Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Dean Liu and Version 1 by Kok Yong Chin.
Various natural compounds can positively influence the skeletal remodelling process, of which naringenin is a candidate. Naringenin is an anti-inflammatory and antioxidant compound found in citrus fruits and grapefruit.
- naringenin
- osteoblasts
- osteoclasts
- osteocytes
1. Introduction
Osteoporosis is a degenerative skeletal condition characterised by reduced bone mass and microstructural deterioration, which subsequently lead to decreased bone strength and an increased fragility fracture risk [1]. Osteoporosis is asymptomatic until it presents as low-trauma fractures of the hip, spinal, proximal humerus, pelvis, and/or wrist [2]. Osteoporosis is more prominent in postmenopausal women because estrogen insufficiency accelerates bone loss [3]. The use of antiresorptive (e.g., bisphosphonates, denosumab, and selective estrogen receptor modulators) and anabolic medications (e.g., teriparatide and abaloparatide) can improve bone mineral density (BMD) and reduce the fracture risk of patients with osteoporosis [4,5][4][5]. However, they come with various side effects [6,7][6][7].
Bone loss occurs when the rate of osteoblastic bone formation is lower than the rate of osteoclastic bone resorption [8]. Various factors could influence the bone turnover process. Inflammation, known to promote bone resorption, is a risk factor for osteoporosis [9]. Proinflammatory cytokines stimulate the expression of receptor activators of nuclear factor-B (RANK) and its functional ligand (RANKL), along with macrophage colony-stimulating factor (M-CSF), which enhance osteoclast formation and function [8]. Furthermore, modifications in redox systems have been linked to the pathogenesis of osteoporosis. Reactive oxygen species (ROS) inhibit osteoblast formation, stimulate apoptosis in osteoblasts and osteocytes, and encourage the formation of osteoclasts [10], all of which result in bone loss and osteoporosis.
Apart from calcium and vitamin D routinely used in osteoporosis prevention [11], dietary antioxidants and anti-inflammatory compounds may slow the progression of osteoporosis [10,12][10][12]. Naringenin (Figure 1) is a flavanone present in citrus fruits, grapes, and tomato skin [13]. Naringenin has been investigated for its antioxidant [14] and anti-inflammatory properties [15]. Previous research found that naringenin suppressed nuclear factor-kappa B (NF-kB) p65 activity and expression in streptozotocin (STZ)-induced diabetic mice [16] and carrageenan-induced paw oedema in rats [17]. In STZ and nicotinamide-induced diabetic rats, naringenin significantly increased the activities of pancreatic enzymatic antioxidants, plasma non-enzymatic antioxidant levels and decreased pancreatic tissue malondialdehyde levels [18]. Meanwhile, another report indicated that naringenin lowered lipid peroxidation and enhanced the activity of antioxidant enzymes, such as superoxide dismutase, catalase, glutathione-s-transferase, glutathione peroxidase and reduced glutathione in the liver of STZ-induced diabetic mice [19]. The studies mentioned above point to the potential of naringenin as an antioxidant and anti-inflammatory agent, which could help to suppress bone loss.
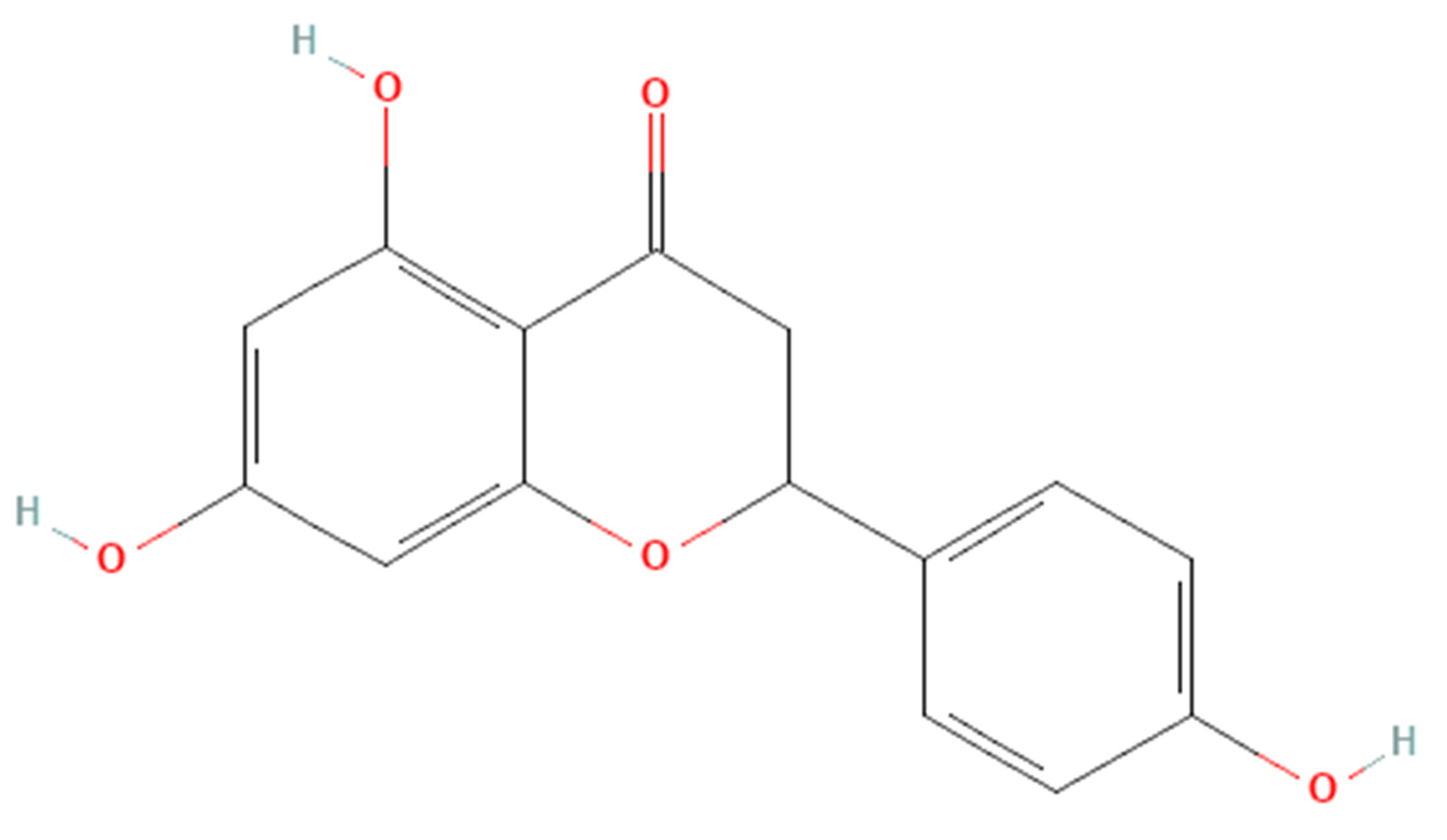
Figure 1. The molecular structure of naringenin.
2. Skeletal Effects of Naringenin on Osteoblasts
The currently available evidence shows that naringenin exerts stimulatory effects on osteoblasts through MAPK, PI3K/Akt, and CXCR4/SDF-1 pathways. Osteoblasts are responsible for osteogenesis by synthesising and mineralising organic bone matrix (osteoid) during skeleton construction and bone remodelling [41][20]. The MAPK cascades regulate Runx2 phosphorylation and transcription, which promote osteoblast differentiation. MAPK pathways and their components, JNK, ERK, and p38, which enforce osteoblastogenesis and establish the non-canonical BMP-2 signal transduction pathways [42,43,44,45][21][22][23][24]. Naringenin stimulates osteogenic gene activation, indicating that it has a stimulatory effect on osteogenic differentiation [21,22][25][26]. Activation of the PI3K/Akt signalling pathway also promotes osteoblast proliferation, differentiation, and bone formation activity [46][27]. Naringenin has been reported to stimulate BMP-2-dependent osteoblastogenesis through the activation of the PI3K/Akt signalling pathway [27][28]. Activation of the CXCR4/SDF-1 signalling pathway is critical in early osteoblastogenesis and its suppression leads to lower bone formation and mineralisation [47,48][29][30].
Osteoclasts are bone resorption cells originating from hematopoietic lineage cells [49][31]. Bone resorption is vital in bone remodelling, but excessive resorption can result in pathological bone loss. Osteoclast differentiation and activation are governed by various hormones and cytokines. The cytokines RANKL and M-CSF, in particular, are required for osteoclastic differentiation [50][32]. To promote osteoclast differentiation, preservation and bone resorption, the M-CSF binds to the colony-stimulating factor 1 receptor, whilst RANKL binds to the RANK receptor [51,52][33][34]. TRAF factors such as TRAF 6 are recruited by RANK-RANKL binding [53][35], leading to the activation of NF-kB, Akt, and MAPKs (ERK/p38/JNK) pathways. Furthermore, the RANKL signalling stimulates c-Fos and then NFATc1, a major switch that plays a role in controlling osteoclast terminal differentiation [54,55][36][37]. Naringenin was reported to reduce M-CSF and RANKL-induced expression of critical markers of osteoclast differentiation markers such as cathepsin K, c-Fos, and NFATc1 [24,25][38][39].
The biological properties of naringenin suggest a broad range of clinical applications. Naringenin decreased CAB-CEJ distance in buccal maxilla and mandible as well as in lingua maxilla and mandible, indicating that naringenin supplementation protects against alveolar bone loss in rats with induced periodontal disease [33][40]. Supplementation of naringenin also improved bone mineral microstructure, mineral, and biomechanical strength [23,27,31,32][28][41][42][43]. However, the bone loss models that have been used to test the effects of naringenin have been limited to OVX and retinol-induced models. Thus, results from other models, such as testosterone deficiency and glucocorticoid models, are indispensable before it is tested on patients with other causes of osteoporosis. Following joint replacement, the abrasive particles initiated by the prosthesis are primarily responsible for osteolysis [56][44]. Naringenin supplementation prevented Ti-particle-induced osteolysis, implying that it may be preferable for treating periprosthetic osteolysis [25][39].
Pharmacokinetics and safety issues of naringenin should be considered before it is used clinically. From the pharmacokinetic aspects, naringenin has very low in vivo bioavailability due to its hydrophobic nature, which limits its practical use. It has a short half-life and is easily converted to its crystalline form, and therefore it is poorly absorbed by the digestive system [57,58,59,60][45][46][47][48]. Previous researchers developed a variety of methods to improve naringenin absorption and low bioavailability, including particle size reduction, complexation with cyclodextrins [61][49], salt formation, solid dispersions [62][50], surfactant usage, nanoparticles, nanocarriers [63][51], and self-emulsifying drug delivery system, as well as prodrug formation [64][52]. Nanotechnology proved to be an efficient way to improve the bioavailability of naringenin by multiple delivery routes to enhance its effectiveness in the treatment of cancer, inflammation, diabetes, liver, brain, and ocular diseases mostly through numerous in vitro and in vivo methods [65][53]. Meanwhile, Rodríguez-Fragoso et al., (2011) [66][54] found out that naringenin inhibits some drug-metabolizing cytochrome P450 enzymes, including CYP3A4 and CYP1A2, potentially leading to drug-drug interactions in the intestine and liver, where phytochemical concentrations are higher. Modification in cytochrome P450 and other enzymatic activity may influence the outcome of drugs that go through extensive first-pass metabolism. An acute toxicity study using Wistar rats reported the lethal dose (LD50) value of naringenin to be 340 mg/kg body weight [67][55]. Using body surface ratio conversation [68][56], the human equivalent dose is 64 mg/kg.
The term “naringenin” was searched for on https://clinicaltrials.gov/ (accessed on 31 August 2022) and the search revealed thirteen registered clinical trials on naringenin. The trials investigate the effects of naringenin on healthy subjects (NCT02627547, NCT04867655, NCT05073523, NCT02380144), hepatitis virus/HCV infection/chronic HCV/Hepatitis C (NCT01091077), energy expenditure/safety issues/glucose metabolism (NCT04697355), safety issues/pharmacokinetics (NCT03582553), subjective cognitive decline (NCT04744922), cardiovascular disease risk factors (NCT00539916), intestinal disease (NCT03032861), metabolic syndrome/vascular compliance/predisposition to cardiovascular disease (NCT04731987), pharmacokinetics of new curcumin formulations/safety of new curcumin formulations (NCT01982734) and cardiovascular risk factor/type-2 diabetes mellitus/insulin sensitivity/metabolic syndrome (NCT03527277). Seven of these clinical trials have been completed, four are still recruiting, one with an unknown status, and lastly one trial is still active but not recruiting. However, no attempt has been made to conduct a human clinical trial to evaluate the impact of naringenin on skeletal diseases. Since pure naringenin has only been studied in limited clinical trials, more research on free drug and naringenin-loaded nanosystems in humans is warranted. Further exploration into the interactions of these nanoformulations with the human body is required before they can be translated into pharmaceuticals and nutraceutical supplements [69][57].
References
- Sozen, T.; Ozisik, L.; Calik Basaran, N. An overview and management of osteoporosis. Eur. J. Rheumatol. 2017, 4, 46–56.
- Cosman, F.; de Beur, S.J.; LeBoff, M.S.; Lewiecki, E.M.; Tanner, B.; Randall, S.; Lindsay, R. Clinician’s Guide to Prevention and Treatment of Osteoporosis. Osteoporos. Int. 2014, 25, 2359–2381.
- Deeks, E.D. Denosumab: A Review in Postmenopausal Osteoporosis. Drugs Aging 2018, 35, 163–173.
- Baber, R.J.; Panay, N.; Fenton, A. 2016 IMS Recommendations on womens midlife health and menopause hormone therapy. Climacteric 2016, 19, 109–150.
- Shoback, D.; Rosen, C.J.; Black, D.M.; Cheung, A.M.; Murad, M.H.; Eastell, R. Pharmacological Management of Osteoporosis in Postmenopausal Women: An Endocrine Society Guideline Update. J. Clin. Endocrinol. Metab. 2020, 105, 587–594.
- Black, D.M.; Rosen, C.J. Clinical Practice. Postmenopausal Osteoporosis. N. Engl. J. Med. 2016, 374, 254–262.
- Sleeman, A.; Clements, J.N. Abaloparatide: A new pharmacological option for osteoporosis. Am. J. Health-Syst. Pharm. 2019, 76, 130–135.
- Lacativa, P.G.S.; de Farias, M.L.F. Osteoporose e inflamação. Arq. Bras. Endocrinol. Metabol. 2010, 54, 123–132.
- Ginaldi, L.; Di Benedetto, M.C.; De Martinis, M. Osteoporosis, inflammation and ageing. Immun. Ageing 2005, 2, 14.
- Domazetovic, V.; Marcucci, G.; Iantomasi, T.; Brandi, M.L.; Vincenzini, M.T. Oxidative stress in bone remodeling: Role of antioxidants. Clin. Cases Miner. Bone Metab. 2017, 14, 209–216.
- Tang, B.M.; Eslick, G.D.; Nowson, C.; Smith, C.; Bensoussan, A. Use of calcium or calcium in combination with vitamin D supplementation to prevent fractures and bone loss in people aged 50 years and older: A meta-analysis. Lancet 2007, 370, 657–666.
- Ekeuku, S.O.; Pang, K.L.; Chin, K.Y. Effects of caffeic acid and its derivatives on bone: A systematic review. Drug Des. Dev. Ther. 2021, 15, 259–275.
- Chen, H.J.; Inbaraj, B.S.; Chen, B.H. Determination of phenolic acids and flavonoids in Taraxacum formosanum kitam by liquid chromatography-tandem mass spectrometry coupled with a post-column derivatization technique. Int. J. Mol. Sci. 2012, 13, 260–285.
- Renugadevi, J.; Prabu, S.M. Naringenin protects against cadmium-induced oxidative renal dysfunction in rats. Toxicology 2009, 256, 128–134.
- Shi, Y.; Dai, J.; Liu, H.; Li, R.R.; Sun, P.L.; Du, Q.; Pang, L.L.; Chen, Z.; Yin, K.S. Naringenin inhibits allergen-induced airway inflammation and airway responsiveness and inhibits NF-κB activity in a murine model of asthma. Can. J. Physiol. Pharmacol. 2009, 87, 729–735.
- Tsai, S.J.; Huang, C.S.; Mong, M.C.; Kam, W.Y.; Huang, H.Y.; Yin, M.C. Anti-inflammatory and antifibrotic effects of naringenin in diabetic mice. J. Agric. Food Chem. 2012, 60, 514–521.
- Chung, T.W.; Li, S.; Lin, C.C.; Tsai, S.W. Antinociceptive and anti-inflammatory effects of the citrus flavanone naringenin. Tzu Chi Med. J. 2019, 31, 81–85.
- Annadurai, T.; Muralidharan, A.R.; Joseph, T.; Hsu, M.J.; Thomas, P.A.; Geraldine, P. Antihyperglycemic and antioxidant effects of a flavanone, naringenin, in streptozotocin-nicotinamide-induced experimental diabetic rats. J. Physiol. Biochem. 2012, 68, 307–318.
- Rashmi, R.; Magesh, S.B.; Ramkumar, K.M.; Suryanarayanan, S.; SubbaRao, M.V. Antioxidant potential of naringenin helps to protect liver tissue from streptozotocin-induced damage. Rep. Biochem. Mol. Biol. 2017, 7, 76–84.
- Tanaka, Y.; Nakayamada, S.; Okada, Y. Osteoblasts and osteoclasts in bone remodeling and inflammation. Curr. Drug Targets Inflamm. Allergy 2005, 4, 325–328.
- Xiao, G.; Gopalakrishnan, R.; Jiang, D.; Reith, E.; Benson, M.D.; Franceschi, R.T. Bone morphogenetic proteins, extracellular matrix, and mitogen-activated protein kinase signaling pathways are required for osteoblast-specific gene expression and differentiation in MC3T3-E1 cells. J. Bone Miner. Res. 2002, 17, 101–110.
- Massagué, J.; Blain, S.W.; Lo, R.S. TGFβ signaling in growth control, cancer, and heritable disorders. Cell 2000, 103, 295–309.
- Nohe, A.; Hassel, S.; Ehrlich, M.; Neubauer, F.; Sebald, W.; Henis, Y.I.; Knaus, P. The mode of bone morphogenetic protein (BMP) receptor oligomerization determines different BMP-2 signaling pathways. J. Biol. Chem. 2002, 277, 5330–5338.
- Guo, X.; Wang, X.F. Signaling cross-talk between TGF-β/BMP and other pathways. Cell Res. 2009, 19, 71–88.
- Ming, L.G.; Ge, B.F.; Wang, M.G.; Chen, K.M. Comparison between 8-prenylnarigenin and narigenin concerning their activities on promotion of rat bone marrow stromal cells’ osteogenic differentiation in vitro. Cell Prolif. 2012, 45, 508–515.
- Ming, L.-G.; Lv, X.; Ma, X.-N.; Ge, B.-F.; Zhen, P.; Song, P.; Zhou, J.; Ma, H.-P.; Xian, C.J.; Chen, K.-M. The Prenyl Group Contributes to Activities of Phytoestrogen 8-Prenynaringenin in Enhancing Bone Formation and Inhibiting Bone Resorption In Vitro. Endocrinology 2013, 154, 1202–1214.
- Xi, J.C.; Zang, H.Y.; Guo, L.X.; Xue, H.B.; Liu, X.D.; Bai, Y.B.; Ma, Y.Z. The PI3K/AKT cell signaling pathway is involved in regulation of osteoporosis. J. Recept. Signal Transduct. 2015, 35, 640–645.
- Wu, J.-B.; Fong, Y.C.; Tsai, H.Y.; Chen, Y.F.; Tsuzuki, M.; Tang, C.H. Naringin-induced bone morphogenetic protein-2 expression via PI3K, Akt, c-Fos/c-Jun and AP-1 pathway in osteoblasts. Eur. J. Pharmacol. 2008, 588, 333–341.
- Xu, J.; Chen, Y.; Liu, Y.; Zhang, J.; Kang, Q.; Ho, K.; Chai, Y.; Li, G. Effect of SDF-1/Cxcr4 Signaling Antagonist AMD3100 on Bone Mineralization in Distraction Osteogenesis. Calcif. Tissue Int. 2017, 100, 641–652.
- Luan, J.; Cui, Y.; Zhang, Y.; Zhou, X.; Zhang, G.; Han, J. Effect of CXCR4 inhibitor AMD3100 on alkaline phosphatase activity and mineralization in osteoblastic MC3T3-E1 cells. Biosci. Trends 2012, 6, 63–69.
- Soysa, N.S.; Alles, N. Osteoclast function and bone-resorbing activity: An overview. Biochem. Biophys. Res. Commun. 2016, 476, 115–120.
- Ono, T.; Nakashima, T. Recent advances in osteoclast biology. Histochem. Cell Biol. 2018, 149, 325–341.
- Suda, T.; Takahashi, N.; Udagawa, N.; Jimi, E.; Gillespie, M.T.; Martin, T.J. Modulation of osteoclast differentiation and function by the new members of the tumor necrosis factor receptor and ligand families. Endocr. Rev. 1999, 20, 345–357.
- Mun, S.H.; Park, P.S.U.; Park-Min, K.H. The M-CSF receptor in osteoclasts and beyond. Exp. Mol. Med. 2020, 52, 1239–1254.
- Bharti, A.C.; Takada, Y.; Shishodia, S.; Aggarwal, B.B. Evidence That Receptor Activator of Nuclear Factor (NF)-κB Ligand Can Suppress Cell Proliferation and Induce Apoptosis through Activation of a NF-κB-independent and TRAF6-dependent Mechanism. J. Biol. Chem. 2004, 279, 6065–6076.
- Takayanagi, H.; Kim, S.; Koga, T.; Nishina, H.; Isshiki, M.; Yoshida, H.; Saiura, A.; Isobe, M.; Yokochi, T.; Inoue, J.; et al. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev. Cell 2002, 3, 889–901.
- Ishida, N.; Hayashi, K.; Hoshijima, M.; Ogawa, T.; Koga, S.; Miyatake, Y.; Kumegawa, M.; Kimura, T.; Takeya, T. Large scale gene expression analysis of osteoclastogenesis in vitro and elucidation of NFAT2 as a key regulator. J. Biol. Chem. 2002, 277, 41147–41156.
- Wang, W.; Li, M.; Luo, M.; Shen, M.; Xu, C.; Xu, G.; Chen, Y.; Xia, L. Naringenin inhibits osteoclastogenesis through modulation of helper T cells-secreted IL-4. J. Cell. Biochem. 2018, 119, 2084–2093.
- Wang, W.; Wu, C.; Tian, B.; Liu, X.; Zhai, Z.; Qu, X.; Jiang, C.; Ouyang, Z.; Mao, Y.; Tang, T.; et al. The inhibition of RANKL-induced osteoclastogenesis through the suppression of p38 signaling pathway by naringenin and attenuation of titanium-particle-induced osteolysis. Int. J. Mol. Sci. 2014, 15, 21913–21934.
- Wood, N. The effects of dietary naringenin supplementation on physiological changes in molar crestal alveolar bone-cemento-enamel junction distance in young rats. J. Med. Food 2005, 8, 31–35.
- Swarnkar, G.; Sharan, K.; Siddiqui, J.A.; Mishra, J.S.; Khan, K.; Khan, M.P.; Gupta, V.; Rawat, P.; Maurya, R.; Dwivedi, A.K.; et al. A naturally occurring naringenin derivative exerts potent bone anabolic effects by mimicking oestrogen action on osteoblasts. Br. J. Pharmacol. 2012, 165, 1526–1542.
- Kaczmarczyk-Sedlak, I.; Wojnar, W.; Zych, M.; Ozimina-Kamińska, E.; Bońka, A. Effect of dietary flavonoid naringenin on bones in rats with ovariectomy-induced osteoporosis. Acta Pol. Pharm.-Drug Res. 2016, 73, 1073–1081.
- Oršolić, N.; Goluža, E.; Dikić, D.; Lisičić, D.; Sašilo, K.; Rodak, E.; Jeleč, Ž.; Lazarus, M.V.; Orct, T. Role of flavonoids on oxidative stress and mineral contents in the retinoic acid-induced bone loss model of rat. Eur. J. Nutr. 2014, 53, 1217–1227.
- Yao, J.; Ma, S.; Feng, W.; Wei, Y.; Lu, H.; Zhong, G.; Wu, Z.; Wang, H.; Su, W.; Li, J. Tanshinone IIA protects against polyethylene particle-induced osteolysis response in a mouse calvarial model. Int. J. Clin. Exp. Pathol. 2018, 11, 4461–4471.
- Kumar, R.P.; Abraham, A. PVP- coated naringenin nanoparticles for biomedical applications—In vivo toxicological evaluations. Chem. Biol. Interact. 2016, 257, 110–118.
- Shpigelman, A.; Shoham, Y.; Israeli-Lev, G.; Livney, Y.D. β-Lactoglobulin-naringenin complexes: Nano-vehicles for the delivery of a hydrophobic nutraceutical. Food Hydrocoll. 2014, 40, 214–224.
- Yang, L.J.; Ma, S.X.; Zhou, S.Y.; Chen, W.; Yuan, M.W.; Yin, Y.Q.; Yang, X.D. Preparation and characterization of inclusion complexes of naringenin with β-cyclodextrin or its derivative. Carbohydr. Polym. 2013, 98, 861–869.
- Martinez, S.E.; Lillico, R.; Lakowski, T.M.; Martinez, S.A.; Davies, N.M. Pharmacokinetic analysis of an oral multicomponent joint dietary supplement (Phycox®) in dogs. Pharmaceutics 2017, 9, 30.
- Shulman, M.; Cohen, M.; Soto-Gutierrez, A.; Yagi, H.; Wang, H.; Goldwasser, J.; Lee-Parsons, C.W.; Benny-Ratsaby, O.; Yarmush, M.L.; Nahmias, Y. Enhancement of naringenin bioavailability by complexation with hydroxypropoyl-β-cyclodextrin. PLoS ONE 2011, 6, e18033.
- Kanaze, F.I.; Kokkalou, E.; Niopas, I.; Georgarakis, M.; Stergiou, A.; Bikiaris, D. Dissolution enhancement of flavonoids by solid dispersion in PVP and PEG matrixes: A comparative study. J. Appl. Polym. Sci. 2006, 102, 460–471.
- Bali, V.; Ali, M.; Ali, J. Nanocarrier for the enhanced bioavailability of a cardiovascular agent: In vitro, pharmacodynamic, pharmacokinetic and stability assessment. Int. J. Pharm. 2011, 403, 46–56.
- Khan, A.W.; Kotta, S.; Ansari, S.H.; Sharma, R.K.; Ali, J. Potentials and challenges in self-nanoemulsifying drug delivery systems. Expert Opin. Drug Deliv. 2012, 9, 1305–1317.
- Teng, H.; Zheng, Y.; Cao, H.; Huang, Q.; Xiao, J.; Chen, L. Enhancement of bioavailability and bioactivity of diet-derived flavonoids by application of nanotechnology: A review. Crit. Rev. Food Sci. Nutr. 2021, 1–16.
- Rodríguez-Fragoso, L.; Martínez-Arismendi, J.L.; Orozco-Bustos, D.; Reyes-Esparza, J.; Torres, E.; Burchiel, S.W. Potential Risks Resulting from Fruit/Vegetable-Drug Interactions: Effects on Drug-Metabolizing Enzymes and Drug Transporters. J. Food Sci. 2011, 76, R112–R124.
- Selvam, M.; Kaliyaperumal, M. Determination of LD 50 of Naringenin for its effects on diabetic nephropathy in rats-A pilot study. J. Chem. Pharm. Res. 2015, 7, 550–553.
- Shin, J.; Seol, I.; Son, C. Interpretation of Animal Dose and Human Equivalent Dose for Drug Development. J. Korean Orient. Med. 2010, 31, 1–7.
- Bhia, M.; Motallebi, M.; Abadi, B.; Zarepour, A.; Pereira-Silva, M.; Saremnejad, F.; Santos, A.C.; Zarrabi, A.; Melero, A.; Jafari, S.M.; et al. Naringenin nano-delivery systems and their therapeutic applications. Pharmaceutics 2021, 13, 291.
More
