Zinc–iron redox flow batteries (ZIRFBs) possess intrinsic safety and stability and have low electrolyte cost. ZBRFB refers to an redox flow batterie (RFB) in which zinc is used as the electrochemically active substance in the electrolyte solutions. The zinc electrode has a reversible anode potential. Zinc ions are stable in both alkaline and acidic environments, even in a neutral electrolyte, and the electrochemical reaction rate is relatively fast.
- zinc–iron
- redox flow battery
- zinc dendrite
- energy storage
- large scale
- carbon electrode
- ion exchange membrane
- electrolyte design
- areal capacity
1. Introduction
2. Characteristics of ZIRFB
2.1. The Basic Principle of ZIRFB
Zinc–iron redox flow batteries (ZIRFBs) has the general characteristics of RFBs. That is to say, the ZIRFBs mainly use the changes in the redox state of active substances in the solutions on both sides of the Fe-based cathode and Zn-based anode to realize the charge–discharge process. A ZIRFB is mainly composed of a stack and two electrolyte storage tanks [33][45]. The electrolyte is stored in a storage tank outside the stack, and then is transported to the inside and outside of the stack by the pump. The redox reaction occurs at the electrodes, and the reactive species flow back to the external storage tank with the electrolyte. The cathode and anode are separated by a separator/membrane, which can optionally allow the supporting electrolyte to pass through to maintain electrolyte balance. The separator/membrane not only separates the half-cells and avoids the cross-mixing of active species, but also provides the required ionic conductivity accompanied by the electrons transfer during the charge–discharge process.2.2. Wide pH Range
Metallic Zn can be electroplated in four various ways with different forms of reactants in the solution depending on the pH from 0 to 16. The relevant reaction equations are as follows:
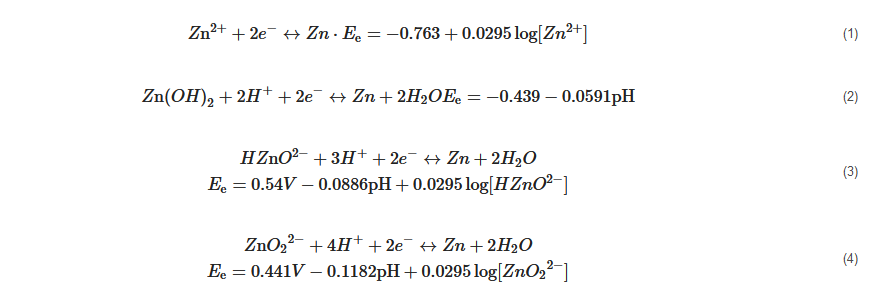
Unlike other RFBs, the electrolyte of ZIRFB can work in a wide pH range. A higher pH value is conducive to the dissolution and deposition of metallic Zn, despite that the Fe2+/Fe3+ redox couple tends to precipitate more easily at high pH. Hence, the appropriate pH range is very important. According to the difference in electrolyte acidity and alkalinity, ZIRFBs are normally divided into three types: alkaline, acidic, and neutral ZIRFBs.
In alkaline ZIRFB, zinc and ferricyanide are used as active substances in the anolyte and catholyte, respectively [34][51]. The system possesses the electrolyte with relatively low cost and high open-circuit voltage (OCV) of 1.74 V. In the discharge state, the anode side is transformed from Zn to zincate solution (alkaline), while the cathode side ferrocyanide is formed from the previous ferricyanide. When charging, it is the opposite process, which is a reversible reaction compared to the discharge process. However, the cycle performance of the ZIRFB is poor due to the issue of zinc dendrites in the alkaline medium.
In theory, the acidic ZIRFB (Ecell = 1.53 V) can have a higher energy density [35][54]. However, in the acidic ZIRFB, the excessive acidity of the solution will affect the deposition of zinc and the hydrolysis of the Fe2+/Fe3+ pair, thus, the hydrogen evolution reaction (HER) is prone to occur. For an acidic system with HAc/NaAc as the buffer solution to keep the pH value of the negative electrolyte between 2–6, a high CE (coulombic/current efficiency) can be realized [36][53].
Compared with alkaline and acidic systems, the neutral ZIRFB system (Ecell = 1.43 V) is mild and non-corrosive, which has lower requirements for the membrane/separator and other components [37][44]. The neutral ZIRFB has a lower battery cost than the other two systems, to a certain extent. Nevertheless, regarding the neutral ZIRFB system, it also has to be taken into account that the hydrolysis of Fen+ ions may lead to the decline of battery cycle performance, which is one of the primary challenges for this type of battery.
2.3. Zinc Dendrites
In comparison to other battery systems, for instance, lead-based and lithium-based batteries, the capacity/energy/power of the liquid–liquid RFBs can be designed independently [33][38][39][45,74,75]. In fact, the ZIRFB is a kind of “half-RFB”. In the electrode reaction, the iron-based active substance on the cathode side is always present in ionic form, while the zinc-based active substance on the anode side is under the plating–stripping process of zinc. This indicates that the power and capacity of the ZIRFBs are not devised flexibly in comparison with the liquid–liquid RFB because the capacity of the ZIRFB is restricted by the surface area of the electrode during the plating–stripping process [40][36]. The essential problem during the plating-stripping transversion is that the zinc dendrites mainly formed during battery charging. The existence of zinc dendrites can easily lead to problems such as a reduction in battery coulombic efficiency (CE) and capacity, and the shortening of battery life. In severe cases, it will impale the separator/membrane and lead to a battery short-circuit. The primary reason is that Zn dendrites are more grievous when the operating current density is high. Under higher current densities, the concentration of zincate or Znn+ in the electrode interface area is extremely low, as the transfer rate of zincate or zinc ions in the electrolyte is obviously slower compared to the reaction rate on the electrode. This may bring about severe concentration polarization [40][36]. Furthermore, the diffusion of zincate/Znn+ tends to realize on the protrusions of the electrode compared to the flat surface, making it easier for zincate or zinc ions to undergo a plating process on the protrusions, and further results in the generation of Zn dendrites. Due to the presence of severe zinc dendrites at high operating current densities, ZIRFB usually operates at relatively lower current densities.2.4. Fe(III) Hydrolysis
The hydrolytic reactions of Fe3+ are much stronger than those of Fe2+ and, consequently, hydrolysis occurs at a much lower pH. There are few reliable investigations of the hydrolytic reactions of Fe2+ because of both the low solubility and its propensity to be oxidised to Fe3+, which can greatly interfere with the ability to measure Fe2+ hydrolysis reactions. There have been several investigations that have examined the hydrolytic reactions of Fe3+, particularly that of the monomeric species, FeOH2+. It is surprising, therefore, to find that a substantial amount of conjecture remains which concerns the stability of the Fe3+ hydrolytic species and phases [41][80]. The hydrolysis reaction of Fe3+ can be described by Reactions (5)–(7) [41][80]. Similar to standard hydrolysis reactions, the interaction of Fe3+ with water takes place in several stages. Firstly, the iron cation reacts with water.3. Research Status of Several Key Problems in ZIRFBs
According to the characteristics of ZIRFBs, the key problems need to be improved including Fe(III) hydrolysis suppression and zinc dendrite prevention, which address the electrode, membrane, and electrolyte optimization, correspondingly.
3.1. Zinc Dendrite Prevention
3.1.1. The 3D Electrode
3.1.2. Improving Membrane/Separator
The membrane/separator is a critical material in RFBs as well, mainly influencing the RFB performance of the battery to a great extent, especially the CE and capacity retention. The membrane/separator divides the negative and positive half-cells to refrain from battery short circuits. Meanwhile, the membrane provides ion transportation pathways to make a conductive circuit to optionally enable H+ or specific ions to pass through, avoiding the crossover between the catholyte and anolyte. To achieve a higher battery, CE requires a higher ionic selectivity of the membrane. Hence, the ideal ion-selective membranes for RFBs should satisfy the following requirements: excellent mechanical properties, high cycle stability, good ionic conductivity and selectivity, and low active species crossover and self-discharge rate. For ZIRFBs, the only concerned metallic ions which may permeate through the membranes and lead to capacity fade are Fen+ and Zn2+. The radius of Fen+ is between 63–92 pm, which is much smaller than that of Zn2+ (139 pm). Hence, the crossover of Fen+ takes place much easier than Zn2+. It was reported that the permeability of the Fen+ ion through Nafion was 5.5 × 10−5 cm2/min, which was 18.9~20.7 times higher than that of the vanadium ion (2.9 × 10−6 cm2/min). For the modification and improvement of membranes for RFB applications, inorganic–organic hybrid membranes and polymer blending composite membranes are widely used to reduce the undesired permeation of metallic ions and improve the ion selectivity of IEMs (ion-exchange membranes) [6][39][6,75]. The main hazard of zinc dendrites is to pierce through the membrane/separator and result in the battery short circuit. To avoid zinc dendrites from piercing the membrane/separator, membranes with high mechanical strength can be selected, such as the PBI (polybenzimidazole) membrane [34][51]. The PBI membrane with heterocyclic rings may ensure the rapid transportation of OH- [34][40][43][36,51,91]. Concurrently, the PBI membrane owns strong mechanical stability and can resist zinc dendrites well, thus, ensuring the long-term cycling stability of alkaline ZIRFBs. At the same time, the use of porous ion-conducting membranes instead of traditional IEMs solves the problem of an increased internal resistance of the membrane due to iron ion pollution, and improves the conductivity of ions from the neutral medium through the membrane, which greatly improves the performance and stability of neutral ZIRFBs.3.1.3. Adding Additives to the Electrolyte
The electrolyte is the source that affects the generation and growth of zinc dendrites. Therefore, the employment of additives into the catholyte/anolyte is a common method to suppress zinc dendrites by direct intervention in the formation of crystal nuclei. Additives can be mainly divided into three categories: metal ions, organic molecules, and polymers. Metallic ions may influence Zn nucleation, and thus, affect the growing process. Therefore, a compact and homogeneous Zn deposit layer is obtained [44][98]. Zhang et al. reported that the aqueous CaCl2 solution containing NH4Cl is appropriate to be a supporting electrolyte [45][99]. Severe Zn dendrites are detected by SEM in 0.1 M ZnCl2 solution. Meanwhile, bulk Zn metal is detected with 1 M NH4Cl as the supporting electrolytes. The cyclic voltammogram (CV) curves show the redox peaks sharpen obviously in the presence of NH4Cl and independent of the amounts of NH4Cl, meaning that the nucleation hysteresis decreases significantly. It can be confirmed that the addition of NH4Cl may promote Z-P/S significantly. However, no prominent Zn dendrite, but only bulk Zn with random holes, is detected in the CaCl2/H2O (3.5 m) solution with 0.5 M NH4Cl. The CV curves are analogous to those in aqueous NH4Cl solutions, but the redox peak currents enhance with the addition of NH4Cl. Therefore, for Z-P/S, a preferable supporting electrolyte has been an aqueous CaCl2 and NH4Cl solution. The charge–discharge curves under various current densities demonstrate a clear plateau with an average voltage of 1.5 V. The CE and EE reach 94% and 75% at 20 mA cm−2, respectively.3.1.4. Flow Field Regulation
The electrolyte flow acts a vital role in Zn dendrites, not only owing to changing the gradient distribution of zinc ions, but also reshaping the orientation of dendritic growth. When electrolyte flow velocity is 50 mL min−1, the species concentration distribution is uniformly obtained by numerical simulation [46][87]. It is very clear that two different zinc-depositing morphologies can be observed under the conditions of the quiescent electrolyte and the flowing electrolyte [47][101]. A higher flow rate of the electrolyte may enhance the transport velocity of Zn2+ which accelerates the diffusion process on the electrode accessory surface and the mass-transfer process in the bulk electrolyte, thus, finally reducing the Zn2+ concentration gradients and constraining the formation and growth of dendrites. Furthermore, a relatively high flow rate results in mitigating dendritic growth, as the formed dendrites are washed away directly by the electrolyte [17].3.2. Fe(III) Hydrolysis Suppression
Both Fe2+ and Fe3+ have hydrolytic reactions in an aqueous solution. It has been reported that the hydrolysis product of iron ions will combine with the sulfonic group in the membrane to increase membrane resistance [48][103]. The hydrolytic reactions of Fe3+ are much stronger than those of Fe2+ and, consequently, occur at a much lower pH [49][104]. However, the hydrolysis of Fe3+ is easier to be suppressed in a hydrochloric acid environment [45][50][99,105]. In the catholyte of acidic ZIRFBs, polymerization takes place more seriously during the Fe2+ oxidation reaction, and ferrihydrite precipitation takes place during the Fe3+ hydrolysis process. The polymerization and hydrolysis reactions are rapidly promoted by enhancing H+/OH− ions formed due to water electrolysis. To address this issue, seven types of Fe2+-complexing ligands are tested and reported, but some issues remain if itswe consider the binding stability and electrochemical performance of the Fe2+-ligand complex [51][82]. It can be concluded that a novel Fe2+-pyridine complexation in the catholyte has been presented for acidic ZIRFBs with a long cycle life and high performance over other Fe2+-complexing ligands. In comparison to other complexing ligands, the Fe2+-complexation with pyridine presents the highest electrochemical activity and reversibility [51][82].3.3. Electrolyte Optimization
3.3.1. Concentration and Additives
By optimizing the composition of the electrolyte, Yuan et al. made the concentration of the Fe(CN)63−/Fe(CN)64− redox couples achieve 1 mol L−1, far exceeding the previously reported concentration (0.4 mol L−1) [34][51]. The high concentration of active redox couples enables the system with a high-energy density. The battery can realize 500 cycles of charge–discharge cycling under 80 and 160 mA cm−2, and still maintain an EE over 80% and CE over 99% at 160 mA cm−2. The results verified the outstanding stability of this system. Most important of all, the functionality of this work is further verified by assembling a kW battery stack at a capital cost of less than USD 90 per kWh.
3.3.2. Zinc–Bromide Complexation
To ensure the long-term operation stability of neutral ZIRFBs, Yang et al. proposed the use of Br- ions to stabilize Zn2+ through complexation interactions in the neutral electrolytes [52][56]. The results of cyclic voltammetry indicate that the redox reversibility has been significantly enhanced between Zn2+ and Zn. To tackle the issue of the sluggish kinetics of the coordination interaction between Br− and Zn2+, ZnBr2 as the electrolyte additive was directly selected to boost the process of complexation. By employing active K3Fe(CN)6 in the catholyte and modified species in the anolyte, the proposed neutral ZIRFB demonstrates excellent efficiencies and cycle stability (without obvious capacity decay) during 2000 cycles (356 h) [52][56].3.3.3. pH
For ZIRFBs, plate electrodes or porous CFs are generally adopted for Z-P/S. During charging, zinc ions or zincate ions are continuously converted to zinc metal, and then, finally, are completely plated on the electrode. Once the deposition is finished on the electrode completely, no further electroplating will be carried out. Further charging will lead to a sharp increase in the charge potential, thus, resulting in the irreversible HER in the negative half-cell. Liu et al. investigated the effect of several inorganic and organic additives on water migration in alkaline ZIRFBs [53][83]. Although all these additives are proved to be effective to suppress water migration, the organic additive, such as xylitol, sorbitol, and mannitol with several hydroxyl groups, can increase the alkalinity of the electrolyte, which in turn, accelerates the corrosion rate of zinc metal and further aggravates the HER of the battery.3.3.4. Mix System
Fe–Cr RFB in the mixed electrolyte was first invented to tackle the cross-contamination issue [13]. Hybrid RFBs, such as Zn–Fe, all-Fe, Sn–Fe, have been widely explored in order to get rid of the bondage of high-cost membrane materials. Zhou et al. reported an Sn–Fe RFB, employed SnCl2 and FeCl2 as both an anolyte and catholyte, and delivered 78.5% of EE and 0.96‰ per cycle of the capacity decay rate at 200 mA cm−2 [54][108]. At present, a long lifetime and a high-power density of Zn–Fe RFBs are achieved through additional operation and structural design.Abbreviations
| RFBs | redox flow batteries |
| ZIRFBs | zinc–iron redox flow batteries |
| ZBRFB | zinc-based RFB |
| VRFB | vanadium RFB |
| R.T. | room temperature |
| OCV | open-circuit voltage |
| OCP | open-circuit potential |
| CEM | cationic exchange membrane |
| IEM | ion exchange membrane |
| n-IEMs | non-ionic membranes |
| PES | poly (ether sulfone) |
| PEG | polyethene glycol |
| SPEEK-K | sulfonated polyether ether ketone |
| PBI | polybenzimidazole |
| BMImCl | 1-butyl-3-methylimidazolium chloride |
| CF | carbon felts |
| Z-P/S | zinc plating/stripping |
| CV | cyclic voltammogram |
| EE | energy efficiency |
| CE | current efficiency |
| VE | voltage efficiency |
| HER | hydrogen evolution reaction |
| SOC | state of charge |
| MC | microporous carbon |
| THEED | N, N, N′ N′-Tetra(2- hydroxyethyl) ethylenediamine |
