Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by I Made Joni and Version 2 by Conner Chen.
According to the literature, 70% of the active ingredients obtained from plants are hydrophobic. New technology has been used as a strategy to increase the bioavailability/bioactivity of phytochemical compounds. In order to develop new nanotechnology-based therapies, the ability to design suitable formulations for drug delivery is of the utmost importance. Phytochemical delivery is essential for effective disease prevention and treatment. These delivery systems include lipid-based delivery systems and polymer-based delivery systems, which have the potential to increase the bioactivity of phytochemical compounds.
- phytochemical
- herbal medicine
- drug delivery
1. Introduction
The traditional use of plants, both used directly and extracted, for medicinal purposes has existed since ancient times [1][2][1,2]. Plants are a source of various phytochemicals and have been used for human health because of their low side effects, low cost, and high acceptance by the general public [3].
Phytochemicals are substances produced by plants (also known as secondary metabolites) that play an important role in traditional medicine. Secondary metabolites have been shown to exhibit various biological activities, which provide a scientific basis for the use of herbs in traditional medicine. They show pharmacological effects that can be used to treat bacterial and fungal infections and even chronic degenerative diseases, such as diabetes and cancer [4][5][4,5].
Herbal medicines are increasingly popular throughout the world and have promising potential to provide treatment, maintain and improve health, as well as prevent and treat several diseases because they are considered safe compared to modern conventional medicines and are more economical [6][7][6,7]. However, most of these biologically active phytochemical constituents have limitations; namely, their absorption and distribution are low, and the target specificity of phytochemicals is generally low, which results in low bioavailability, resulting in decreased biological activity. In addition, large doses are required to produce the activity of these phytochemical compounds, and also some of these phytochemical compounds are sensitive to acidic conditions and have low stability phytochemical compounds [6][8][9][10][11][6,8,9,10,11]. These limits hinder their clinical application.
Puerarin is the main active compound isolated from the roots of Pueraria lobata (Willd.) Ohwi, which has broad pharmacological activity. Puerarin is used for cardiovascular disease, diabetes, osteonecrosis, Parkinson’s disease, Alzheimer’s disease, endometriosis, and cancer [12]. Puerarin has a low solubility in water: 0.46 mg/mL. The maximum solubility of puerarin occurs in a phosphate buffer of pH 7.4 at 7.56 mg/mL [13][14][13,14]. The low solubility limits the application of puerarin. In recent years, research on increasing the bioavailability of puerarin has grown rapidly. Various nanotechnology has been investigated to increase the bioavailability of puerarin, one of which is the solid lipid nanoparticle (SLN) carrier system. Compared to puerarin suspension, SLN-puerarin is absorbed rapidly. This is supported by a shorter Tmax. In addition, SLN-puerarin showed more than threefold bioavailability compared to puerarin suspension [15][16][15,16].
Nanotechnology-based delivery systems function as drug carriers that can overcome the various limitations that herbal medicines face, including increasing the bioavailability and bioactivity of phytochemicals. The approach using nanotechnology can be a promising innovative technology that is applied to phytochemical constituents, increasing the phytotherapy efficiency of herbal medicines. The development of an efficient and safe drug delivery system is the goal of various researchers. Recent developments in the field of nanotechnology have led to renewed interest in herbal medicinal formulations. Several delivery system approaches, such as phytosomes, solid lipid nanoparticles (SLN), nanostructured lipid carriers (NLC), polymeric nanoparticles, nanoemulsions, etc., have been proposed. Nanoparticles have been used to modify and improve the pharmacokinetic properties of different drugs, so the nanotechnology approach is expected to increase the bioavailability and bioactivity of herbal medicines [6][10][17][18][6,10,17,18].
2. Nanotechnology-Based Drug Delivery System for Phytochemical Compounds
According to the literature, 70% of the active ingredients obtained from plants are hydrophobic [3]. New technology has been used as a strategy to increase the bioavailability/bioactivity of phytochemical compounds. In order to develop new nanotechnology-based therapies, the ability to design suitable formulations for drug delivery is of the utmost importance. Phytochemical delivery is essential for effective disease prevention and treatment. These delivery systems include lipid-based delivery systems and polymer-based delivery systems, which have the potential to increase the bioactivity of phytochemical compounds (Figure 12) [19][20][21][22,24,25].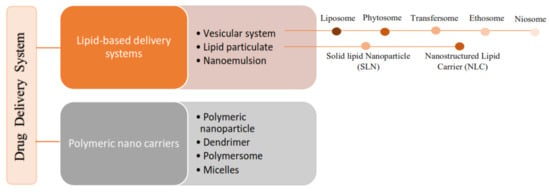
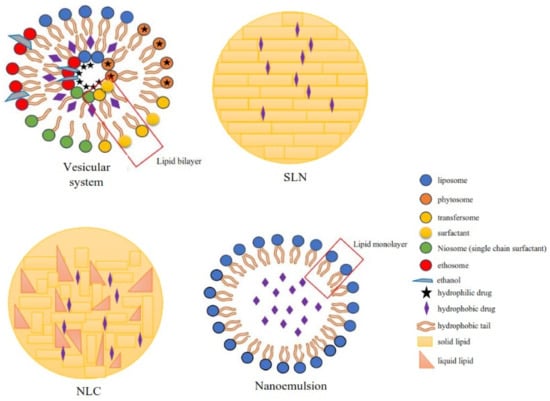
Figure 23.
Classification of the lipid-based delivery system.
2.1. Phytosomes Increasing the Activity of Phytochemical Compounds
Sinigrin is a glucosinolate found in the Brassicaceae family [41][62]. Mazumder et al. formulated sinigrin into a phytosome delivery system, intending to increase bioavailability and overcome the problem of the solubility of sinigrin, which was then assessed through its activity. It is known that sinigrin has wound-healing activity [42][49]. In a study conducted by Mazumder et al. (2016), the wound-healing activity of sinigrin was compared with the activity of the phytosome–sinigrin complex [41][62] Sinigrin-phytosome showed a significant wound-healing effect when compared to pure sinigrin. After 42 h, the phytosome–sinigrin complex healed 100% of the wounds, whereas pure sinigrin only showed 71% wound closure [42][49]. This proves that sinigrin-phytosome increases wound-healing activity.
Taxifolin, also known as dihydroquercetin, is a bioactive flavonoid that has an antioxidant effect [43][50]. Taxifolin can eliminate the excess of free radicals, enhancing immune functioning and reducing cancer cell formation in the human body [44][63]. From a literature study, it was shown that taxifolin has anticancer and antiproliferative effects on murine skin fibroblasts and human breast cancer cells [45][64]. However, taxifolins have limitations in their hydrophilicity and have a large molecular size, limiting their clinical application [46][65].
Kumar, et al. (2021) conducted a study in which a taxifolin ethyl acetate fraction derived from Cedrus deodara bark extract was loaded into a phytosome delivery system, with aims to evaluate the antioxidant and anticancer activity of taxifolin [43][50]. Phytosomes can increase the lipophilicity of the active compound so that they enhance the absorption of the active substance and increase its ability to cross biological membranes [47][66]. Based on the research of Kumar, et al. (2021), phytosomes showed high antioxidant activity with a lower IC50 value compared to the ethyl acetate and the standard. The results of the TB assay and MTT assay using the MCF7 cell line helped to conclude that the phytosome dilution (100 mg) showed significant anticancer activity compared to the ethyl acetate fraction [43][50].
Silybin is one of the main polyphenols that has been shown to exhibit many biological activities, including protecting the liver from oxidative stress and cancer formation as well as ongoing inflammatory processes [48][51]. However, this silybin has poor water solubility and low gastrointestinal absorption [49][67]. In order to overcome these limitations, Chi et al. (2020) conducted a study in which silybin was loaded into the delivery system of phytosome-nanosuspensions to enhance the hepatoprotective activity of silybin, which was tested in CCl4-induced model mice [48][51].
The induction using CCl4 resulted in an increase in the serum levels of ALT, AST, and AKP in the positive controls. Mice treated with silybin phytosome-nanosuspensions (SPc-NPs) showed significant reductions in ALT, AST, and AKP after CCl4 induction. In addition, histology of the liver sections from the mice treated with SPc-NPs was performed. The histology results showed clearly that SPc-NPs prevented hepatocyte necrosis, and also provided fewer fatty acid particles compared to the silybin-treated group. This is evidence of the enhanced hepatoprotective effect of Silybin phytosome-nanosuspensions [48][51].
Apigenin is a hydrophobic flavonoid compound that exhibits many biological activities, such as antioxidant, antimicrobial, anti-inflammatory, antiviral, antidiabetic, etc. [50][51][52][53][68,69,70,71]. Apigenin is reported to have excellent antioxidant and hepatoprotective properties. However, apigenin has limitations, namely, poor water solubility, fast metabolism, and low oral bioavailability, thus limiting its clinical application [54][55][72,73]. In order to overcome the limitations of apigenin, Telage et al. (2016) conducted a study where apigenin was loaded into a phytosome delivery system [56][52].
After being loaded into the phytosome delivery system, the solubility of apigenin-phytosome (APLC) in water increased compared to pure apigenin. In addition, the release of APLC was higher than that of pure apigenin, and the bioavailability of APLC increased compared to pure apigenin; this could be seen from the parameters Cmax, Tmax, and AUC. Apigenin activity as an antioxidant was tested by CCl4-induced hepatotoxicity models in rats; APLC showed significant antioxidant activity compared to free apigenin. This can be seen from the significant decrease in antioxidant activity indicators, such as glutathione, superoxide dismutase, catalase, and lipid peroxidase [56][52].
Quercetin is known to have poor absorption when given orally. In order to overcome this problem, El-Fattah et al. (2017) loaded quercetin into a phytosome delivery system to increase absorption and increase quercetin activity. Quercetin, as a phytoestrogen, is known to stimulate estrogen receptors (ERα and ERβ) [57][74]. In this study, quercetin was used for hormone replacement therapy [58][53].
Menopause symptoms that occur in women (aged 45–55 years) include an increase in food intake and body weight, metabolic dysfunction, bone density loss, diabetes, impaired muscle function, hyperlipidemia, psychological and mood changes, increased inflammatory markers, and oxidative stress, which can result in cell membrane lipid peroxidation and protein and DNA damage [59][75] Therefore, hormone replacement therapy has been the standard approach to relieve these symptoms.
The estrogenic activity of quercetin was observed at doses of 10 and 50 mg/kg/day using a mouse model that was ovariectomized for four weeks. These estrogenic activities include inflammation, oxidative stress, lipid profile, blood sugar levels, and bone and weight gain [58][53].
Treatment with quercetin-phytosome showed an increase in serum inorganic calcium phosphorus and glutathione content, as well as an improvement in the lipid profile. In addition, qurcetin-phytosome was found to decrease serum alkaline phosphatase, acid phosphatase, malondialdehyde, tumor necrosis factor-alpha, and blood sugar levels [58][53].
Quercetin-phytosome at a dose of 50 mg/kg showed better results than that of free quercetin. This is due to the better absorption of quercetin-phytosome. The results showed that antioxidant, anti-inflammatory, hypolipidemic, and other activities increased after quercetin was loaded into the phytosome delivery system [58][53].
Singh et al. (2018) carried out a gingerol formulation that was loaded into a phytosome delivery system, which was then complexed with chitosan to overcome the problem of respiratory tract infections [60][54]. Gingerol is a polyphenol derived from Zingiber officinale, which has many biological activities, one of which can protect cells against oxidative stress. In addition, gingerols can boost the immune system caused by free radicals, viruses, etc. However, these gingerols exhibit low bioavailability and water solubility profiles [61][62][63][76,77,78].
2.2. Polymeric Nanoparticle to Increase the Activity of Phytochemical Compounds
In a study conducted by Rani et al. (2020), the formulation of piperine-loaded nanocapsules (PNCs) was carried out to determine the resulting antitrypanosomal activity against Trypanosoma evansi, which causes trypanosomiasis [64][59]. Piperine is an alkaloid compound and the main bioactive component in pepper. Piperine has been reported to exhibit antioxidant, antitumor, antiasthmatic, antipyretic, analgesic, anti-inflammatory, antibacterial, and antifungal activities. In addition, piperine has been reported to induce toxic and apoptotic effects in protozoan parasites [65][66][88,89]. However, piperine has limitations, including low water solubility, fast metabolism, and undergoes systemic elimination [66][67][89,90]. This can be overcome by a nanotechnology-based delivery system; in this case, piperine is loaded into polymer nanocapsules using the emulsion-diffusion method [64][59].
Piperine loaded into a nanocapsule has a sustained release compared to pure piperine. The Piperine and PNCs were tested for growth inhibition studies in an axenic culture for three days. Piperine loaded into NCs (PNCs) showed significantly more antitrypanosomal activity with a 3.0-fold lower IC50 value than pure piperine (IC50 PNCs 5.04 M; IC50 piperine 14.45 M) [64][59]. Piperine nanoencapsulation, in the form of nanocapsules, showed a more significant antitrypanosomal effect than pure piperine. This is due to the increased absorption, bioavailability, and sustained release of piperine from the delivery system.
Jatropha pelargoniifolia (JP) is a medicinal plant rich in phenolics and flavonoids, which could be the major contributors to oxidative defense mechanisms in human cells. JP is widely used in traditional medicine because of its broad therapeutic activity [68][91]. However, JP has limitations, including poor solubility, bioavailability, and stability, and is sensitive to gastric acid pH so it cannot be administered in conventional dosage forms [69][70][71][92,93,94]. Alqahtani et al. (2021) conducted a study to overcome these limitations with nanotechnology, where JP-loaded chitosan nanoparticles (JP-CSNPs) were investigated for their antioxidant activity with antibacterial and anticancer potential [72][58].
The antioxidant activity of JP-CSNP was evaluated by a 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay. The results showed that the antioxidant activity of JP-CSNP was slightly higher than that of the pure extract. The DPPH results showed that JP-CSNP had higher antimicrobial activity against Gram-positive bacteria with a 1.6-fold lower IC50 than the empty nanoparticles. The anticancer activity of the extracts of JP and JP-CSNP was tested using A549 adenocarcinoma human alveolar cells. The IC50 value of JP-CSNPs was two times lower than that of the JP extract [72][58]. Based on the results of the DPPH test and cytotoxic test on A549 human lung adenocarcinoma cells, it was shown that the Jatropha pelargoniifolia (JP) contained in chitosan nanoparticles antimicrobial and anticancer potential, where its activity is slightly higher than the free extract.
