Macrophages, one of the body’s most abundant populations of leukocytes, are mainly derived from the yolk sac during embryogenesis and are found in almost every tissue that plays an essential role during mammalian development. They are specialized phagocytes, large vacuolated cells with abundant cytoplasm containing lysosomal granules. Wnt signaling is a conserved pathway across species. It is involved in various essential tasks by regulating cell differentiation, proliferation, stem cell development, immune cell functions, and tissue repair. Evidence for the Wnt system’s pivotal role is that aberrant alterations of this molecular pathway are involved in multiple human disorders and pathologies, such as congenital abnormalities, autoimmune diseases, and cancer. Wnt and macrophages in the most immunologically active lung, liver, intestine, kidney, heart, and skin are discussed.
- injury
- repair
- Wnt
1. Introduction
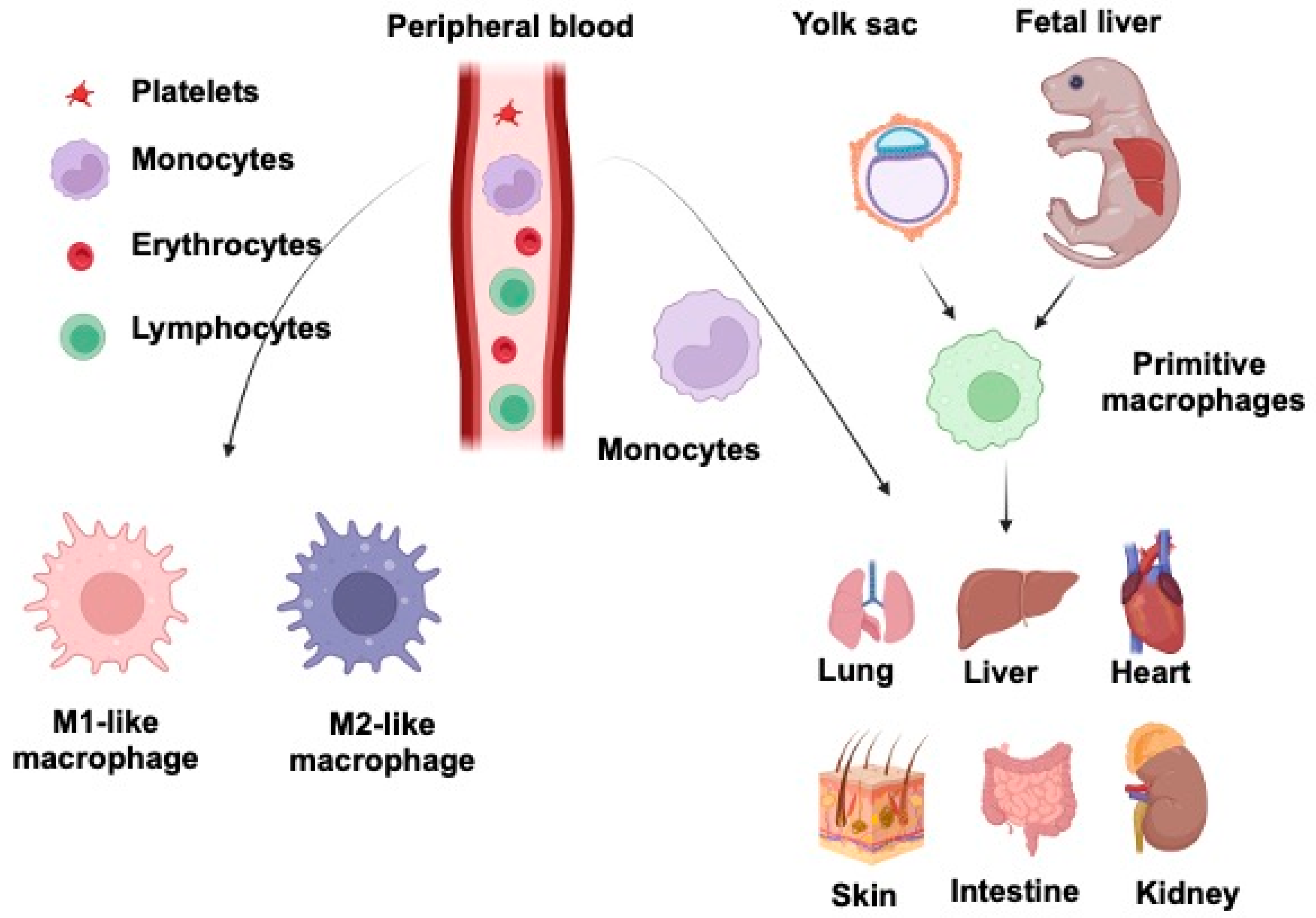
2. Macrophages and Wnt Signaling in Lung Injury and Repair
3. Macrophages and Wnt Signaling in Liver Injury and Repair
4. Macrophages and Wnt Signaling in Intestine Injury and Repair
5. Macrophages and Wnt Signaling in Kidney Injury and Repair
6. Macrophages and Wnt Signaling in Heart Injury and Repair
Macrophages and Wnt ligands are independently associated with cardiac development, reaction to cardiac injury, and repair [77][40]. Furthermore, Wnt signaling functions diversely in cardiovascular development and disease processes [78][41]. Monocytes and monocyte-derived macrophages are known to play important roles in the development of atherosclerosis and coronary heart disease, as well as in the immune response against cardiac ischemia [79,80][42][43]. After the heart is damaged, Wnt signaling is reactivated. There is increasing evidence that reactivation of the canonical Wnt signaling negatively affects infarct healing associated with cardiomyocyte death and cardiac fibrosis [79][42].7. Macrophages and Wnt Signaling in Skin Injury and Repair
Macrophages are well known to play essential roles and coordinate in all stages of the skin wound healing process [83,84,85][44][45][46]. A skin injury can provide an ideal model for studying the role of the innate immune system between regeneration and fibrotic healing. Recently, the wound-induced hair neogenesis (WIHN) model, which can induce fibrotic scarring, was used to investigate the potential role of macrophages in determining healing fate by Gay and colleagues [86][47]. Their results showed that late wound macrophages phagocytosed the dermal Wnt inhibitor SFRP4 to establish sustained Wnt activity, leading to fibrosis. In addition, the phagocytosis of SFRP4 by macrophages in the human hidradenitis suppurativa was related to the recovery of fibrotic skin. These results revealed that macrophages could change the fate of skin wound healing by regulating major signaling pathways via phagocytosis. Macrophages are known to regulate developmental vascularization through non-canonical Wnt signaling and are associated with wound angiogenesis. Stefater III and colleagues [87][48] showed that wound macrophages use the Wnt-Flt1 signaling pathway via Flt1, a receptor for vascular endothelial growth factor A. Calcineurin is an important mediator in regulating wound response. Thus, they found that macrophages use Wnt-Calcineurin-Flt1 signaling to inhibit angiogenesis and slow repair. To investigate the effect of perifollicular macrophage-derived Wnt on the activation of hair follicle stem cells (HF-SCs) and the induction of anagen (the active growth phase of hair follicles) in the hair cycle in mice, Castellana and colleagues [88][49] used and injected subcutaneously into mice a liposome containing IWP-2, a specific hydrophobic small molecule inhibitor of Wnt.References
- Varol, C.; Mildner, A.; Jung, S. Macrophages: Development and tissue specialization. Annu. Rev. Immunol. 2015, 33, 643–675.
- Hussell, T.; Bell, T.J. Alveolar macrophages: Plasticity in a tissue-specific context. Nat. Rev. Immunol. 2014, 14, 81–93.
- Mowat, A.M.; Scott, C.; Bain, C. Barrier-tissue macrophages: Functional adaptation to environmental challenges. Nat. Med. 2017, 23, 1258–1270.
- Cheng, P.; Li, S.; Chen, H. Macrophages in Lung Injury, Repair, and Fibrosis. Cells 2021, 10, 436.
- Puttur, F.; Gregory, L.G.; Lloyd, C.M. Airway macrophages as the guardians of tissue repair in the lung. Immunol. Cell Biol. 2019, 97, 246–257.
- Liegeois, M.; Legrand, C.; Desmet, C.J.; Marichal, T.; Bureau, F. The interstitial macrophage: A long-neglected piece in the puzzle of lung immunity. Cell. Immunol. 2018, 330, 91–96.
- Schyns, J.; Bureau, F.; Marichal, T. Lung Interstitial Macrophages: Past, Present, and Future. J. Immunol. Res. 2018, 2018, 5160794.
- Cosin-Roger, J.; Ortiz-Masià, M.D.; Barrachina, M.D. Macrophages as an Emerging Source of Wnt Ligands: Relevance in Mucosal Integrity. Front. Immunol. 2019, 10, 2297.
- Byrne, A.J.; Maher, T.M.; Lloyd, C.M. Pulmonary Macrophages: A New Therapeutic Pathway in Fibrosing Lung Disease? Trends Mol. Med. 2016, 22, 303–316.
- Hou, J.; Shi, J.; Chen, L.; Lv, Z.; Chen, X.; Cao, H.; Xiang, Z.; Han, X. M2 macrophages promote myofibroblast differentiation of LR-MSCs and are associated with pulmonary fibrogenesis. Cell Commun. Signal. 2018, 16, 89.
- Cao, Z.; Lis, R.; Ginsberg, M.; Chavez, D.; Shido, K.; Rabbany, S.Y.; Fong, G.-H.; Sakmar, T.; Rafii, S.; Ding, B.-S. Targeting of the pulmonary capillary vascular niche promotes lung alveolar repair and ameliorates fibrosis. Nat. Med. 2016, 22, 154–162.
- Sennello, J.A.; Misharin, A.V.; Flozak, A.S.; Berdnikovs, S.; Cheresh, P.; Varga, J.; Kamp, D.W.; Budinger, G.S.; Gottardi, C.J.; Lam, A.P. Lrp5/beta-Catenin Signaling Controls Lung Macrophage Differentiation and Inhibits Resolution of Fibrosis. Am. J. Respir. Cell Mol. Biol. 2017, 56, 191–201.
- Schaale, K.; Brandenburg, J.; Kispert, A.; Leitges, M.; Ehlers, S.; Reiling, N. Wnt6 is expressed in granulomatous lesions of Mycobacterium tuberculosis-infected mice and is involved in macrophage differentiation and proliferation. J. Immunol. 2013, 191, 5182–5195.
- Zhou, B.; Magana, L.; Hong, Z.; Huang, L.S.; Chakraborty, S.; Tsukasaki, Y.; Huang, C.; Wang, L.; Di, A.; Ganesh, B.; et al. The angiocrine Rspondin3 instructs interstitial macrophage transition via metabolic-epigenetic reprogramming and resolves inflammatory injury. Nat. Immunol. 2020, 21, 1430–1443.
- Li, P.; He, K.; Li, J.; Liu, Z.; Gong, J. The role of Kupffer cells in hepatic diseases. Mol. Immunol. 2017, 85, 222–229.
- Dou, L.; Shi, X.; He, X.; Gao, Y. Macrophage Phenotype and Function in Liver Disorder. Front. Immunol. 2019, 10, 3112.
- Irvine, K.M.; Clouston, A.D.; Gadd, V.L.; Miller, G.C.; Wong, W.-Y.; Melino, M.; Maradana, M.R.; MacDonald, K.; Lang, R.A.; Sweet, M.J.; et al. Deletion of Wntless in myeloid cells exacerbates liver fibrosis and the ductular reaction in chronic liver injury. Fibrogenesis Tissue Repair 2015, 8, 19.
- Carpino, G.; Nobili, V.; Renzi, A.; De Stefanis, C.; Stronati, L.; Franchitto, A.; Alisi, A.; Onori, P.; De Vito, R.; Alpini, G.; et al. Macrophage Activation in Pediatric Nonalcoholic Fatty Liver Disease (NAFLD) Correlates with Hepatic Progenitor Cell Response via Wnt3a Pathway. PLoS ONE 2016, 11, e0157246.
- Akcora, B.O.; Storm, G.; Bansal, R. Inhibition of canonical WNT signaling pathway by beta-catenin/CBP inhibitor ICG-001 ameliorates liver fibrosis in vivo through suppression of stromal CXCL12. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 804–818.
- Jiang, A.; Okabe, H.; Popovic, B.; Preziosi, M.E.; Pradhan-Sundd, T.; Poddar, M.; Singh, S.; Bell, A.; England, S.G.; Nagarajan, S.; et al. Loss of Wnt Secretion by Macrophages Promotes Hepatobiliary Injury after Administration of 3,5-Diethoxycarbonyl-1, 4-Dihydrocollidine Diet. Am. J. Pathol. 2019, 189, 590–603.
- Cui, J.; Li, M.; Liu, W.; Zhang, B.; Sun, B.; Niu, W.; Wang, Y. Liver kinase B1 overexpression controls mycobacterial infection in macrophages via FOXO1/Wnt5a signaling. J. Cell. Biochem. 2019, 120, 224–231.
- Au, D.T.; Migliorini, M.; Strickland, D.K.; Muratoglu, S.C. Macrophage LRP1 Promotes Diet-Induced Hepatic Inflammation and Metabolic Dysfunction by Modulating Wnt Signaling. Mediat. Inflamm. 2018, 2018, 7902841.
- Yang, Y.; Ye, Y.C.; Chen, Y.; Zhao, J.L.; Gao, C.C.; Han, H.; Liu, W.C.; Qin, H.Y. Crosstalk between hepatic tumor cells and macrophages via Wnt/beta-catenin signaling promotes M2-like macrophage polarization and reinforces tumor malignant behaviors. Cell Death Dis. 2018, 9, 793.
- Debebe, A.; Medina, V.; Chen, C.Y.; Mahajan, I.M.; Jia, C.; Fu, D.; He, L.; Zeng, N.; Stiles, B.W.; Chen, C.L.; et al. Wnt/beta-catenin activation and macrophage induction during liver cancer development following steatosis. Oncogene 2017, 36, 6020–6029.
- Na, Y.R.; Stakenborg, M.; Seok, S.H.; Matteoli, G. Macrophages in intestinal inflammation and resolution: A potential therapeutic target in IBD. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 531–543.
- Ruder, B.; Becker, C. At the Forefront of the Mucosal Barrier: The Role of Macrophages in the Intestine. Cells 2020, 9, 2162.
- Saha, S.; Aranda, E.; Hayakawa, Y.; Bhanja, P.; Atay, S.; Brodin, N.P.; Li, J.; Asfaha, S.; Liu, L.; Tailor, Y.; et al. Macrophage-derived extracellular vesicle-packaged WNTs rescue intestinal stem cells and enhance survival after radiation injury. Nat. Commun. 2016, 7, 13096.
- Roger, J.C.; Ortiz-Masia, M.D.; Calatayud, S.; Hernandez, C.; Álvarez, A.; Hinojosa, J.; Esplugues, J.V.; Barrachina, M.D. M2 macrophages activate WNT signaling pathway in epithelial cells: Relevance in ulcerative colitis. PLoS ONE 2013, 8, e78128.
- Cosín-Roger, J.; Ortiz-Masia, M.D.; Calatayud, S.; Hernandez, C.; Esplugues, J.V.; Barrachina, M.D. The activation of Wnt signaling by a STAT6-dependent macrophage phenotype promotes mucosal repair in murine IBD. Mucosal Immunol. 2016, 9, 986–998.
- Hernández, C.; Barrachina, M.D.; Cosín-Roger, J.; Ortiz-Masiá, D.; Álvarez; Terrádez, L.; Nicolau, M.J.; Alós, R.; Esplugues, J.V.; Calatayud, S. Progastrin represses the alternative activation of human macrophages and modulates their influence on colon cancer epithelial cells. PLoS ONE 2014, 9, e98458.
- Cao, Q.; Harris, D.C.H.; Wang, Y. Macrophages in kidney injury, inflammation, and fibrosis. Physiology 2015, 30, 183–194.
- Huen, S.C.; Cantley, L.G. Macrophages in Renal Injury and Repair. Annu. Rev. Physiol. 2017, 79, 449–469.
- Chen, T.; Cao, Q.; Wang, Y.; Harris, D.C. M2 macrophages in kidney disease: Biology, therapies, and perspectives. Kidney Int. 2019, 95, 760–773.
- Wen, Y.; Yan, H.-R.; Wang, B.; Liu, B.-C. Macrophage Heterogeneity in Kidney Injury and Fibrosis. Front. Immunol. 2021, 12, 681748.
- Lin, S.-L.; Li, B.; Rao, S.; Yeo, E.-J.; Hudson, T.E.; Nowlin, B.T.; Pei, H.; Chen, L.; Zheng, J.J.; Carroll, T.J.; et al. Macrophage Wnt7b is critical for kidney repair and regeneration. Proc. Natl. Acad. Sci. USA 2010, 107, 4194–4199.
- Lever, J.P.; Hull, T.D.; Boddu, R.; Pepin, M.; Black, L.M.; Adedoyin, O.O.; Yang, Z.; Traylor, A.M.; Jiang, Y.; Li, Z.; et al. Resident macrophages reprogram toward a developmental state after acute kidney injury. JCI Insight 2019, 4, e125503.
- Feng, Y.; Ren, J.; Gui, Y.; Wei, W.; Shu, B.; Lu, Q.; Xue, X.; Sun, X.; He, W.; Yang, J.; et al. Wnt/beta-Catenin-Promoted Macrophage Alternative Activation Contributes to Kidney Fibrosis. J. Am. Soc. Nephrol. 2018, 29, 182–193.
- Feng, Y.; Liang, Y.; Ren, J.; Dai, C. Canonical Wnt Signaling Promotes Macrophage Proliferation during Kidney Fibrosis. Kidney Dis. 2018, 4, 95–103.
- Feng, Y.; Liang, Y.; Zhu, X.; Wang, M.; Gui, Y.; Lu, Q.; Gu, M.; Xue, X.; Sun, X.; He, W.; et al. The signaling protein Wnt5a promotes TGFbeta1-mediated macrophage polarization and kidney fibrosis by inducing the transcriptional regulators Yap/Taz. J. Biol. Chem. 2018, 293, 19290–19302.
- Lafuse, W.P.; Wozniak, D.J.; Rajaram, M.V.S. Role of Cardiac Macrophages on Cardiac Inflammation, Fibrosis and Tissue Repair. Cells 2020, 10, 51.
- Hermans, K.C.; Blankesteijn, W.M. Wnt Signaling in Cardiac Disease. Compr. Physiol. 2015, 5, 1183–1209.
- Tao, H.; Yang, J.-J.; Shi, K.-H.; Li, J. Wnt signaling pathway in cardiac fibrosis: New insights and directions. Metabolism 2016, 65, 30–40.
- Foulquier, S.; Daskalopoulos, E.P.; Lluri, G.; Hermans, K.C.M.; Deb, A.; Blankesteijn, W.M. WNT Signaling in Cardiac and Vascular Disease. Pharmacol. Rev. 2018, 70, 68–141.
- Minutti, C.M.; Knipper, J.A.; Allen, J.E.; Zaiss, D.M.W. Tissue-specific contribution of macrophages to wound healing. Semin. Cell Dev. Biol. 2017, 61, 3–11.
- Gieseck, R.L., 3rd; Wilson, M.S.; Wynn, T.A. Type 2 immunity in tissue repair and fibrosis. Nat. Rev. Immunol. 2018, 18, 62–76.
- Malsin, E.S.; Kim, S.; Lam, A.P.; Gottardi, C.J. Macrophages as a Source and Recipient of Wnt Signals. Front. Immunol. 2019, 10, 1813.
- Gay, D.; Ghinatti, G.; Guerrero-Juarez, C.F.; Ferrer, R.A.; Ferri, F.; Lim, C.H.; Murakami, S.; Gault, N.; Barroca, V.; Rombeau, I.; et al. Phagocytosis of Wnt inhibitor SFRP4 by late wound macrophages drives chronic Wnt activity for fibrotic skin healing. Sci. Adv. 2020, 6, eaay3704.
- Stefater, J.A., 3rd; Rao, S.; Bezold, K.; Aplin, A.C.; Nicosia, R.F.; Pollard, J.W.; Ferrara, N.; Lang, R.A. Macrophage Wnt-Calcineurin-Flt1 signaling regulates mouse wound angiogenesis and repair. Blood 2013, 121, 2574–2578.
- Castellana, D.; Paus, R.; Perez-Moreno, M. Macrophages contribute to the cyclic activation of adult hair follicle stem cells. PLoS Biol. 2014, 12, e1002002.
