The coenzyme Q10 is a naturally occurring benzoquinone derivative widely prescribed as a food supplement for different physical conditions and pathologies. Thanks to its favourable combination of functional activity and safety profile, it is widely prescribed for an ever increasing number of physical conditions [1,2].
Ageing, myopathy, cardiomyopathy, high blood pressure, dyslipidemia, migraine, diabetes, infertility, Friedreich's ataxia, and neurologic disorders like Parkinson’s and Huntington’s diseases, are but a few examples that today prompt practitioners to prescribe CoQ10 [3]. To this already extensive list, fibromyalgia has recently been added, once it has been clear that CoQ10 deficiency and mitochondrial dysfunction are both implicated in its pathophysiology.
12. Fibromyalgia: Key Characteristics and Therapeutic Approaches
Fibromyalgia (FM) is a pathology characterized by chronic and widespread musculoskeletal pain, often associated with asthenia and fatigue and a large set of somatic and neuro-vegetative symptoms. Sleep disorders are also present, characterized by frequent nocturnal awakenings which exacerbate the fatigue condition. In particular, the so-called alpha-delta anomaly is considered to be specific to FM, as once deep sleep is reached, characterized by delta waves at the electroencephalogram, there is a sharp return to superficial sleep, characterized by alpha waves. Cognitive disorders are present in the majority of patients, being related to difficulty in concentration and short-term memory loss
[1][5].
To date, the prevalence of FM is estimated between 5 and 7%, while the incidence level ranges between 7–11 cases per 1000 people per year. It is more frequent in women than in men, with 11 vs. 7 cases per 1000 people, respectively
[2][3][6,7]. Moreover, it can develop at any age. Actually, a juvenile fibromyalgia syndrome (JFM) is known constituting a complex condition that affects approximately 2–7% of school-aged children showing chronic musculoskeletal and diffuse pain, fatigue, and sleep and mood disturbances
[4][8]. At the same time, FM can also be diagnosed in people at an advanced age. According to some reports, the prevalence of FM reaches a peak between the age of 55–65 years, and the main challenge is a timely and correct diagnosis as patients are frequently identified with rheumatoid arthritis, arthrosis, and rheumatic polymyalgia
[5][9]. Obesity is also commonly associated with FM
[6][10], with a prevalence ranging from 47 to 73%
[7][11]. Stress, depression, anxiety, chronic sleep deprivation, and low physical activity have been linked to an increase in body weight in FM patients. Vincent and co-workers recently assessed the complex relationship between BMI, physical, and psychological factors in these patients but failed to highlight a clear relationship. In fact, they found that with an increase in BMI, it becomes more difficult for the patient to engage in physical activity, leading to a worsening of the symptoms of FM
[7][11].
Currently, the diagnosis of FM is carried out according to the American College of Rheumatology (ACR) 2016 criteria, based on the scores of the widespread pain index (WPI) and the symptom severity scale (SSS)
[8][12]. Mainly based on clinical parameters, these diagnostic criteria frequently overlap with those of other diseases, thus, making FM hard to uncover. In fact, on average, a period of 2.3 years from the first complaint is necessary to get to a definitive diagnosis, excluding those diseases having symptoms, but not causes, common to FM by the evaluation of selected markers.
For some time now, researchers have been calling for specific biomarkers. As thoroughly reviewed by Ablin and co-workers, it should be possible to highlight both a predisposition to FM (from a genetic point of view) and the manifestations of the disease. However, those investigated so far, including serological alterations and instrumental investigations, are for research purposes only
[9][13].
The etiology of FM has not yet been fully understood, and uncertainty still exists concerning the pathophysiological framework. The preeminent hypothesis calls into question a dysregulation in the mechanisms of pain control by the central nervous system (CNS)
[10][11][12][13][14][15][16][17][18][14,15,16,17,18,19,20,21,22], while according to other studies, FM would be supported by an inflammation of the small peripheral fibers
[19][23].
Anyhow, FM is clearly characterized by mitochondrial dysfunction
[20][24]. Sprott and co-workers demonstrated a change in the number and size of mitochondria in patients with symptomatic FM
[21][25], and this evidence has been corroborated by additional studies, proving reduced mitochondrial DNA concentrations and CoQ
10 levels accompanied by the increased expression of pro-inflammatory interleukins IL-1β and IL-18, higher levels of TNF-α
[22][26], and the activation of NLRP3 (NOD-like receptor family, pyrin domain containing 3) and caspase-1. Also, Bullon and co-workers demonstrated that AMPK was not phosphorylated in the fibroblasts of FM patients, and this was in charge of decreased mitochondrial biogenesis, reduced oxygen consumption, decreased antioxidant-enzyme-expression levels, and mitochondrial dysfunction. Moreover, AMPK impairment also results in a marked NLRP3 inflammasome protein activation and a subsequent increase in the serum levels of IL-1β and IL-18
[23][24][27,28].
The multifaceted nature of FM requires a multimodal and multidisciplinary approach, mainly aimed at reducing the severity of the symptoms. However, on the basis of the scientific evidence available to date, there are no fully satisfying protocols. Moreover, poor adherence to the therapeutic indications and the presence of comorbidities are two very frequent factors that can modulate the worsening of symptomatology, leading to its chronicity
[25][26][27][28][29,30,31,32].
Over the last decade, the use of personalized dietary regimes has become increasingly important. The supplementation of minerals, such as selenium, zinc, iron, and magnesium, but also vitamins like B9, B12, A, and D, as well as antioxidants, is pursued to complement the nutritional strategy, avoiding nutritional deficits, taking control of oxidative stress, and supporting the immune system
[29][30][31][32][33][33,34,35,36,37]. Among the plethora of compounds exploited for their antioxidant properties, CoQ
10 has the preeminent role of supplementing FM patients, as is thoroughly discussed below.
23. Coenzyme Q10 as a Key Functional Derivative
Coenzyme Q (CoQ) is a widely distributed naturally occurring lipophilic benzoquinone, firstly discovered by Crane and co-workers more than 60 years ago in beef mitochondria
[34][38]. From a chemical point of view, the molecule is characterized by the presence of a quinoid structure, bearing a main side chain comprised of the repetition of several isoprenyl units. As for humans and many mammals, 10 residues are featured in the predominant form of the coenzyme, while other homologous forms are also present. Most of the CoQ
10 in our body is produced endogenously, but a small amount is also ingested daily through the diet, being mainly present in meat and fish and, in much lower quantities, in some vegetables. Due to its wide prevalence in living organisms, this coenzyme is further referred to as ubiquinone
[35][39].
Since the first pioneering study by Crane and co-workers, the crucial function of CoQ10 in the mechanisms of ATP production at the mitochondrial level was deciphered and acknowledged. In particular, the intense research activity carried out by Peter Mitchell, who, thanks to these studies, was awarded the Nobel Prize, clarified CoQ10 role as a key cofactor in the oxidative phosphorylation.
As thoroughly reviewed by several authors
[36][37][38][40,41,42], CoQ
10 acts as a mobile electron carrier in the electron transfer chain at the inner mitochondrial membrane, but it is also located in blood lipoproteins and cell membranes, where it plays a key antioxidant role once in its reduced form. Indeed, as CoQ
10H
2, it is able to quench free radicals and regenerate α-tocopherol and ascorbate
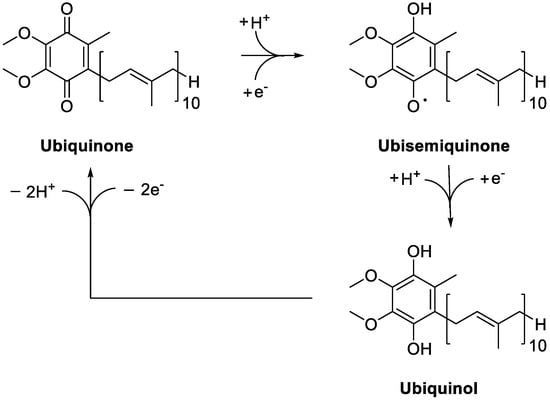
[39][40][41][43,44,45]. Several NAD(P)H-dependent reductases ensure the generation of ubiquinol from ubiquinone, possibly via the ubisemiquinone intermediate, depending on the oxidation state of the cofactor (
Figure 12). They include NAD(P)H:quinone reductase 1 (NQO1), catalyzing the reduction of the coenzyme to CoQH
2 through a two-electron reaction, and NADH-cytochrome
b5 reductase and NADPH-cytochrome P450 reductase, which are one-electron reductases
[42][43][44][45][46][47][48][46,47,48,49,50,51,52]. Combined with ubiquinol, these enzymes give rise to the trans-plasma membrane antioxidant system, contributing to the maintenance of the cell antioxidant activities.
Figure 12.
Redox cycling of Coenzime Q
10
.
In addition to redox activity, more recent data revealed that CoQ
10 influences the expression of a significant number of genes. Investigated in HeLa cells by Gorelick and co-workers, it was proved to alter 264 sequences, enriching, in particular, lipid-related genes
[49][53]. Using the human intestinal cell line CaCo-2, Groneberg and co-workers demonstrated that CoQ
10 treatment increases the expression of 694 genes encoding proteins involved in cell signaling, intermediary metabolism, transport, transcription control, disease mutation, phosphorylation, embryonal development, and binding
[50][51][54,55], thus, confirming the crucial functional role of this compound.
The number of investigations aiming at demonstrate the effectiveness of CoQ10 in the management of FM has been increasing over time, outlining a positive causal relationship between the amount of its supplementation and the relief in FM symptoms, first and foremost the feeling of fatigue. Therefore, according on the outcomes obtained so far, CoQ10 might be reasonably regarded as the gold standard supplement for people affected by FM.
However, a closer look at the available data highlights the lack of a sound and clear scientific basis justifying this trumpeted claim. Actually, most, but not all, the authors highlighted reduced levels of CoQ10 in FM patients, therefore the consensus of the scientific community on this condition is not unanimous. Moreover, scientific investigations claiming the functional efficacy of this supplementation suffers from clear and obvious limits. In particular, besides biased reading of the experimental outcomes, the number of patients enrolled in the studies are often limited, even consisting of a single patient [94], thus inadequate to provide evidences expendable for the whole FM population. Therefore, prospective and randomized trial with hundreds of patients per study group are now required, to corroborate the efficacy of CoQ10 in the management of FM observed in pilot studies.