You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 3 by Camila Xu and Version 2 by Camila Xu.
Gelatin methacryloyl (GelMA)-based composites are evolving three-dimensional (3D) networking hydrophilic protein composite scaffolds with high water content. These protein composites have been devoted to biomedical applications due to their unique abilities, such as flexibility, soft structure, versatility, stimuli-responsiveness, biocompatibility, biodegradability, and others.
- GelMA
- nanoparticles
- nanomaterials
- carbon nanomaterials
1. Introduction
The natural process of bone regeneration may be affected by significant bone defects, patient co-morbidities, and inflammatory disorders, which decrease the self-healing capacity of the skeletal system [1]. In bone defects, the healing process can be divided into three main stages: (i) cartilage generation, (ii) biomineralization in the cartilage occurs, and (iii) bone formation. However, with severe bone defects, which physical accidents can cause, the regeneration process cannot occur without surgical interventions. A way to solve bone defects is with bone tissue engineering (BTE) [2]. Tissue engineering focuses on using biodegradable and porous scaffolds that allow cells to adhere and proliferate. Consequently, the necessary conditions are created for forming structures like the extracellular matrix (ECM) [3]. The process of BTE starts with the migration and recruitment of osteoprogenitor cells. Then, proliferation and differentiation of these cells occur, forming a matrix, and finally, bone remodeling occurs [4]. The natural bone tissue comprises macro, micro, and nanostructures made of organic and inorganic compounds [2]. The use of nanomaterials in scaffolds helps mimic the biological nanostructure of the ECM [4]. The existing different ways and strategies implemented in bone tissue engineering include biological, osteoinductive, and hybrid materials, as well as cell therapy [5]. Biocompatibility, mechanical qualities, and biodegradability are unique properties that make biomaterials one of the most suitable strategies for BTE. Several biomedical applications of biomaterials consist of joint replacements [6], contact lenses [7], drug delivery systems [8], tissue engineering [9], and others. Specifically, hydrogels can swell in water multiple times their original weight without dissolution. They resemble the natural ECM; hence they are optimal for recreating conditions for in vitro cell culture and can provide three-dimensional (3D) support for tissue formation [10][11]. Hydrogels are promising in repairing cartilage injuries, skull defects, and arthritis due to their tunable mechanical strength, favorable compatibility, and bioactivity. For this reason, they are considered good candidates for the BTE field [12].
Several hydrogels are developed for bone tissue engineering. Among them, methacryloyl functionalized gelatin (GelMA) is one of the famous biomaterials. GelMA is a derivative of gelatin. Likewise, gelatin is derived from collagen. This makes gelatin-based scaffolds great candidates for biomedical applications since collagen is the most abundant protein found in the skin’s bones, cartilage, and connective tissue [10]. Gelatin has multiple advantages over collagen, these include a lower antigenicity, higher solubility and it is less immunogenic. Gelatin is an attractive polymer since it is economically accessible and highly available. It preserves the bioactive groups of collagens that promote cell attachment and remodeling: the arginyl-glycine-aspartic acid (RGD) sequences and the matrix metalloproteinase (MMP) target sequences [13]. However, for the gelatin to be applied, it needs to be crosslinked since it is not stable at body temperature and in an aqueous system. Modification of gelatin with methacrylic groups is needed to synthesize GelMA. This allows crosslinking to form hydrogels with specific properties. The sequences of RGD and MMP are not affected by derivatization. Therefore, GelMA is a good choice for bone tissue engineering and regenerative medicine.
There exist multiple methods for synthesizing GelMA. Even though the investigation is made to improve these methods, they all follow the general process of Van Den Bulcke [14]. The procedure consists of reacting gelatin with methacrylic anhydride in the presence of phosphate buffer (pH 7.5) at 50 °C. This results in modified gelatin (GelMA) prepolymer. For the preparation of the hydrogel, crosslinking of the gelatin methacrylamide must occur in an aqueous medium in the presence of a water-soluble photoinitiator [11]. The physicochemical properties of the synthesized GelMA depend on two main factors: the level of methacrylation and the source of gelatin. GelMA’s gelatin is usually obtained from mammalian sources like porcine or bovine skin. However, the skin of these animals is restricted by several factors. Like some major religions, around 40% of the population worldwide follows one of these religions. An alternative is the use of marine sources such as fish skin, which can be found as a waste product, and is not restricted by religions [15]. The first synthesis of GelMA was reported in 2000 by Van Den Bulcke et al. [11]. Since then, the publications on GelMA have increased. The rate of studies about GelMA for bone tissue engineering can also be seen with a search on the database of Scopus using the keywords gelatin PRE/1 methacryloyl and another search using the exact keywords and adding the keywords bone and tissue. The results of this research made on the 27th of May 2022 can be presented in a graph (Figure 1).
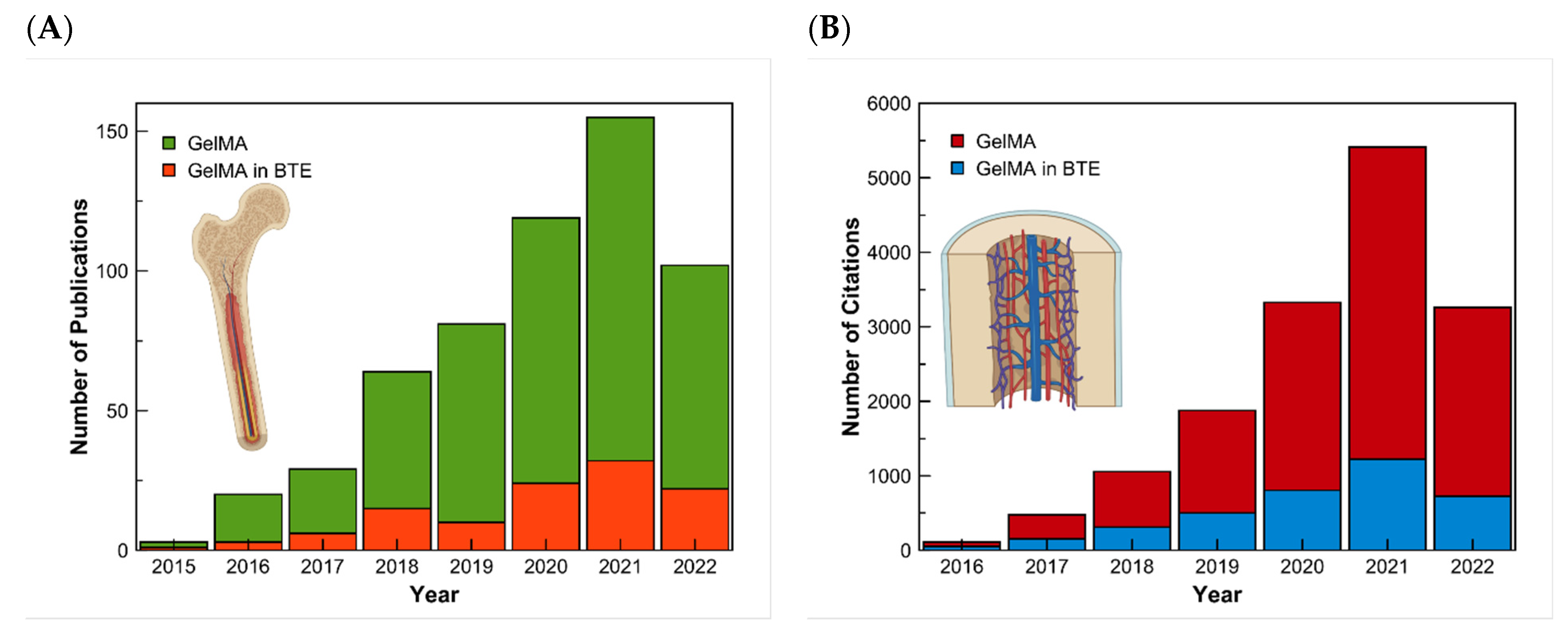
Figure 1. Research results in the Scopus Database for GelMA and GelMA in bone tissue engineering. (A) Results of the number of articles published, and (B) the number of citations from 2015 to 2022, respectively.
Hybrid hydrogel systems can be formed by mixing GelMA with nanomaterials, such as graphene oxide and carbon nanotubes, for specific biological applications [16]. Nanomaterial refers to the material whose scale reaches the nanoscale in every direction. Once the material is in the nanoscale, its properties differ significantly from those of its conventional form [17]. According to their functionality, different nanomaterials can be categorized into polymers, metals, ceramics, and composites, where they can replace and/or restore specific biological aspects [18]. In the biomedical field, certain nanomaterials can help to repair damaged body tissue and organs, readjusting body functions. Some nanomaterials have antiviral, anticancer, antioxidant, and antidiabetic activity such as selenium nanoparticles, therefore, they have been used for tackling SARS-CoV-2 [19]. Nanomaterials and nanostructures can also be used as antimicrobial surfaces, solving critical issues in implantable medical devices like the appearance of biofilms, which can lead to infectious diseases or even the death of the patient [20].
Recent BTE developments have focused on the 3D printing of nanomaterials used to fabricate custom scaffolds, depending on their application in the body [21]. Scaffold is a type of implant that can be used for drug delivery, overcoming the limitations of conventional drug delivery systems. Aside from a controlled release, scaffolds can be used for the treatment, diagnosis, and regenerative therapy of diseases. The best option to explore these advantages is to use a biodegradable scaffold, for example, GelMA, because it does not require surgery to be removed from the body [22]. The microstructure of the skeletal muscle tissue allows bones to have a strong structure and to perform the needed functions in our body. This allows the manipulation of GelMA to form a similar arrangement to the highly aligned myotubes that form the microstructure of bones [23]. The gel manipulation, the macromolecular characteristics, and the polymer network structures are changed. The current interest of the scientific community is to enhance the mechanical and adhesive properties of such gels [24].
2. Nanoparticle-Incorporated GelMA Nanocomposites
Due to their unique structures and properties, 3D hydrogels and nanoparticles have shown a very high potential for medical and diagnostic applications. The expeditious development of biomedicine increased the demand for multifunctional hydrogels with enhanced mechanical properties [25]. Table 1 summarizes the latest advances of nanoparticles with GelMA to solve the limitations of other GelMA hydrogels. The information in the table is explained in more detail in Section 2.1, Section 2.2, Section 2.3 and Section 2.4, including restrictions or further studies that could be made.Table 1. Recent advances of nanoparticles-integrated GelMA composites for bone tissue engineering.
| Function | Type of Nanoparticle | Limitations Solved by the Nanoparticle | Target Application | Ref. |
|---|---|---|---|---|
| Improve physical and/or biological properties of GelMA | Sr-NPs | Most available bio-inks do not support the post-printing maturation tissue process | Nanocomposite bio-ink for 3D bioprinting | [26] |
| BCP-NPs | Nanocomposites of HA or β-TCP have limitations for bone regeneration. | GelMA nanocomposite to treat significant defects in bones | [27] | |
| Mg-PCL | Increase physical stability and biological functionality | Nanocomposite bio-ink for 3D bioprinting | [28] | |
| LPN | Weak rheological properties and soft 3D structure | GelMA nanocomposite bio link | [29] | |
| PDA | Most photothermal agents are not suitable for mild PTT | Composite for PTT | [30] | |
| Controlled drug release in GelMA hydrogels | Nanoliposomes | Rapidly release of drugs with GelMA | Promising bio link | [31] |
| MSNs loaded with MF | MF dilutes rapidly | Injectable hydrogel for craniomaxillofacial bone regeneration. | [32] | |
| MSN | Some available bio-inks do not have nanosized minerals present in bones | Nanocomposite bio-ink for 3D bioprinting | [33] | |
| Fabrication of periosteum with GelMA | CaPs | Most artificial periostea focus only on osteogenesis activity ignoring angiogenesis capability. | Artificial periosteum with osteogenesis and angiogenesis capability | [34] |
| nHAMA | Most artificial periosteum focuses only on osteogenesis activity ignoring angiogenesis capability. | Artificial periosteum with osteogenesis and angiogenesis capability | [35] | |
| Imaging GelMA scaffolds | Au-NPs | GelMA scaffolds can not be monitored once implanted in vivo. Only newly formed bones can be imaged through CT. | Contrast agents for CT imaging. | [36] |
| SMNP | Photoacoustic and fluorescence imaging of cartilage scaffolds have poor resolution | Contrast agents for MRI imaging | [37] |
2.1. Biocompatibility and Physicochemical Characteristics of Nanoparticles Embedded GelMA Composites
Alcala-Orozco et al., synthesized strontium nanoparticles (Sr-NPs) that created star-like structures via multistage self-assembly. These nanoparticles were further incorporated into a GelMA solution via short vortex spinning, forming a nanocomposite bio-ink (Sr-GelMA) of Sr nanoparticles and low concentration (5 w/v%) GelMA. The Sr-GelMA nanocomposite targets the current physical and biological limitations of available bio-inks by modulating rheological properties. Sr nanoparticles have been demonstrated to have good biocompatibility; therefore, they have been used before for bone regeneration. The incorporation of Sr to GelMA did not affect the crosslinking reaction. The Sr-GelMA nanocomposite preserved its physical properties, such as pore size and swelling ratio, compared to GelMA alone. However, there was a significant increase in viscosity that enhanced printability. With this bio-ink, 3D hydrogel discs were printed via extrusion. Tests were made in these scaffolds, demonstrating the activity of osteogenic differentiation of human mesenchymal stromal cells (hMSCs) and mineral deposition. Further studies could test the influence of Sr-GelMA nanocomposite in other cell types, such as endothelial cells, chondrocytes, and osteoclasts [26]. In another study, GelMA nanocomposites made of synthetic bones were developed to treat large defect bones. They are made of compounds like hydroxyapatite (HA) and β-tricalcium phosphate (β-TCP). Even though these compounds have favorable characteristics to improve bone function, they have limitations. For example, after the GelMA with HA hydrogels has degraded, HA remains in place, so it is not entirely replaced by new bone. In the case of β-TCP/GelMA hydrogels, complete bone regeneration occurs, but β-TCP is easily degraded in the body. To target these disadvantages, in 2021 a GelMA hydrogel composite was made with biphasic calcium phosphate nanoparticles (BCP-NPs). The mixture consists of HA and β-TCP at (60/40 wt%). The final hydrogel has stability against biodegradation. The use of BCP-NPs improved cell differentiation and improved the mechanical properties of the hydrogel [27]. Previously, biphasic calcium phosphate nanoparticles-loaded hyaluronic acid/gelatin as well as chitosan/gelatin hydrogels have been used for BTE [38][39]. Further research could compare in vitro and in vivo the performance of these three different hydrogels with BCP-NPs. In the same year, Alcala-Orozco et al., fabricated a bio-ink consisting of GelMA, magnesium hydroxide nanoparticles (Mg), and polycaprolactone (PCL). This biological ink was aimed to have strong physical stability as well as natural functionality. The results demonstrated that the Mg-PCL degraded faster than standard PCL, a positive outcome for in vivo implant degradation and bone regeneration. Another good result was that the combination of Mg-PCL enhanced osteogenic differentiation. This regenerative bone scaffold is promising for skeletal tissue regeneration [28]. Additionally, in 2019, Cidonio et al., investigated the benefits of incorporating nanoclay in the GelMA bio-ink. The study focused on including laponite (LPN) nanoparticles, and the results displayed an enhancement in shape fidelity retention. With the LPN-GelMA bio-ink, the human bone marrow stromal cells proliferated with a significant increase in cell number over 21 days compared to GelMA alone. The bio-ink was implanted ex vivo in a chick chorioallantoic membrane (CAM) model to test this gel. The outcome of this test was that the bio-ink constructs could integrate correctly in the vascular chick embryo after 7 days of incubation. The overall results showed the potential application of the cell-laden hydrogel in hard and soft tissue reparation, but further research is needed [29]. Photothermal therapy (PTT) has excellent potential as a bone regeneration method. PTT is based on using light absorbers that create heat from light energy. Polydopamine nanoparticles (PDA) can be used as photothermal agents. They have advantages like good biocompatibility, mild photothermal effect, and easy preparation. PDA can be found in various morphologies. However, in the study of Wu et al., in 2022, spherical PDA was used for the synthesis of a GelMA/poly(methyl methacrylate)/polydopamine nanoparticles hydrogel (GelMA/PMMA/PDA) (Figure 32). This composite was made via free-radical polymerization. poly(methyl methacrylate) (PMMA) was used as an additive to improve mechanical properties and stabilize the structure. Biocompatibility tests were made in vivo. The effects of GelMA/PMMA/PDA hydrogel with mild PTT were tested with a rat cranial defect model. In vitro studies showed that the hydrogel has good biocompatibility, induces osteogenic differentiation, and has an excellent photothermal effect. The hydrogel reached 44.1 °C, and the temperature suitable for bone repair is between 40 °C to 43 °C. In vivo studies showed that the hydrogel maintained a stable structure for the first four weeks and started degradation after the four weeks. It was concluded that the hydrogel with PTT promotes the regeneration of bone defects. Further research is still needed to understand the mechanism of bone repair by mild photothermal hydrogels [30].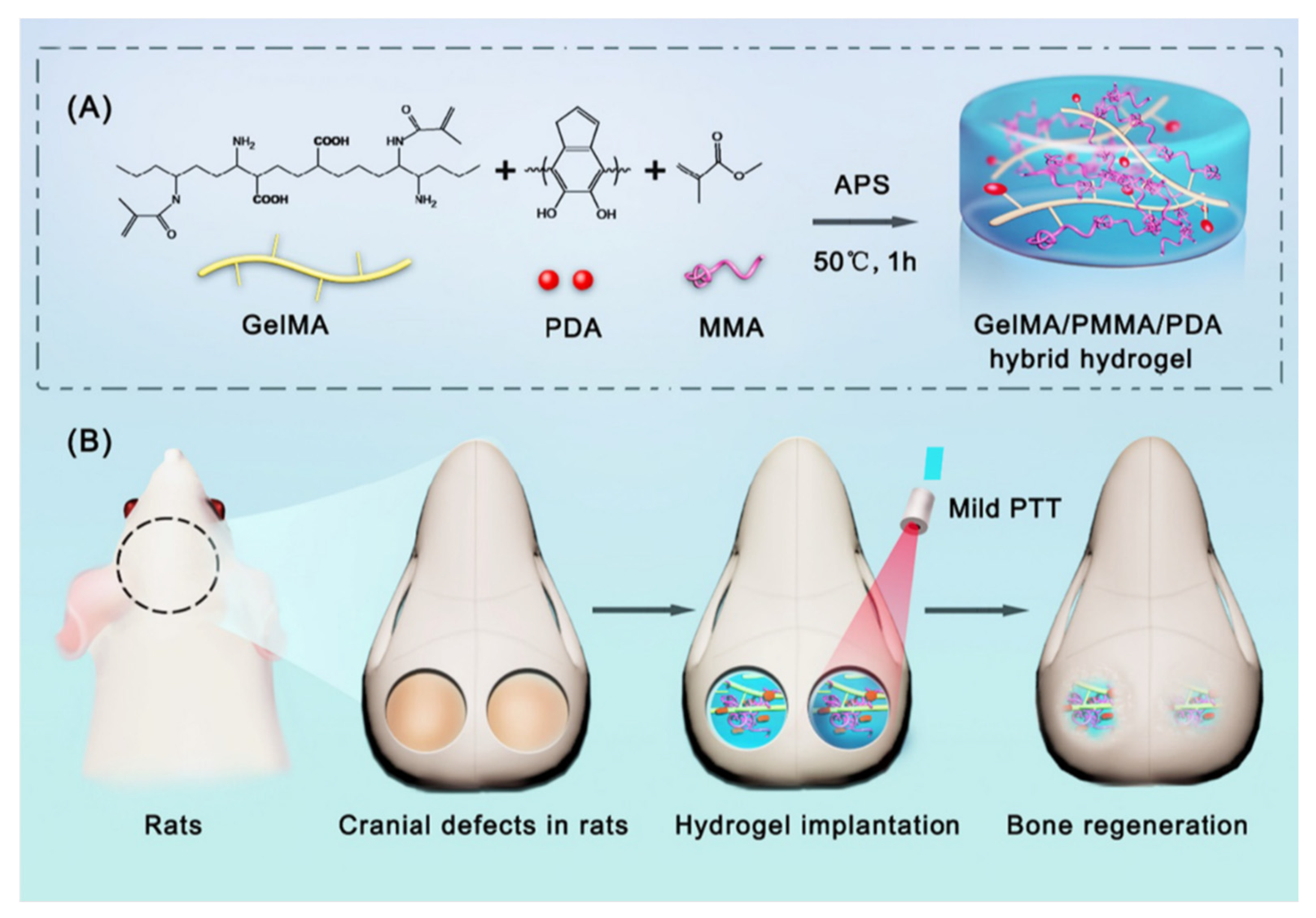
2.2. Nanoparticle Incorporated GelMA-Based Drug Delivery System for Bone Regeneration
Another type of nanoparticle is soft nanoparticles like nanoliposomes. Nanoliposomes can be included in a GelMA matrix to form a nano-functionalized platform. Elkhoury et al., 2020 synthesized nanoliposomes with salmon lecithin and loaded them with naringin. This compound can induce stem cells’ osteodifferentiation. However, it requires an extensive metabolism. GelMA hydrogels can release drugs in a few hours due to their big pores. Nevertheless, if the drugs are loaded into nanoliposomes, the release can be controlled and prolonged for several days. In the study, when de-loaded nanoliposomes were incorporated into the GelMA matrix, a controlled drug release and high encapsulation efficiency were obtained. Additionally, there were no signals of cytotoxicity toward human mesenchymal/stromal stem cells. The mechanical and rheological properties of GelMA improved and the swelling ratio and hydrophilic character decreased. GelMA hydrogel with naringin-loaded nanoliposomes can be a promising bio-ink. Further studies will focus on the optimization of the nanocomposite hydrogel as a bio-ink and its osteodifferentiation ability in 2D and 3D cell cultures [31]. Mesoporous silica nanospheres (MSNs) enhance the osteogenic potential of hydrogels and can alter their mechanical properties. The mineralization of bone marrow stromal cells can be stimulated with MSNs. They have multiple characteristics that help them function as carriers of bioactivity, such as large surface area, pore volume, and good biocompatibility. In the work of Qu et al. in 2021, MSNs were carboxylated, resulting in MSNs-COOH. Then, these nanospheres were loaded with metformin (MF), a drug that supports bone formation used in type 2 diabetes, resulting in Mf-MSNs-COOH nanospheres. One of the main challenges with MF is that it can rapidly dilute if there is no optimal storage and drug release control. The loaded nanospheres were added to a GelMA solution to address this problem, and photocrosslinking was used to make the hybrid hydrogel of MF-MSNs-COOH/GelMA. The incorporation of MSNs-COOH obtained the highest compressive modulus and swelling ratios to GelMA in 1.5 mg/mL. The cytocompatibility was examined with human exfoliated deciduous teeth (SHEDS). It was concluded that MF-MSNs-COOH/GelMA is a promising hydrogel for injectable bone regeneration therapy, focused on craniomaxillofacial applications. Further studies are still required to find the adequate concentration of Metformin for MSNs in GelMA [32]. Another study of nanocomposites as bio-inks was made by Tavares et al. in 2021. In this research, a multi-bioactive nanocomposite bio-ink was made with GelMA, human bone marrow-derived mesenchymal stem cells (hBM-MSC), and functionalized mesoporous silica nanoparticles (MSN). As MSN has a mesoporous structure, it can act as a nanocarrier. Dexamethasone (Dex), calcium (Ca), and phosphate (P) ions were incorporated into the MSN. The final functionalized nanoparticles (MSNCaPDex) can promote stem cell osteogenic differentiation with just one administration. The manufactured 3D bio-ink was composed of the bone matrix’s major significant organic (GelMA) and inorganic (MSNCaPDex) components. It showed promising results like the autonomous promotion of pro-osteogenic differentiation without adding another osteogenic supplementation. This bio-ink is an excellent option for 3D extrusion bioprinting. Further studies should focus on the living constructs after implantation in bone defects to test if the bioactive and pro-osteogenic capabilities are maintained [33].2.3. Nanoparticles Incorporated GelMA Composites for Biomimetic Hydrogel Periosteum
The periosteum is a membrane located at the outer surface of bones. It can work as a source of growth factor due to its rich cellular composition, like differentiated osteoprogenitor cells, mesenchymal cells, and osteoblasts. When there is a high-energy trauma, bone defects can occur, and the periosteum can be damaged. This membrane provides blood to the bone, in case of damage, bone healing will be delayed [40]. Several artificial periostea have been made (Figure 43), but most of them only focus on the osteogenic activity, overlooking angiogenesis activity [34]. In 2020 Liu et al., made a mimicking periosteum. The hybrid biomimetic periosteum was made with electrospinning by using organic and inorganic materials. The inorganic part was composed of calcium phosphate nanoparticles (CaPs). The emulsion method synthesized these nanoparticles with a diameter of 50.7 ± 2.3 nm. CaPs were further incorporated into GelMA, the organic component, with electrospinning. Using inorganic components helps in the regeneration of hard tissue, improves the stiffness and mechanical strength of the system, and enhances osteogenic differentiation. CaPS also provide bioactivity to the system since they can work as drug carriers, and their released ions are essential in the process of bone formation. The final hybrid hydrogel fibers showed controlled ions release for over 10 days, mineralization appeared on the surface of the fibers after coincubation with simulated body fluid (SBF), and osteogenesis and angiogenesis capabilities were verified with human umbilical vein endothelial and MC3T3-E1 cells [34].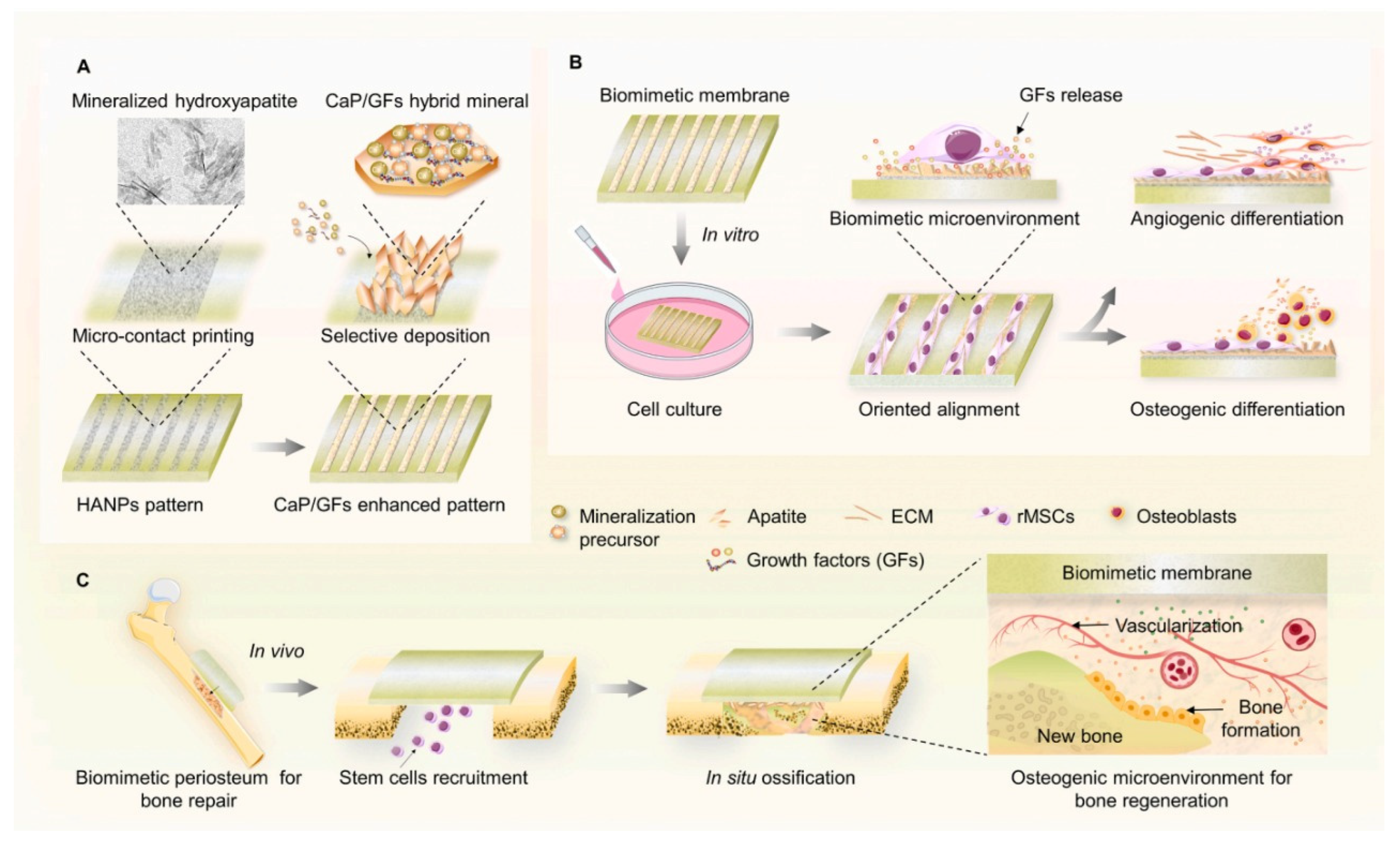
Figure 43. Schematic illustration of the synthesis of a mimicking periosteum membrane with nanoparticles. (A) Use of apatite selective deposition for the generation of a biomimetic membrane with mineralized micropattern. Illustration for the potential effects of the periosteum-mimetic membrane with the mineralized pattern on (B) stem cell differentiation in vitro, and (C) vascularized osteogenesis in vivo. Retrieved from [41]. Reproduced from [41] with permission from Elsevier, copyright©2021.
2.4. Nanoparticles-Incorporated GelMA Scaffolds for Bioimaging
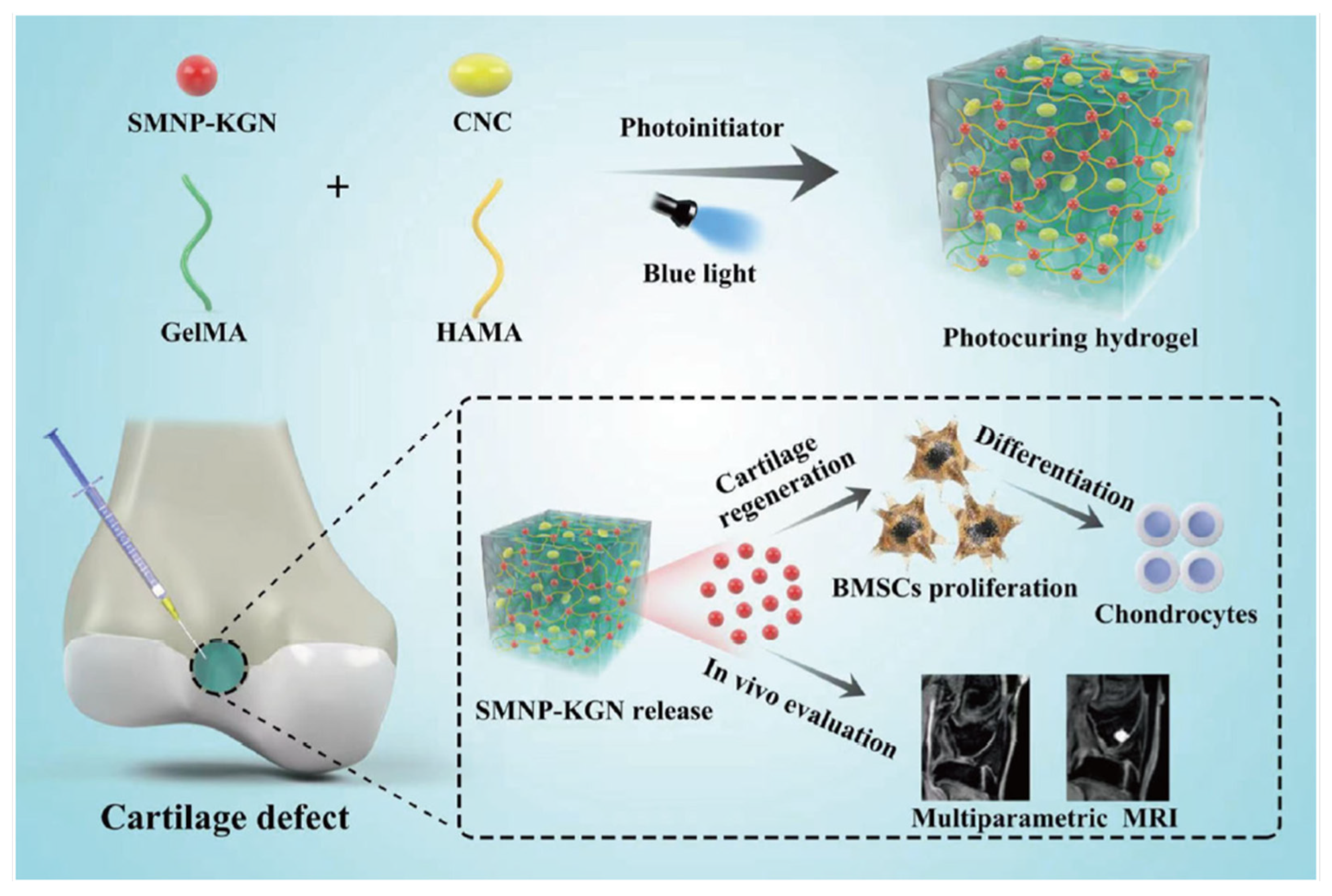
In the work of Celikkin et al. in 2019, gold nanoparticles (Au-NPs) have been used in GelMA as a contrast agent. BTE is progressing toward clinical applications, hence the importance of imaging methods, such as computed tomography (CT), to evaluate the results of tissue-engineered constructs. The X-ray attenuation shows the material’s link to its density and atomic number. Therefore, mineralized tissues will have high contrast while soft tissues, composed mainly of water, have low contrast. Hydrogels, like GelMA, have a weak CT signal. By incorporating Au nanoparticles into GelMA pre-polymer solution, 3D GelMA-AuNPs scaffolds were obtained. Different concentrations of Au nanoparticles were used, and the best mechanical, enhanced radiopacity, and cytocompatibility performance, occurred with 0.16 mM 60 nm Au nanoparticles. With the incorporation of Au nanoparticles, the attenuation was increased. The scaffolds had osteogenic features and good cytocompatibility. These results demonstrate that Au nanoparticles can help image GelMA through CT, not only the newly formed bone. Further studies could include the effect of having Au-NPs in the composites discussed in Section 2.1., Section 2.2. and Section 2.3. [36] Some studies have been made attempting to promote chondrocyte differentiation using kartogenin (KGN). Still, the results showed that KGN by itself will present a loss of KGN or absorption into the circulatory system. Another problem with the traditional surgical procedures for articular cartilage remains unsatisfactory. Some studies have tried to find noninvasive methods to monitor the functionalization and degradation of tissue engineering scaffolds in vivo. One example is fluorescence imaging, but due to the poor resolution of the image, further application of this method is limited [42]. For this reason, Chen et al., designed a blended hydrogel scaffold system loaded with synthetic melanin nanoparticles (SMNP) and KGN for theragnostic purposes. The results demonstrated that this SMNP-KGN/Gel had strong mechanical properties, thermal stability, and enhanced magnetic resonance imaging (MRI). The release of the drug KGN could make bone-derived mesenchymal stem cells proliferate and differentiate in vivo (Figure 54) [37].References
- Oliveira, C.; Leeuwenburg, S.; Mano, J.F. New Insights into the Biomimetic Design and Biomedical Applications of Bioengineered Bone Microenvironments. APL Bioeng. 2021, 5, 041507.
- Dong, Z.; Yuan, Q.; Huang, K.; Xu, W.; Liu, G.; Gu, Z. Gelatin Methacryloyl (GelMA)-Based Biomaterials for Bone Regeneration. RSC Adv. 2019, 9, 17737–17744.
- Costa-Pinto, A.R.; Correlo, V.M.; Sol, P.C.; Bhattacharya, M.; Charbord, P.; Delorme, B.; Reis, R.L.; Neves, N.M. Osteogenic Differentiation of Human Bone Marrow Mesenchymal Stem Cells Seeded on Melt Based Chitosan Scaffolds for Bone Tissue Engineering Applications. Biomacromolecules 2009, 10, 2067–2073.
- Li, X.; Wang, L.; Fan, Y.; Feng, Q.; Cui, F.-Z.; Watari, F. Nanostructured Scaffolds for Bone Tissue Engineering. J. Biomed. Mater. Res. A 2013, 101A, 2424–2435.
- El-Husseiny, H.M.; Mady, E.A.; El-Dakroury, W.A.; Zewail, M.B.; Noshy, M.; Abdelfatah, A.M.; Doghish, A.S. Smart/Stimuli-Responsive Hydrogels: State-of-the-Art Platforms for Bone Tissue Engineering. Appl. Mater. Today 2022, 101560.
- Sobajima, A.; Okihara, T.; Moriyama, S.; Nishimura, N.; Osawa, T.; Miyamae, K.; Haniu, H.; Aoki, K.; Tanaka, M.; Usui, Y.; et al. Multiwall Carbon Nanotube Composites as Artificial Joint Materials. ACS Biomater. Sci. Eng. 2020, 6, 7032–7040.
- Borandeh, S.; Alimardani, V.; Sadat Abolmaali, S.; Seppälä, J. Graphene Family Nanomaterials in Ocular Applications: Physicochemical Properties and Toxicity. Chem. Res. Toxicol. 2021, 34, 1386–1402.
- Ali Saleemi, M.; Hosseini Fouladi, M.; Voon Chen Yong, P.; Chinna, K.; Kumari Palanisamy, N.; Hwa Wong, E. Toxicity of Carbon Nanotubes: Molecular Mechanisms, Signaling Cascades, and Remedies in Biomedical Applications. Chem. Res. Toxicol. 2020, 34, 24–46.
- Hoon Jeong, S.; Kim, M.; Yeon Kim, T.; Kim, H.; Hyeon Ju, J.; Kwang Hahn, S. Supramolecular Injectable Hyaluronate Hydrogels for Cartilage Tissue Regeneration. ACS Appl. Bio. Mater. 2020, 3, 5040–5047.
- Sun, M.; Sun, X.; Wang, Z.; Guo, S.; Yu, G.; Yang, H. Synthesis and Properties of Gelatin Methacryloyl (GelMA) Hydrogels and Their Recent Applications in Load-Bearing Tissue. Polymer 2018, 10, 1290.
- Van Den Bulcke, A.I.; Bogdanov, B.; De Rooze, N.; Schacht, E.H.; Cornelissen, M.; Berghmans, H. Structural and Rheological Properties of Methacrylamide Modified Gelatin Hydrogels. Biomacromolecules 2000, 1, 31–38.
- Xue, X.; Hu, Y.; Deng, Y.; Su, J.-C. Recent Advances in Design of Functional Biocompatible Hydrogels for Bone Tissue Engineering. Adv. Funct. Mater. 2021, 31.
- Kurian, A.G.; Singh, R.K.; Patel, K.D.; Lee, J.H.; Kim, H.W. Multifunctional GelMA Platforms with Nanomaterials for Advanced Tissue Therapeutics. Bioact. Mater. 2022, 8, 267–295.
- di Muzio, L.; Cienzo, F.; Paolicelli, P.; Petralito, S.; Garzoli, S.; Brandelli, C.; Trilli, J.; Antonietta Casadei, M. A Convenient Strategy to Synthesize Highly Tunable Gelatin Methacryloyl with Very Low Gelation Temperature. Eur. Polym. J. 2021, 154, 110538.
- Elkhoury, K.; Morsink, M.; Tahri, Y.; Kahn, C.; Cleymand, F.; Shin, S.R.; Arab-Tehrany, E.; Sanchez-Gonzalez, L. Synthesis and Characterization of C2C12-Laden Gelatin Methacryloyl (GelMA) from Marine and Mammalian Sources. Int. J. Biol. Macromol. 2021, 183, 918–926.
- Yue, K.; Trujillo-de Santiago, G.; Alvarez, M.M.; Tamayol, A.; Annabi, N.; Khademhosseini, A. Synthesis, Properties, and Biomedical Applications of Gelatin Methacryloyl (GelMA) Hydrogels. Biomaterials 2015, 73, 254–271.
- Zhang, J.; Chen, H.; Zhao, M.; Liu, G.; Wu, J. 2D Nanomaterials for Tissue Engineering Application. Nano Res. 2020, 13, 2019–2034.
- Fattahi Nafchi, R.; Ahmadi, R.; Heydari, M.; Reza Rahimipour, M.; Jafar Molaei, M.; Unsworth, L. In Vitro Study: Synthesis and Evaluation of Fe3O4/CQD Magnetic/Fluorescent Nanocomposites for Targeted Drug Delivery, MRI, and Cancer Cell Labeling Applications. Langmuir 2022, 38, 3804–3816.
- Singh, A.; Singh, P.; Kumar, R.; Kaushik, A. Exploring Nanoselenium to Tackle Mutated SARS-CoV-2 for Efficient COVID-19 Management. Front. Nanotechnol. 2022, 4.
- Mostafavi, E.; Dubey, A.K.; Walkowiak, B.; Kaushik, A.; Ramakrishna, S.; Teodori, L. Antimicrobial Surfaces for Implantable Cardiovascular Devices. Curr. Opin. Biomed. Eng. 2022, 23, 100406.
- Qu, M.; Wang, C.; Zhou, X.; Libanori, A.; Jiang, X.; Xu, W.; Zhu, S.; Chen, Q.; Sun, W.; Khademhosseini, A. Multi-Dimensional Printing for Bone Tissue Engineering. Adv. Health Mater. 2021, 10, 2001986.
- Chavda, V.P.; Jogi, G.; Paiva-Santos, A.C.; Kaushik, A. Biodegradable and Removable Implants for Controlled Drug Delivery and Release Application. Expert Opin. Drug Deliv. 2022, 19, 1177–1181.
- Boularaoui, S.; Shanti, A.; Lanotte, M.; Luo, S.; Bawazir, S.; Lee, S.; Christoforou, N.; Khan, K.A.; Stefanini, C. Nanocomposite Conductive Bioinks Based on Low-Concentration GelMA and MXene Nanosheets/Gold Nanoparticles Providing Enhanced Printability of Functional Skeletal Muscle Tissues. ACS Biomater. Sci. Eng. 2021, 7, 5810–5822.
- Montazerian, H.; Baidya, A.; Haghniaz, R.; Davoodi, E.; Ahadian, S.; Annabi, N.; Khademhosseini, A.; Weiss, P.S. Stretchable and Bioadhesive Gelatin Methacryloyl-Based Hydrogels Enabled by in Situ Dopamine Polymerization. ACS Appl. Mater. Interfaces 2021, 13, 40290–40301.
- Zhao, F.; Yao, D.; Guo, R.; Deng, L.; Dong, A.; Zhang, J. Composites of Polymer Hydrogels and Nanoparticulate Systems for Biomedical and Pharmaceutical Applications. Nanomaterials 2015, 5, 2054–2130.
- Alcala-Orozco, C.R.; Mutreja, I.; Cui, X.; Kumar, D.; Hooper, G.J.; Lim, K.S.; Woodfield, T.B.F. Design and Characterisation of Multi-Functional Strontium-Gelatin Nanocomposite Bioinks with Improved Print Fidelity and Osteogenic Capacity. Bioprinting 2020, 18, e00073.
- Choi, J.-B.; Kim, Y.-K.; Byeon, S.-M.; Park, J.-E.; Bae, T.-S.; Jang, Y.-S.; Lee, M.-H. Fabrication and Characterization of Biodegradable Gelatin Methacrylate/Biphasic Calcium Phosphate Composite Hydrogel for Bone Tissue Engineering. Nanomaterials 2021, 11, 617.
- Alcala-Orozco, C.R.; Mutreja, I.; Cui, X.; Hooper, G.J.; Lim, K.S.; Woodfield, T.B.F. Hybrid Biofabrication of 3D Osteoconductive Constructs Comprising Mg-Based Nanocomposites and Cell-Laden Bioinks for Bone Repair. Bone 2022, 154, 116198.
- Cidonio, G.; Alcala-Orozco, C.R.; Lim, K.S.; Glinka, M.; Mutreja, I.; Kim, Y.-H.; Dawson, J.I.; Woodfield, T.B.F.; Oreffo, R.O.C. Osteogenic and Angiogenic Tissue Formation in High Fidelity Nanocomposite Laponite-Gelatin Bioinks. Biofabrication 2019, 11, 035027.
- Wu, Y.; Zhang, X.; Tan, B.; Shan, Y.; Zhao, X.; Liao, J. Near-Infrared Light Control of GelMA/PMMA/PDA Hydrogel with Mild Photothermal Therapy for Skull Regeneration. Biomater. Adv. 2022, 133, 112641.
- Elkhoury, K.; Sanchez-Gonzalez, L.; Lavrador, P.; Almeida, R.; Gaspar, V.; Kahn, C.; Cleymand, F.; Arab-Tehrany, E.; Mano, J.F. Gelatin Methacryloyl (GelMA) Nanocomposite Hydrogels Embedding Bioactive Naringin Liposomes. Polymer 2020, 12, 2944.
- Qu, L.; Dubey, N.; Ribeiro, J.S.; Bordini, E.A.F.; Ferreira, J.A.; Xu, J.; Castilho, R.M.; Bottino, M.C. Metformin-Loaded Nanospheres-Laden Photocrosslinkable Gelatin Hydrogel for Bone Tissue Engineering. J. Mech. Behav. Biomed. Mater. 2021, 116, 104293.
- Tavares, M.T.; Gaspar, V.M.; Monteiro, M.V.; Farinha, J.P.S.; Baleizão, C.; Mano, J.F. GelMA/Bioactive Silica Nanocomposite Bioinks for Stem Cell Osteogenic Differentiation. Biofabrication 2021, 13, 035012.
- Liu, W.; Bi, W.; Sun, Y.; Wang, L.; Yu, X.; Cheng, R.; Yu, Y.; Cui, W. Biomimetic Organic-Inorganic Hybrid Hydrogel Electrospinning Periosteum for Accelerating Bone Regeneration. Mater. Sci. Eng. C 2020, 110, 110670.
- Yang, Y.; Xu, T.; Zhang, Q.; Piao, Y.; Bei, H.P.; Zhao, X. Biomimetic, Stiff, and Adhesive Periosteum with Osteogenic–Angiogenic Coupling Effect for Bone Regeneration. Small 2021, 17, 2006598.
- Celikkin, N.; Mastrogiacomo, S.; Walboomers, X.F.; Swieszkowski, W. Enhancing X-Ray Attenuation of 3D Printed Gelatin Methacrylate (GelMA) Hydrogels Utilizing Gold Nanoparticles for Bone Tissue Engineering Applications. Polymer 2019, 11, 367.
- Chen, C.; Huang, S.; Chen, Z.; Liu, Q.; Cai, Y.; Mei, Y.; Xu, Y.; Guo, R.; Yan, C. Kartogenin (KGN)/Synthetic Melanin Nanoparticles (SMNP) Loaded Theranostic Hydrogel Scaffold System for Multiparametric Magnetic Resonance Imaging Guided Cartilage Regeneration. Bioeng. Transl. Med. 2022; e10364, early view.
- Faruq, O.; Kim, B.; Padalhin, A.R.; Lee, G.H.; Lee, B.-T. A Hybrid Composite System of Biphasic Calcium Phosphate Granules Loaded with Hyaluronic Acid–Gelatin Hydrogel for Bone Regeneration. J. Biomater. Appl. 2017, 32, 433–445.
- Nie, L.; Wu, Q.; Long, H.; Hu, K.; Li, P.; Wang, C.; Sun, M.; Dong, J.; Wei, X.; Suo, J.; et al. Development of Chitosan/Gelatin Hydrogels Incorporation of Biphasic Calcium Phosphate Nanoparticles for Bone Tissue Engineering. J. Biomater. Sci. Polym. Ed. 2019, 30, 1636–1657.
- Gupta, S.; Kumar Teotia, A.; Qayoom, I.; Ahmad Shiekh, P.; Muntazir Andrabi, S.; Kumar, A. Periosteum-Mimicking Tissue-Engineered Composite for Treating Periosteum Damage in Critical-Sized Bone Defects. Biomacromolecules 2021, 22, 3237–3250.
- Yang, G.; Liu, H.; Cui, Y.; Li, J.; Zhou, X.; Wang, N.; Wu, F.; Li, Y.; Liu, Y.; Jiang, X.; et al. Bioinspired Membrane Provides Periosteum-Mimetic Microenvironment for Accelerating Vascularized Bone Regeneration. Biomaterials 2021, 268, 120561.
- Xiao, S.; Lin, Y.; Tang, Y.; Lv, Z.; Chen, L. Real-Time Quantification of Cartilage Degeneration by GAG-Targeted Cationic Nanoparticles for Efficient Therapeutic Monitoring in Living Mice. Mol. Pharm. 2021, 18, 1444–1454.
More