing and clearance of pathogenic microorganisms in the lung epithelial mucosa [54][55][56] | Upregulated by IL-4, IL-13 and downregulated by IFN-γ, and it plays an important role in recognizing and clearance of pathogenic microorganisms in the lung epithelial mucosa [119,120,121] |