You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 1 by Long Ming and Version 2 by Conner Chen.
Cardiovascular diseases (CVDs) are one of the leading causes of death worldwide. Accumulating evidences have highlighted the importance of exosomes and non-coding RNAs (ncRNAs) in cardiac physiology and pathology.
- cardiovascular disease
- exosomes
- ncRNA
- heart disease
- mRNA
1. Introduction
Cardiovascular diseases (CVDs) represent one of the major causes of death annually and poses a serious burden to the healthcare sector of the society. The World Health Organization estimates that the number of people succumbing to CVDs may cross almost 25 million by 2030 [1]. With the advancements in healthcare systems and infrastructure, the quality of life of CVDs patients has improved substantially. Nevertheless, despite such interventions, the prevalence of heart failure (HF) still remains relatively high. As a matter of fact, cardiac tissues are composed of different types of cells which work in perfect harmony with each other owing to various delicate inter- and intra-cellular communication systems between these cells. This homeostasis is basically achieved through regulated orchestration of various signaling pathways involving autocrine, paracrine, and endocrine release of chemicals/mediators in a feedback loop system. Nevertheless, when this homeostasis is perturbed, pathological conditions are inevitable, and CVDs represent such a multifaceted phenomenon with wide range of pathologies. Accumulating evidences have highlighted the importance of exosomes and non-coding RNAs (ncRNAs) in cardiac physiology and pathology [2][3][4][2,3,4]. It is widely accepted that exosomes and ncRNAs play crucial role in maintenance of the normal cellular function and their potential as prospective biomarkers and therapeutic candidates are rapidly increasing.
2. General Introduction of Exosomes
Extracellular vesicles (EVs) are membranous lipid assemblies, which carries a variety of cellular cargo including lipids, proteins, nucleic acids, metabolites, and so on [5]. Generally, these EVs are categorized based on their size and the nature of their biogenesis [6]; nevertheless, there is some overlap within this nomenclature leading to some contradiction [7]. As of yet, there are no set rules to fully categorize EVs. As a result, the International Society of Extracellular Vesicles has advocated the generic term “EVs” for the vesicles released from the cell [8]. Nevertheless, broadly speaking, there are two major classes namely microvesicles (MVs) and exosomes. MVs are also known as ectosomes, microparticles, or shedding vesicles, are vesicles having size ranging from ∼100–1000 nm and are formed from the outward budding of the plasma membrane [9][10][9,10]; whereas, exosomes are the vesicles ranging from ∼40 to 120 nm and are formed through a complex process that involves inward budding of endosomes [10][11][12][10,11,12]. Since the discovery of EVs, intensive research has been on-going; nevertheless, as of yet the biology of these EVs especially exosomes are not completely understood. It has been envisaged that exosomes are virtually being released from almost every cell type and they basically facilitate transport of various molecular entities, including nucleic acids, proteins, lipids, and metabolites, both locally and systemically [5][13][14][15][16][17][5,13,14,15,16,17]. Research in the frontiers of exosomes are rapidly increasing; basically a PubMed search with the keyword “exosomes” shows more than thousands of literature been published on the subject, highlighting their importance in the present scenario. Accumulating evidences have ascertained their imperative role in the context of cardiovascular physiology and pathology [18][19][20][18,19,20]. The origin and evolutionary perspective of exosomes and their primordial origin remains enigmatic and understanding of its plausible relation with single celled organism also remains relatively obscure. Exosomes which were once thought to be merely associated with the recycling machinery of the cell, playing role in cellular homeostasis, have undergone pragmatic shift in the field of translational medicine. They are released from wide spectrum of cells, including immune cells such as B cells, T cells, dendritic cells and stem cells, and are present in various biological fluids, such as cerebrospinal fluid, serum, saliva, urine, etc. Evidence has shown that exosomes are mechanistically and functionally diverse from its canonical counterpart and are also more heterogeneous, depending upon its origin [21]. Persistent to its endosomal origin, studies have shown the presence of major lipid rafts components consisting of ceramide, cholesterol, sphingomyelin, phosphoglycerides, long and saturated fatty-acyl chains, etc., in the exosomes. Additionally, since exosomes and multivesicular bodies (MVBs) generally originate with the aid of endosomal sorting complex required for transport (ESCRT) pathway, the proteins related to ESCRT are very prevalent and, in fact, many of them, such as HSP70, HSP90, TSG101, Alix, and tetraspanin family proteins, are considered “signature proteins” of exosomes. This, however, does not imply the absence of any other proteins since exosomes can also arise independent of classical ESCRT pathway and also it is to be noted that they act as a carrier for various protein molecules; thus, their protein profile seems to be wide and varied depending on the conditions. Recent studies have highlighted the importance of membrane proteins in the exosomes which can be leveraged to understand their origin, their preferred cellular destination and pathology of diseased state [21]. In addition to lipids and proteins, exosomes also comprise nucleic acid molecules, including mRNA, miRNA, lncRNA, circRNA, etc., as discussed below. A representative figure highlighting the biogenesis of exosomes and the typical structure of exosomes are presented in Figure 1. As a matter of fact, it is in general consensus that once these exosomes are secreted from the parent cell, they interact with the recipient/responder cells through various mechanism including clathrin-mediated endocytosis, lipid-raft mediated, and/or caveolin-mediated endocytosis, receptor-ligand mediated internalization, phagocytosis or micropinocytosis, and/or direct fusion with the plasma membrane. Lately, it has been evident that these pathways are not mutually explicit and plausibly could co-exist for the internalization of a same population of exosomes [10][22][10,22]. For example, Isabella et al., 2009 showed exosome uptake by melanoma cells through the plasma membrane fusion [23]. Similarly, another study identified exosome uptake in neurosecretory PC12 through clathrin-mediated endocytosis [24]. Perhaps, through these mechanisms, these exosomal particles modulate the activity of the recipient cells. The mechanism of exosome uptake is shown in classical cellular cargo transport physiology [10][25][10,25]. Further, it has been envisaged that the mode and level of internalization of exosomes by different cells varies widely depending on the cell type and environmental conditions. Unfortunately, but not surprisingly, it has been highlighted that the uptake of exosomes is highest in fibroblast cells and least in cardiomyocytes. Nevertheless, the underlying intricacies regulating exosomal targeting/internalization by cardiomyocyte still remains incompletely understood. Interestingly, Eguchi et al., highlighted that stem cell-derived exosomes containing the anti-apoptotic miRNA-214 are up-taken by the cardiac cells through clathrin-mediated endocytosis [26]. With paucity in the literature underlying molecular intricacies in exosomal internalization and interaction in cardiac cells, not much could be ascertained in the present scenario. Albeit certain speculations could be made based on the understanding obtained from reports on exosomes cell interaction with other cell types. It is envisaged that alteration in its profile gives plethora of information in relation to perturbation in the physiological homeostasis of the body. Interestingly, multiple lines of studies have shown that exosomes with their signature molecules plausibly act as an excellent and minimally invasive biomarker for diagnosis and prognosis of various diseases in general and CVDs in particular. To this end, much literature reviews are available highlighting the potential of exosomal signatures molecules as intriguing biomarkers for variety of pathological conditions including CVDs [27][28][29][27,28,29].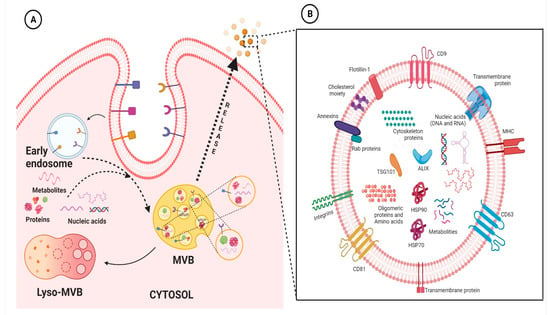
Figure 1. Representative figure highlighting the biogenesis of exosomes (A) and the typical structure of exosomes (B). Basically, exosome biogenesis starts with the inward vagination of the cellular membrane to form early endosomes. Thereafter, the intraluminal vesicles (ILVs) are formed, and the endosomes mature to multivesicular bodies (MVBs). MVBs fuse with the cellular membrane to release ILVs into the extracellular space, where thereafter they are denoted as exosomes. On the other hand, these MVBs can fuse with lysosomes of the cell, resulting in the degradation of ILVs (A). Exosomes contain various molecular entities, including nucleic acids (DNA and/or RNA), membrane anchored-proteins, cytosolic proteins, and lipids (B). The figures are prepared with the BioRender Software (biorender.com).
3. Exosomes in Cardiac Physiology and Pathology
As a matter of fact, exosome-mediated crosstalk amongst various cell types in heart tissues have been highlighted to play crucial role in the maintenance of cardiac homeostasis, as well as in the pathogenesis of cardiac diseases [27][30][27,30]. It is well recognized that in response to various stresses, heart tissue undergoes cardiac remodeling and development of cardiac hypertrophy, apoptosis, and fibrotic responses, which eventually contribute to HF [31][32][31,32]. Albeit, understanding the molecular intricacies underlying cardiac remodeling is one of the main challenges in cardiovascular medicine. However, it has been highlighted that these responses, in part, involves vesicle-mediated cellular cross talk among cardiomyocytes and other cells in the myocardium [33][34][33,34]. Reports have shown that cardiac cells under stress have increased secretion of exosomes and the exosomal content/composition are also altered; all these aspects eventually activate or suppress various molecular signaling in the recipient cells [30][35][30,35]. Interestingly, Lyu and group have highlighted that cardiac fibroblast (CF)-derived exosomes enhanced Renin–Angiotensin System (RAS) signaling in cardiomyocytes; and it was found that attenuation of these exosome secretion considerably reversed Angiotensin II-induced cardiac injuries [36]. Similarly, researchers have highlighted that CF-derived exosomes, which were plausibly enriched with miRNAs, ensues in induction of hypertrophic responses[37]; whereas Yang et al., highlighted that exosomes derived from cardiomyocytes ensued in cardiac fibrosis through myocyte-fibroblast cross-talk [38]. Li and group has shown that plasma exosomal seemingly regulates inflammatory responses during cardiopulmonary bypass surgery through plausible involvement of miR-223 [39]. These studies explicitly highlighted the importance of exosomes in cardiac homeostasis and disease biology. In addition to playing an imperative role in maintaining cardiac homeostasis and pathophysiology; they have been highlighted to endow with potentials to revolutionize cell based therapeutic intervention against CVDs by being a potential means of cell free therapeutic strategy [40]. Accordingly, in the subsequent section, newer area into the exploration of exosomes as cell free therapeutic intervention, intriguing drug delivery platform, and novel biomarkers for CVDs had been discussed.
4. Exosomes-Based Therapeutic Interventions against CVDs
Over the years, efforts have continuously been laid down to develop effective therapeutic strategies that would certainly improve the quality of the CVDs clientele. Newer therapeutic strategies are being developed, focusing not only to protect the heart tissue but also to regenerate the myocardium. To this end, accumulating evidence has highlighted the potential of stem cell therapies against CVDs; nevertheless, as of yet, these therapies refrain from showing promising results in clinical trials. Meanwhile, it has been envisaged that most of the favorable outcomes of the transplanted cells were usually indirect. Reports have highlighted that when mesenchymal stem cells (MSCs) were injected in animal model, only 6% of the injected cells were finally being retained in the infarct site [41]. It has been argued that the transplanted cells may secrete various factors/mediators, including extracellular vesicles (EVs), exosomes, growth factors, etc., that might actually play important role in mediating the beneficial effects of cell therapy. This has reinforced the holistic and emerging view of exosomes as an alternative and viable therapy. Nevertheless, despite many promising studies, the precise mechanism of exosome induced perturbations in the recipient cell still remains poorly understood. Meanwhile, taking note of other aspects, a forward leap in the arena of exosomes-based therapeutic interventions has been development of synthetic exosomes with drug delivery potentials, especially the bio-engineered targeted exosomes as detailed in the subsequent sections. Interestingly, many studies clearly indicated that exosomes in general and engineered exosomes in particular have opened newer frontier in arena of intriguing drug delivery platform and there is a high probably that these strategies may find a prosperous status in biomedical sciences in near future.
(A) Exosomes as Cell-Free Therapeutic Strategies against CVDs
Owing to their various intriguing characteristics, they are increasingly being employed as a means of cell-free therapeutic interventions for myriads of obstinate diseases, including CVDs [40]. Accumulating evidence has reported that exosomes from cardiosphere-derived stem cells (CDCs) have been shown to simulate the therapeutic effects of CDCs to a large extend in animal models of heart disease [42][43][44][45][42,43,44,45]. They have been underscored to modulate cardiomyocyte hypertrophic and apoptotic responses, induce angiogenesis, and stimulate endogenous cardiomyocyte proliferation [46]. Interestingly, Zhu and the group have reported the application of human umbilical cord mesenchymal stem cell (UMSC) derived exosomes against aging related cardiac complications. In their study, the authors have ascertained that UMSC derived exosomes through the release of novel metastasis-associated lung adenocarcinoma transcript 1 (MALAT1) lncRNA suppressed aging-related cardiac complications through subsequent attenuation of NF-κB/TNF-α signaling cascade [47]. Further, it has been highlighted that exosomes produced by CDCs have been demonstrated to stimulate myocardial regeneration via transportation of miRNA to the cardiac cells [42][44][48][42,44,48]. In addition, Limana and group have demonstrated that exosomal from pericardial fluid considerably improved myocardial performance following myocardial infarction (MI) and has ascertained that exosomal protein clustering, an important mediator of TGF-β signaling, was plausibly responsible for the underlying cardiac protective effects [49]. Interestingly, these discoveries rationalize the use of exosomes as intriguing therapeutic intervention against CVDs.
(B) Bio-Engineered Exosomes as Next-Generation Therapeutic Intervention
As a matter of fact, exosomes have been comprehended as an important cellular communication agent embodying potentials to transport diverse range of molecular entities within the biological system [50][51][50,51]. Because of their intrinsic ability to delivery molecular entities, they are considered as a promising drug delivery system (DDS) for various bioactive compounds and small molecular drugs and has been demonstrated to considerably improve their pharmacological properties against various diseases in general, and CVDs in particular. Compared with conventional drug delivery platforms, such as micelles, microemulsion, nanospheres, liposomes, and metallic nano-particulate system; exosomes offer many desirable advantages, such as lower toxicity, lower immunogenicity, high stability in circulation, better biocompatibility, and biological barrier permeability, which makes them attractive platforms for efficient delivery of therapeutic agents. Interestingly, exosomes have been used to deliver therapeutic drug and small molecules to many tissues, including the heart [52][53][54][55][56][57][58][52,53,54,55,56,57,58]. In fact, in recent years, engineered exosomes has been harnessed for targeted co-delivery of chemotherapeutics drug and RNA in fight against various diseases [59]. Nevertheless, exosomes in analogy with other drug delivery platforms also suffer from the drawback of endocytosis by the mononuclear phagocyte system (MPS). It has been highlighted that, when unmodified/neat exosomes were administrated systemically in animal model, they were found preferentially accumulated in the MPS organs such as liver, kidney, and spleen, which, thereafter, were rapidly cleared by bile excretion, renal filtration, and/or were phagocytized, leading to minimal accumulation of the therapeutics in the intended tissues or organs and undue delivery to un-intended tissues [60]. This bio-distribution profile and off-target effects limited the clinical acceptability of the unmodified exosomes [60][61][62][60,61,62]. Therefore, attempts have been made to modify exosomes for effective targeting to desired tissue. One method that has been harnessed is modification of exosomes with homing ligands or peptides, which confers them targeting capability to tissues or organs carrying the corresponding receptors. In cardiovascular system, several homing ligands/peptides are been explored for targeted therapy [52][63][64][65][52,63,64,65]. Moreover, many peptides endowed with homing potential to different cardiovascular systems, such as normal cardiomyocyte, ischemia/reperfusion injured cardiomyocytes, the vascular system etc. offers exciting avenues for exosome targeting ligands [63][64][66][67][68][63,64,66,67,68]. Interestingly, exosomes can be derived from an individual differentiated hematopoietic stem cells (HSC) and used for tissue-targeted cargo delivery through the expression of tissue-specific peptides. Thereafter, by loading miRNA and/or siRNA of the targeted gene, these modified tissue targeted exosomes can selectively regulate gene expression in the specific tissue corresponding to the homing peptides. Interestingly, Vandergriff et al., developed an infarct-targeting exosomes, through the use of cardiac homing peptide (CHP: CSTSMLKAC (IMTP)) to increase the efficacy and decrease the effective dose of intravenously delivered exosomes [63][64][63,64]. They basically conjugated cardiac stem cell-derived exosomes with cardiac homing peptide IMTP through a click chemistry approach using dioleoylphosphatidyl ethanolamine N-hydroxy succinimide linker. Interestingly, increased retention of the IMTP-exosomes within the ischemia/reperfusion injured heart tissues were observed to a considerable extent and improvement in cardiac function was also achieved thereof [69]. Similarly, molecular cloning and lentivirus packaging techniques were employed to engineer exosomal enriched membrane protein, i.e., Lamp2b fused with ischemic myocardium-targeting peptide IMTP. Such a fusion resulted in peptides being displayed on the surface of exosomes. Interestingly, these IMTP-exosomes displayed efficient internalization by hypoxia-injured embryonic cardiomyocyte H9c2 cells compared to blank-exosomes and subsequent increased accumulation in ischemic heart tissue were also obtained [65]. Meanwhile, attenuation of the inflammatory, apoptotic, and fibrotic responses was observed and enhanced vasculogenesis, and improved cardiac function were detected following IMTP-exosome treatment in ischemic heart. Further, Mentkowski and Lang bio-engineered a cardiomyocyte targeted exosomes that demonstrated improved cardiac retention in in vivo system [52]. Further, Mentkowski and Lang bio-engineered a cardiomyocyte targeted exosomes that demonstrated improved cardiac retention in an in vivo system [52]. To this end, the researcher selected a cardiomyocyte-specific peptide (CardioMyocyte Peptide (CMP): WLSEAGPVVTVRALRGTGS) [63][70][63,70]; which has proven ability to specifically target cardiac tissues [53][69][71][72][53,69,71,72]. The researcher ligated this CMP to the extra-exosomal N-terminus of Lamp2b. Interestingly, these cardiac-targeted CDC exosomes showed improved uptake into cardiac cells in an in vitro model; thereby leading to improved cardiac retention in in vivo system and, eventually, reduced cardiac apoptosis [52]. It has been envisaged that decorating the surfaces of the exosomes with homing ligand/entities will certainly reduce the time exosomes require to reach the therapeutic concentration in targeted tissues, and will considerably reduce the off-target effect, thereby leading to enhanced therapeutic potential. For detailed outline for the generation and isolation of the engineered exosomes; readers are advised to go through various previously published articles [52][59][65][68][69][73][52,59,65,68,69,73]. An overview of procedures for generation of engineered exosomes for specific targeting of the therapeutic molecules to desired tissue along with the workflow of differential ultracentrifugation for the isolation of the exosome are represented in Figure 2.
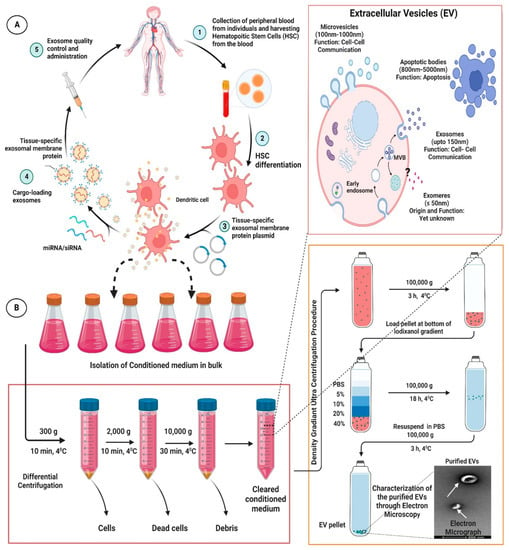

Figure 2. Representative figure highlighting the procedures for generation of engineered/modified exosomes for specific targeting of the therapeutic molecules to desired tissue (A) along with the workflow of differential ultracentrifugation for exosome isolation (B). The figures are prepared with the BioRender Software (biorender.com).
5. Exosomes as Prospective Biomarkers for CVDs
Accumulating evidences have shown that exosomes contain diverse biological contents that plausibly is a reflection of a particular state of the system [74]. Along these lines, the vast repertoire of molecular entities that are packaged within exosomes, their versatile appearance in nearly all body fluids marks their potential candidature for prospective novel non-invasive biomarkers [75].
Amongst the exosomes content, exosomes proteins and RNA molecules especially miRNA are increasingly been reported as promising biomarkers [76]. In fact, exosomal miRNAs have been the most studied for their role as novel biomarkers for CVDs. A distinct miRNA profile has been reported by various workers in CVD patients compared to normal individuals. To this end, Matsumoto and group reported that p53-responsive circulating exosomes miRNAs viz. hsa-miR-192, hsa-miR-194 and hsa-miR-34a, were considerably upregulated in the serum of acute MI clienteles that have experienced development of HF in short period. This study highlights the importance of these exo-miRNA as plausible prognostic biomarkers for acute MI [77]. Further, studies have also shown that serum exosomal miR-9 and miR-124 levels were significantly higher in stroke patients. Concomitantly, circulating exosomal miR-9 and miR-124 might be promising biomarkers for stroke diagnosis [78]. Further, Gidlof and colleagues have demonstrated that upregulation of plasma levels of hsa-miR-208b and hsa-miR-499-5p corresponded to increase in the risk of HF, highlighting their prognostic biomarker potential [79].
Further, studies have envisaged the importance of various other exosomal proteins for prospective biomarkers for CVDs. To this end, Pironti et al., have reported that circulating exosomes induced by cardiac pressure overload contain functional angiotensin II type 1 receptors (AT1Rs); they have envisaged that the transfer of AT1Rs plausibly deteriorates cardiac function during blood pressure (BP) overload, thus, could help in analyzing the prognosis of the pressure overload diseased patients [80]. Similarly, the adenosine 2A receptors and dopamine receptors have also been packed within EVs and transferred to other cells, leading to an increase in BP and cardiac remodeling thereof [81]. These findings seemingly highlight for usage of these exosomal proteins as prognostic biomarkers for hypertension clienteles.
