Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Jessie Wu and Version 3 by Jessie Wu.
The periodontium is a complex system composed of gingiva, periodontal ligament (PDL), cementum, and alveolar bone, featuring a hierarchically compartmentalized architecture. The homeostasis of this system is maintained by the PDL, a specialized connective tissue, which is located between the cementum and alveolar bone and articulates (gomphosis) the teeth to the jaws. Embryologically, PDL derives from the dental follicle cells under the guidance of Hertwig’s epithelial root sheath (HERS), which secrete numerous epithelium-derived factors before obliterating almost completely.
- cell sheet
- 3D printing
- periodontal ligament
- periodontal tissue engineering
1. Introduction
Periodontal ligament (PDL) is a fibrous network with a thickness ranging between 100 and 400 µm and is characterized by an extensive blood supply and a neural network [1]. PDL is constituted by a heterogeneous population of cells (namely PDL cells) that includes periodontal ligament fibroblasts (PDLFs), which represent by far the largest population and are responsible for the deposition and maintenance of the extracellular matrix (ECM) and periodontal ligament stem cells (PDLSCs), showing both osteogenic and tendo/ligamentogenic characteristics. Collagen type I and, in lesser amounts, type III constitute cross-banded fibrils, named Sharpey’s fibers, that provide mechanical support and are usually classified as dentinogingival, transseptal, or alveolodental (forming the bulk of proper PDL fibers) [1]. In particular, fibers oblique or perpendicular to the long axis of the tooth are thought to play pivotal roles in eliciting adaptive responses during mastication and occlusion [1]. Among all the fibers, the horizontal ones withstand the greatest loads and exhibit the greatest strain under mastication [2], (Figure 1). The collagen fibers are generally aligned according to a periodic crimped pattern [3] that prevents ligament overextension [4][5]. Sharpey’s fibers anchor mostly to acellular cementum, a mineralized layer (50–300 μm thickness) covering the tooth dentin surface. PDL cells are arranged along PDL fibers so that the long cellular axis is parallel to the main fiber bundles of the PDL [6][7]. The presence of a particular type of elastic fibers named oxtytalan, made of fimbrillins, that form a network running parallel to cementum and are thought to interact with vessels and neural fibers is also noteworthy [8]. In a healthy subject, PDL covers the tooth root almost entirely, and a tight epithelial seal within the gingival sulcus prevents microorganisms from reaching the PDL. This delicate system is compromised by the onset of periodontal disease (PD), which affects in its severe form about 10% of adults, ranking sixth among the most prevalent diseases in the world [9]. PD starts as a localized and reversible inflammation of the gingiva (gingivitis) due to dental plaque, and, when untreated, it may become chronic periodontitis, which is characterized by the progressive destruction of the tooth-supporting tissues, i.e., cementum, PDL, and bone [10][11].
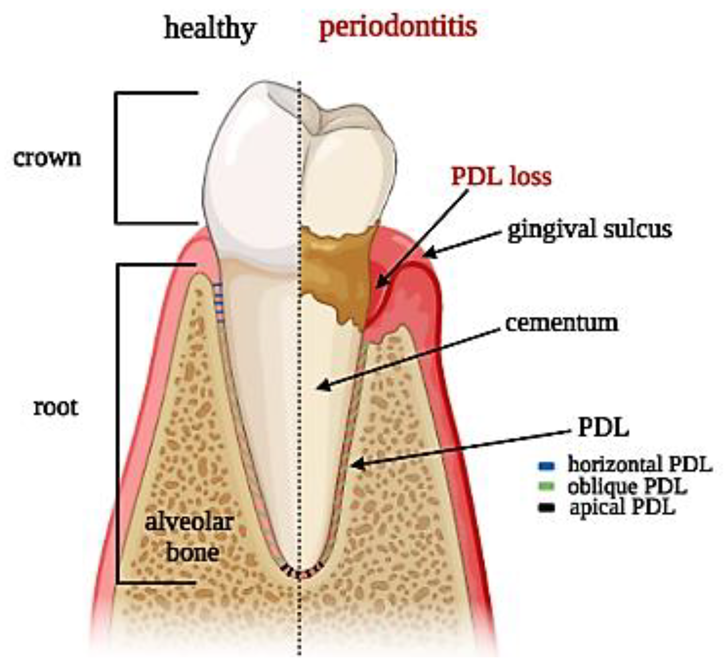
Figure 1. Tooth section showing periodontal ligament anatomy: superficial and deep periodontium along with the typical distribution of PDL fibers in both physiological and pathological conditions.
The complex architecture of the periodontal apparatus (Figure 1), including dual-tissue interfaces (alveolar bone–PDL and PDL–cementum of the tooth root), is difficult to regenerate due to the small dimensions of the PDL and the challenging oral environment [12]. Any strategy aiming at periodontal regeneration should entail studying the specific events that guide the formation and remodeling of the PDL as well as understanding the intimate bond of tissue histology and function.
2. Biomimetic Scaffolds to Reproduce the Micro-Environment of Periodontal Ligament
Traditionally, periodontal regeneration achieved through guided tissue regeneration (GTR) has been based on the concept of avoiding epithelial invasion of the bone defect to be treated by means of barrier membranes to allow PDL and bone repopulation of the dental root. Thus, a specific avenue of research has been paved toward the improvement of these membranes from the original non-resorbable expanded polytetrafluoroethylene (e-PTFE) [13] to the high technology level of recent developments, such as that proposed by Nasajpour et al. [14]. In parallel, the promising potential unleashed by tissue engineering, which relies upon combining biomaterials functioning as scaffolds and stem cells, has opened a range of new therapeutic strategies in the periodontal field. In a pioneering proof of concept, Sonoyama et al. proposed PDLSCs, the resident stem cells of PDL, in association with hydroxyapatite (HA) and tricalciumphosphate (TCP) for forming cementum and PDL-like structures [15]. More recently, Shi et al., after culturing PDLSCs in osteogenic conditions and seeding them on a biphasic calcium phosphate scaffold, observed a periodontal regeneration in the recipient animal constituted by new bone formation and PDL-organized fibers correctly inserted into adjacent cementum and bone, along with neo-vascularization, after 12 weeks [16]. Given these premises, it has become increasingly evident that the PDL is itself the key to attaining the complete regeneration of the periodontium, since this thin tissue of less than 500 μm interconnecting dental root and alveolar bone [17][18] through a series of collagen fiber bundles, is obliterated in PD. Many research efforts have been and are currently spent on identifying the best methods for fabricating biomimetic 3D scaffolds able to reproduce the PDL microenvironment. In Table 1, reswearchers report some relevant in vivo studies.
Table 1.
Representative in vivo studies using different scaffolds and PDLSCs to regenerate the periodontal complex.
| Type of Scaffold | Cell Type | Production Method | Outcome | Reference |
|---|---|---|---|---|
| PGA or PCL+ βTCP | OBs, PDLSCs | Cell sheet technology | Periodontal complex | [19][20] |
| Human tooth root | PDLSCs, HUVEC | Cell sheet technology | PDL fibers | [21] |
| PCL | PDLSCs | 3D printing of fiber-guided scaffolds | Enhancement of the bone volume fraction and of tissue mineral density | [22][23] |
| collagen/bioactive glass/chitosan membrane | PDLSCs | electrospinning | Periodontal complex | [24] |
| PCL/PLGA+BMP-2,-7, CTGF | PDLSCs | 3D printing | Cementum-like layer formation | [25] |
| iTE scaffold (core/shell fibrous super-assembled framework+ BMP-2, bFGF) | PDLSCs | iTE | Periodontal complex | [26] |
2.1. Cell Sheet Technology
One of the first tissue engineering approaches the cell sheet technology, based on culturing the cells in hyper-confluency until they produce their own extracellular matrix (ECM) by forming a cell sheet [27]. Proposed by Okano et al., this technique entails the use of poly-N-isopropyl acrylamide (PIPA Am) as a convenient cell substrate capable of both supporting the growth of a cell monolayer at 37 °C and releasing it below 20 °C without any enzymatic degradation [28]. The adhesion of the cell sheet to the root surface was enhanced through the preservation of the integrin–fibronectin complex [29]. In 2009, Iwata et al. isolated canine PDLSCs and seeded them on temperature-responsive culture dishes until sheet formation. Three-layered PDL cell sheets supported with woven poly glycolic acid (PGA) were transplanted to the exposed dental root surfaces, and bone defects were filled with porous β-TCP, inducing both the regeneration of new bone and the connection of cementum with well-oriented collagen fibers [19]. In 2012, Vaquette et al. combined fused deposition modeling with electrospinning, obtaining a biphasic scaffold with compartments through bone and PDL sheets, demonstrating that the presence of cell sheets promoted periodontal fiber attachment and cementum-like cells [20]. Takahashi et al. demonstrated that PIPA Am is useful for fabricating a brush surface with selective patterns capable of supporting cell growth while preserving orientation [30]. To overcome the pitfalls of single cell sheets in large-scale tissue injuries, Raju et al. proposed 3D complex cell sheets composed of multiple types of cells, attaining the functional connection of collagen fibers to the tooth root and alveolar bone [31]. Similarly, the co-culture of PDLSCs and human umbilical vein endothelial cells (HUVECs) allowed the generation of 3D cell sheet constructs that were wrapped around human tooth roots and implanted into the subcutaneous layer of mice. The presence of HUVECs contributed to the regulation of the thickness of PDL, which was thicker than in mice treated with PDLSCs alone [21]. An unsolved issue of PDL cell sheet technology [32], however, is achieving the directional control of the fibrous network within the constructs. To verify this crucial role of the ECM, as a proof of concept, microfibrous scaffolds were prepared by removing the cellular component from tooth slices using sodium dodecyl sulfate and Triton X-100 and supporting the repopulation and differentiation of PDL cells [33].
2.2. The 3D Printing
Among the most promising techniques implemented to physically control the orientations of PDL, researchers have focused recently on additive manufacturing, a technique that allows one to precisely control the macro- and micro-structure of the scaffolds [34][35]. Ideally, this technique can build complex tissues by depositing different materials layer by layer following 3D digital models and can even embed cells directly within the constructs during the fabrication in a process called bio-printing [36]. Hence, the main advantage over traditional tissue engineering protocols relies in the possibility of fine-tuning the creation of tissues to be akin to that of the native cellular micro-environment [37][38]. This approach may be used as a sophisticated mean to reproduce proper fiber orientation, creating specific micro-grooved surfaces for aligning human PDL cells with high predictability [23]. Such is the case of polymeric 3D scaffolds capable of replicating the peculiar micro-patterned histological architecture [39]. In vitro, this arrangement could be maintained for prolonged periods of time in the presence of growing cell populations [40].
2.2.1. Synthetic Polymers and Surface Modifications of Printed Scaffolds
Among the materials suitable for 3D printing, polycaprolactone (PCL) is widely used due to its convenient rheological, mechanical, and biological features [41]. PCL scaffolds endowed with meso/microscale architectural features were also fabricated to form de novo bone–ligament–cementum complexes in vivo [22]. A 3D-printed bone region with grooved pillars seeded with fibroblasts overexpressing bone morphogenetic protein (BMP)-7 was covered with a tooth dentin segment, and was subsequently positioned subcutaneously in a murine model, with a very encouraging outcome [22].
To improve cell adhesion efficiency on PCL, various surface modification treatments aimed at reducing its hydrophobic interface have been proposed, such as graphene oxide (GO), oxygen plasma, and gelatin coatings (Figure 2) [42][43]. Through plasma treatment, it is possible to variate the surface roughness of nanosized PCL scaffolds, conveniently modulating cell adhesion [44]. Moreover, through electrospinning technique, PCL allows the preparation of nanofibrils or nanocellulose membranes, which can be utilized to encapsulate and carry drugs. For instance, membranes made of PCL encapsulated in gelatin nanocellulose were prepared, and magnesium oxide nanoparticles were incorporated inside them. This system showed high biocompatibility and hydrophilicity that promoted PDLSCs proliferation rates [43].
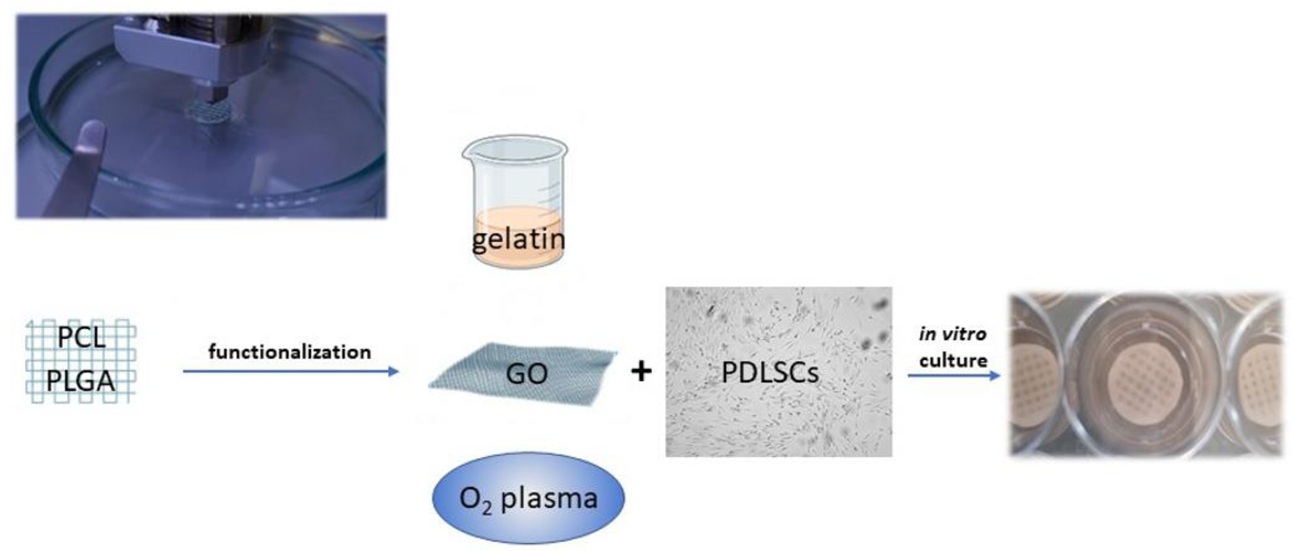
Figure 2. Polymers, such as PCL and PGLA, can be used to print scaffolds functionalized to promote PDLSC adhesion and proliferation. Different strategies of functionalization can be adopted: gelatin nanocellulose can be mixed with PCL or used as an envelope to incorporate other nanoparticles; graphene oxyde (GO) coating increases the hydrophilicity of the PCL surface; oxygen plasma variates the surface roughness.
Vera-Sánchez et al. studied the biocompatibility and potential of a composite coating with GO to induce differentiation of human PDLSCs [45], showing that the GO coating technology increases the hydrophilicity of the PCL surface, promoting cell adhesion. Additionally, poly(d,l-lactide-co-glycolide)/hyaluronic acid PLGA/HA biodegradable microcarriers were treated with GO, improving osteogenic differentiation of stem cells [46]. PCL is fundamental in allowing the printability of the scaffold since it can be conveniently integrated with proper hydrogels functioning as cell carriers and possibly other components, such as a mineralized compartment in a multilayered construct. From an anecdotal point of view, a human case of a large periodontal bone defect treated with a 3D-printed PCL-based scaffold and enriched with platelet-derived growth factors has been reported [47]. Unfortunately, the scaffold was removed after 13 months due to exposure and bacterial contamination. This unsuccessful outcome likely depended on the slow resorbability of PCL and the geometry of the construct, which was too bulky and scarcely interconnected.
Melt electrowriting (MEW), a novel technology particularly suitable for PCL [48], is expected to overcome the common limitations of 3D-printed scaffolds, such as porosity not being well matched to tissue, poor resolution, and inflexible shapes [49]. MEW enables the fabrication of micron- to nanodiameter filaments arranged in highly ordered architectures [50] within multicompartmental scaffolds [51] that may mimic the biochemical composition and/or structural organization of the hierarchical structure of the periodontium, incorporating not only PDL but also its interfacial tissues [52]. By presenting selectively regulatory cues within each compartment, multicompartmental scaffolds can guide cells to form the tissue types desired within the anatomical locations designed [53], promoting cell/tissue in-growth [54]. In 2022, the research group led by William V. Giannobile proposed the design of tricompartmental scaffolds obtained via MEW. Thereby, human PDLFs and primary osteoblasts were co-cultured, achieving “a mineral gradient from calcified to uncalcified regions with PDL-like insertions within the transition region” [55]. As the authors claim, their “process effectively recapitulates the key feature of interfacial tissues in periodontium”, offering “a fundament for engineering periodontal tissue constructs with characteristic 3D microenvironments similar to native tissues”.
2.2.2. Natural Polymers
A viable and natural alternative to PCL is collagen, the primary extracellular PDL protein, which has been employed widely as grafting material owing to its outstanding biocompatibility [56][57]. Unfortunately, collagen alone is not easily printable because of its low viscosity and denaturation temperature [58]. To address this problem, a novel technology called freeform reversible embedding of suspended hydrogels (FRESH) was introduced whereby collagen was deposited in a hydrogel that functioned as a transient mold to be removed non-destructively afterwards [59]. FRESH allowed the printing of collagen parts of human hearts with a satisfactory resolution (20–200 μm) [60].
A good candidate combining antibacterial properties and printability is chitosan, a natural biodegradable polysaccharide already used for guided tissue regeneration [61][62]. A nanohydroxyapatite–chitosan scaffold combined with PDLSCs resulted in effective promotion of bone regeneration in a calvaria bone repair model [63]. In 2018, Varoni et al. [64] prepared a tri-layer scaffold characterized by highly oriented channels aimed at guiding PDL fiber growth through electrochemical deposition. The other compartments were produced with medium- and low-molecular-weight chitosan for regenerating gingiva and bone, respectively. Excellent results supported the feasibility of this resorbable tri-layered structure both in vitro, with high cell survival rates, and in vivo, achieving selective differentiation in terms of mineralized deposits. From this perspective, the development of a new chitosan-based bioink incorporating cellulose nanocrystals, although it has only been tested on murine pre-osteoblasts, is to be regarded as most interesting [65] since it could extend the application of 3D bioprinting for periodontal regeneration to a natural polymer.
2.2.3. Hydrogels
The above-described natural polymers, collagen and chitosan, along with fibrin can also be used as hydrogels, being biodegradable and biocompatible and resembling the original ECM components. The ideal carrier is meant to mimic the ECM, which forms an intricate fibrillar architecture, and can also deeply affect PDLSC colonizing capabilities. Indeed, when seeded on a fibrin sponge, PDLSCs produced abundant ECM, that was positively stained by Alizarin Red S [66]. The effect of a biomimetic electrospun fish–collagen/bioactive–glass/chitosan composite nanofiber membrane (Col/BG/CS) on periodontal regeneration was investigated, showing that the composite membrane promoted cell growth and osteogenic gene expression in vitro, but it was also effective in promoting PDL and bone formation in a canine model [24].
The combination of collagen and methacrylate has garnered growing interest owing to the suitability of the latter for 3D printing; indeed, by adding methacrylate, the collagen may crosslink via UV light in a more controlled way in lieu of using thermal crosslinking. A customized 3D cell-laden hydrogel array with a gradient of gelatin methacrylate (GelMA) and poly(ethylene glycol) (PEG) dimethacrylate compositions showed that the higher the ratio of PEG was, the better the performance of the PDLSCs in cell proliferation and cell spreading on the scaffold [67]. Nonetheless, as inert ECM-based scaffolds alone may be poorly efficient for generating durable tissue repair, they are usually functionalized to release active compounds. PDLSCs sheets combined with platelet-rich plasma were useful for increasing the production of ECM and enhancing cell differentiation [68]. PDLSCs engineered to overexpress platelet-derived growth factor-BB showed increased osteogenic power and were tested in a rat model to induce alveolar bone regeneration [69][70]. The presence of signaling molecules, such as connective tissue growth factor (CTGF), BMP-2, and BMP-7 promote tissue regeneration and cementogenic differentiation [71][72][73]. These three factors have been incorporated into 3D-printed PLGA microspheres, and the results indicated that BMP-7 triggered thicker cementum-like layers, better integration with the dentin surface, and higher expression of cementum protein-1 [25]. In situ tissue engineering (iTE) allowed the production of a iTE-scaffold made with a PLGA/poly (L-lactic acid (PLLA) shell/core structure and functionalized to allow a sequential delivery of b fibroblast growth factor (bFGF), which promotes regeneration of the periodontium [74] and BMP-2, significantly facilitating stem cell homing, proliferation, and periodontal bone regeneration [26][75]. This iTE-scaffold, implanted in a rat model of periodontal defect, demonstrated an anti-inflammatory response, provided adequate blood supply, and achieved the desired bone repair [76]. Regrettably, over the years, safety concerns have hindered the usage of bioactive molecules such as BMP-2 at the high concentrations required to be effective [77], somehow questioning the classic paradigm of tissue engineering based on cells/scaffolds/signaling cues and favoring the development of smart materials [78].
References
- de Jong, T.; Bakker, A.D.; Everts, V.; Smit, T.H. The intricate anatomy of the periodontal ligament and its development: Lessons for periodontal regeneration. J. Periodontal Res. 2017, 52, 965–974.
- Ortun-Terrazas, J.; Cegonino, J.; Santana-Penin, U.; Santana-Mora, U.; Perez Del Palomar, A. Approach towards the porous fibrous structure of the periodontal ligament using micro-computerized tomography and finite element analysis. J. Mech. Behav. Biomed. Mater. 2018, 79, 135–149.
- Gathercole, L.J.; Keller, A. Crimp morphology in the fibre-forming collagens. Matrix 1991, 11, 214–234.
- Maceri, F.; Marino, M.; Vairo, G. A unified multiscale mechanical model for soft collagenous tissues with regular fiber arrangement. J. Biomech. 2010, 43, 355–363.
- Szczesny, S.E.; Driscoll, T.P.; Tseng, H.Y.; Liu, P.C.; Heo, S.J.; Mauck, R.L.; Chao, P.G. Crimped Nanofibrous Biomaterials Mimic Microstructure and Mechanics of Native Tissue and Alter Strain Transfer to Cells. ACS Biomater. Sci. Eng. 2017, 3, 2869–2876.
- Cho, M.I.; Garant, P.R. Development and general structure of the periodontium. Periodontology 2000 2000, 24, 9–27.
- Bosshardt, D.D.; Bergomi, M.; Vaglio, G.; Wiskott, A. Regional structural characteristics of bovine periodontal ligament samples and their suitability for biomechanical tests. J. Anat. 2008, 212, 319–329.
- Tsuruga, E.; Irie, K.; Sakakura, Y.; Yajima, T. Expression of fibrillins and tropoelastin by human gingival and periodontal ligament fibroblasts in vitro. J. Periodontal Res. 2002, 37, 23–28.
- Peres, M.A.; Macpherson, L.M.D.; Weyant, R.J.; Daly, B.; Venturelli, R.; Mathur, M.R.; Listl, S.; Celeste, R.K.; Guarnizo-Herreno, C.C.; Kearns, C.; et al. Oral diseases: A global public health challenge. Lancet 2019, 394, 249–260.
- Lourenco, T.G.; Heller, D.; Silva-Boghossian, C.M.; Cotton, S.L.; Paster, B.J.; Colombo, A.P. Microbial signature profiles of periodontally healthy and diseased patients. J. Clin. Periodontol. 2014, 41, 1027–1036.
- Feres, M.; Teles, F.; Teles, R.; Figueiredo, L.C.; Faveri, M. The subgingival periodontal microbiota of the aging mouth. Periodontology 2000 2016, 72, 30–53.
- Sowmya, S.; Mony, U.; Jayachandran, P.; Reshma, S.; Kumar, R.A.; Arzate, H.; Nair, S.V.; Jayakumar, R. Tri-Layered Nanocomposite Hydrogel Scaffold for the Concurrent Regeneration of Cementum, Periodontal Ligament, and Alveolar Bone. Adv. Healthc. Mater. 2017, 6, 1601251.
- Lekovic, V.; Kenney, E.B.; Kovacevic, K.; Carranza, F.A., Jr. Evaluation of guided tissue regeneration in Class II furcation defects. A clinical re-entry study. J. Periodontol. 1989, 60, 694–698.
- Nasajpour, A.; Mandla, S.; Shree, S.; Mostafavi, E.; Sharifi, R.; Khalilpour, A.; Saghazadeh, S.; Hassan, S.; Mitchell, M.J.; Leijten, J.; et al. Nanostructured Fibrous Membranes with Rose Spike-Like Architecture. Nano Lett. 2017, 17, 6235–6240.
- Sonoyama, W.; Liu, Y.; Fang, D.; Yamaza, T.; Seo, B.M.; Zhang, C.; Liu, H.; Gronthos, S.; Wang, C.Y.; Wang, S.; et al. Mesenchymal stem cell-mediated functional tooth regeneration in swine. PLoS ONE 2006, 1, e79.
- Shi, H.; Zong, W.; Xu, X.; Chen, J. Improved biphasic calcium phosphate combined with periodontal ligament stem cells may serve as a promising method for periodontal regeneration. Am. J. Transl. Res. 2018, 10, 4030–4041.
- Bartold, P.M.; Gronthos, S.; Ivanovski, S.; Fisher, A.; Hutmacher, D.W. Tissue engineered periodontal products. J. Periodontal. Res. 2016, 51, 1–15.
- Iwata, T.; Yamato, M.; Zhang, Z.; Mukobata, S.; Washio, K.; Ando, T.; Feijen, J.; Okano, T.; Ishikawa, I. Validation of human periodontal ligament-derived cells as a reliable source for cytotherapeutic use. J. Clin. Periodontol. 2010, 37, 1088–1099.
- Iwata, T.; Yamato, M.; Tsuchioka, H.; Takagi, R.; Mukobata, S.; Washio, K.; Okano, T.; Ishikawa, I. Periodontal regeneration with multi-layered periodontal ligament-derived cell sheets in a canine model. Biomaterials 2009, 30, 2716–2723.
- Vaquette, C.; Fan, W.; Xiao, Y.; Hamlet, S.; Hutmacher, D.W.; Ivanovski, S. A biphasic scaffold design combined with cell sheet technology for simultaneous regeneration of alveolar bone/periodontal ligament complex. Biomaterials 2012, 33, 5560–5573.
- Panduwawala, C.P.; Zhan, X.; Dissanayaka, W.L.; Samaranayake, L.P.; Jin, L.; Zhang, C. In vivo periodontal tissue regeneration by periodontal ligament stem cells and endothelial cells in three-dimensional cell sheet constructs. J. Periodontal Res. 2017, 52, 408–418.
- Pilipchuk, S.P.; Monje, A.; Jiao, Y.; Hao, J.; Kruger, L.; Flanagan, C.L.; Hollister, S.J.; Giannobile, W.V. Integration of 3D Printed and Micropatterned Polycaprolactone Scaffolds for Guidance of Oriented Collagenous Tissue Formation In Vivo. Adv. Healthc. Mater. 2016, 5, 676–687.
- Park, C.H.; Rios, H.F.; Jin, Q.; Sugai, J.V.; Padial-Molina, M.; Taut, A.D.; Flanagan, C.L.; Hollister, S.J.; Giannobile, W.V. Tissue engineering bone-ligament complexes using fiber-guiding scaffolds. Biomaterials 2012, 33, 137–145.
- Zhou, T.; Liu, X.; Sui, B.; Liu, C.; Mo, X.; Sun, J. Development of fish collagen/bioactive glass/chitosan composite nanofibers as a GTR/GBR membrane for inducing periodontal tissue regeneration. Biomed. Mater. 2017, 12, 055004.
- Cho, H.; Tarafder, S.; Fogge, M.; Kao, K.; Lee, C.H. Periodontal ligament stem/progenitor cells with protein-releasing scaffolds for cementum formation and integration on dentin surface. Connect. Tissue Res. 2016, 57, 488–495.
- Ding, T.; Li, J.; Zhang, X.; Du, L.; Li, Y.; Li, D.; Kong, B.; Ge, S. Super-assembled core/shell fibrous frameworks with dual growth factors for in situ cementum-ligament-bone complex regeneration. Biomater. Sci. 2020, 8, 2459–2471.
- Owaki, T.; Shimizu, T.; Yamato, M.; Okano, T. Cell sheet engineering for regenerative medicine: Current challenges and strategies. Biotechnol. J. 2014, 9, 904–914.
- Okano, T.; Yamada, N.; Sakai, H.; Sakurai, Y. A novel recovery system for cultured cells using plasma-treated polystyrene dishes grafted with poly(N-isopropylacrylamide). J. Biomed. Mater. Res. 1993, 27, 1243–1251.
- Hasegawa, M.; Yamato, M.; Kikuchi, A.; Okano, T.; Ishikawa, I. Human periodontal ligament cell sheets can regenerate periodontal ligament tissue in an athymic rat model. Tissue Eng. 2005, 11, 469–478.
- Takahashi, H.; Nakayama, M.; Itoga, K.; Yamato, M.; Okano, T. Micropatterned thermoresponsive polymer brush surfaces for fabricating cell sheets with well-controlled orientational structures. Biomacromolecules 2011, 12, 1414–1418.
- Raju, R.; Oshima, M.; Inoue, M.; Morita, T.; Huijiao, Y.; Waskitho, A.; Baba, O.; Inoue, M.; Matsuka, Y. Three-dimensional periodontal tissue regeneration using a bone-ligament complex cell sheet. Sci. Rep. 2020, 10, 1656.
- Tsumanuma, Y.; Iwata, T.; Washio, K.; Yoshida, T.; Yamada, A.; Takagi, R.; Ohno, T.; Lin, K.; Yamato, M.; Ishikawa, I.; et al. Comparison of different tissue-derived stem cell sheets for periodontal regeneration in a canine 1-wall defect model. Biomaterials 2011, 32, 5819–5825.
- Son, H.; Jeon, M.; Choi, H.J.; Lee, H.S.; Kim, I.H.; Kang, C.M.; Song, J.S. Decellularized human periodontal ligament for periodontium regeneration. PLoS ONE 2019, 14, e0221236.
- Chen, H.; Han, Q.; Wang, C.; Liu, Y.; Chen, B.; Wang, J. Porous Scaffold Design for Additive Manufacturing in Orthopedics: A Review. Front. Bioeng. Biotechnol. 2020, 8, 609.
- Park, C.H.; Kim, K.H.; Rios, H.F.; Lee, Y.M.; Giannobile, W.V.; Seol, Y.J. Spatiotemporally controlled microchannels of periodontal mimic scaffolds. J. Dent. Res. 2014, 93, 1304–1312.
- Oliveira, N.K.; Salles, T.H.C.; Pedroni, A.C.; Miguita, L.; D’Avila, M.A.; Marques, M.M.; Deboni, M.C.Z. Osteogenic potential of human dental pulp stem cells cultured onto poly-epsilon-caprolactone/poly (rotaxane) scaffolds. Dent. Mater. 2019, 35, 1740–1749.
- Thattaruparambil Raveendran, N.; Vaquette, C.; Meinert, C.; Samuel Ipe, D.; Ivanovski, S. Optimization of 3D bioprinting of periodontal ligament cells. Dent. Mater. 2019, 35, 1683–1694.
- Zhang, L.; Yang, G.; Johnson, B.N.; Jia, X. Three-dimensional (3D) printed scaffold and material selection for bone repair. Acta Biomater. 2019, 84, 16–33.
- Park, C.H. Biomaterial-Based Approaches for Regeneration of Periodontal Ligament and Cementum Using 3D Platforms. Int. J. Mol. Sci. 2019, 20, 4364.
- Park, C.H.; Oh, J.H.; Jung, H.M.; Choi, Y.; Rahman, S.U.; Kim, S.; Kim, T.I.; Shin, H.I.; Lee, Y.S.; Yu, F.H.; et al. Effects of the incorporation of epsilon-aminocaproic acid/chitosan particles to fibrin on cementoblast differentiation and cementum regeneration. Acta Biomater. 2017, 61, 134–143.
- Niinomi, M. Metallic biomaterials. J. Artif. Organs 2008, 11, 105–110.
- Park, J.; Park, S.; Kim, J.E.; Jang, K.J.; Seonwoo, H.; Chung, J.H. Enhanced Osteogenic Differentiation of Periodontal Ligament Stem Cells Using a Graphene Oxide-Coated Poly(epsilon-caprolactone) Scaffold. Polymers 2021, 13, 797.
- Peng, W.; Ren, S.; Zhang, Y.; Fan, R.; Zhou, Y.; Li, L.; Xu, X.; Xu, Y. MgO Nanoparticles-Incorporated PCL/Gelatin-Derived Coaxial Electrospinning Nanocellulose Membranes for Periodontal Tissue Regeneration. Front. Bioeng. Biotechnol. 2021, 9, 668428.
- Jeon, H.; Lee, H.; Kim, G. A surface-modified poly(varepsilon-caprolactone) scaffold comprising variable nanosized surface-roughness using a plasma treatment. Tissue Eng. Part C Methods 2014, 20, 951–963.
- Vera-Sanchez, M.; Aznar-Cervantes, S.; Jover, E.; Garcia-Bernal, D.; Onate-Sanchez, R.E.; Hernandez-Romero, D.; Moraleda, J.M.; Collado-Gonzalez, M.; Rodriguez-Lozano, F.J.; Cenis, J.L. Silk-Fibroin and Graphene Oxide Composites Promote Human Periodontal Ligament Stem Cell Spontaneous Differentiation into Osteo/Cementoblast-Like Cells. Stem Cells Dev. 2016, 25, 1742–1754.
- Fu, C.; Yang, X.; Tan, S.; Song, L. Enhancing Cell Proliferation and Osteogenic Differentiation of MC3T3-E1 Pre-osteoblasts by BMP-2 Delivery in Graphene Oxide-Incorporated PLGA/HA Biodegradable Microcarriers. Sci. Rep. 2017, 7, 12549.
- Rasperini, G.; Pilipchuk, S.P.; Flanagan, C.L.; Park, C.H.; Pagni, G.; Hollister, S.J.; Giannobile, W.V. 3D-printed Bioresorbable Scaffold for Periodontal Repair. J. Dent. Res. 2015, 94, 153S–157S.
- Kade, J.C.; Dalton, P.D. Polymers for Melt Electrowriting. Adv. Healthc. Mater. 2021, 10, e2001232.
- Latimer, J.M.; Maekawa, S.; Yao, Y.; Wu, D.T.; Chen, M.; Giannobile, W.V. Regenerative Medicine Technologies to Treat Dental, Oral, and Craniofacial Defects. Front. Bioeng. Biotechnol. 2021, 9, 704048.
- Eichholz, K.F.; Hoey, D.A. Mediating human stem cell behaviour via defined fibrous architectures by melt electrospinning writing. Acta Biomater. 2018, 75, 140–151.
- Vaquette, C.; Pilipchuk, S.P.; Bartold, P.M.; Hutmacher, D.W.; Giannobile, W.V.; Ivanovski, S. Tissue Engineered Constructs for Periodontal Regeneration: Current Status and Future Perspectives. Adv. Healthc. Mater. 2018, 7, e1800457.
- Ivanovski, S.; Vaquette, C.; Gronthos, S.; Hutmacher, D.W.; Bartold, P.M. Multiphasic scaffolds for periodontal tissue engineering. J. Dent. Res. 2014, 93, 1212–1221.
- Gonzalez-Fernandez, T.; Rathan, S.; Hobbs, C.; Pitacco, P.; Freeman, F.E.; Cunniffe, G.M.; Dunne, N.J.; McCarthy, H.O.; Nicolosi, V.; O’Brien, F.J.; et al. Pore-forming bioinks to enable spatio-temporally defined gene delivery in bioprinted tissues. J. Control. Release 2019, 301, 13–27.
- Abbasi, N.; Abdal-Hay, A.; Hamlet, S.; Graham, E.; Ivanovski, S. Effects of Gradient and Offset Architectures on the Mechanical and Biological Properties of 3-D Melt Electrowritten (MEW) Scaffolds. ACS. Biomater. Sci. Eng. 2019, 5, 3448–3461.
- Yao, Y.; Raymond, J.E.; Kauffmann, F.; Maekawa, S.; Sugai, J.V.; Lahann, J.; Giannobile, W.V. Multicompartmental Scaffolds for Coordinated Periodontal Tissue Engineering. J. Dent. Res. 2022, 101, 1457–1466.
- Lee, C.H.; Singla, A.; Lee, Y. Biomedical applications of collagen. Int. J. Pharm. 2001, 221, 1–22.
- van den Bos, T.; Tonino, G.J. Composition and metabolism of the extracellular matrix in the periodontal ligament of impeded and unimpeded rat incisors. Arch. Oral. Biol. 1984, 29, 893–897.
- Bozec, L.; Odlyha, M. Thermal denaturation studies of collagen by microthermal analysis and atomic force microscopy. Biophys. J. 2011, 101, 228–236.
- Hinton, T.J.; Jallerat, Q.; Palchesko, R.N.; Park, J.H.; Grodzicki, M.S.; Shue, H.J.; Ramadan, M.H.; Hudson, A.R.; Feinberg, A.W. Three-dimensional printing of complex biological structures by freeform reversible embedding of suspended hydrogels. Sci. Adv. 2015, 1, e1500758.
- Lee, A.; Hudson, A.R.; Shiwarski, D.J.; Tashman, J.W.; Hinton, T.J.; Yerneni, S.; Bliley, J.M.; Campbell, P.G.; Feinberg, A.W. 3D bioprinting of collagen to rebuild components of the human heart. Science 2019, 365, 482–487.
- Mota, J.; Yu, N.; Caridade, S.G.; Luz, G.M.; Gomes, M.E.; Reis, R.L.; Jansen, J.A.; Walboomers, X.F.; Mano, J.F. Chitosan/bioactive glass nanoparticle composite membranes for periodontal regeneration. Acta Biomater. 2012, 8, 4173–4180.
- Qasim, S.B.; Delaine-Smith, R.M.; Fey, T.; Rawlinson, A.; Rehman, I.U. Freeze gelated porous membranes for periodontal tissue regeneration. Acta Biomater. 2015, 23, 317–328.
- Ge, S.; Zhao, N.; Wang, L.; Yu, M.; Liu, H.; Song, A.; Huang, J.; Wang, G.; Yang, P. Bone repair by periodontal ligament stem cellseeded nanohydroxyapatite-chitosan scaffold. Int. J. Nanomed. 2012, 7, 5405–5414.
- Varoni, E.M.; Vijayakumar, S.; Canciani, E.; Cochis, A.; De Nardo, L.; Lodi, G.; Rimondini, L.; Cerruti, M. Chitosan-Based Trilayer Scaffold for Multitissue Periodontal Regeneration. J. Dent. Res. 2018, 97, 303–311.
- Maturavongsadit, P.; Narayanan, L.K.; Chansoria, P.; Shirwaiker, R.; Benhabbour, S.R. Cell-Laden Nanocellulose/Chitosan-Based Bioinks for 3D Bioprinting and Enhanced Osteogenic Cell Differentiation. ACS Appl. Bio Mater. 2021, 4, 2342–2353.
- Trubiani, O.; Orsini, G.; Zini, N.; Di Iorio, D.; Piccirilli, M.; Piattelli, A.; Caputi, S. Regenerative potential of human periodontal ligament derived stem cells on three-dimensional biomaterials: A morphological report. J. Biomed. Mater. Res. A 2008, 87, 986–993.
- Ma, Y.; Ji, Y.; Huang, G.; Ling, K.; Zhang, X.; Xu, F. Bioprinting 3D cell-laden hydrogel microarray for screening human periodontal ligament stem cell response to extracellular matrix. Biofabrication 2015, 7, 044105.
- Xu, Q.; Li, B.; Yuan, L.; Dong, Z.; Zhang, H.; Wang, H.; Sun, J.; Ge, S.; Jin, Y. Combination of platelet-rich plasma within periodontal ligament stem cell sheets enhances cell differentiation and matrix production. J. Tissue Eng. Regen. Med. 2017, 11, 627–636.
- Pan, J.; Deng, J.; Luo, Y.; Yu, L.; Zhang, W.; Han, X.; You, Z.; Liu, Y. Thermosensitive Hydrogel Delivery of Human Periodontal Stem Cells Overexpressing Platelet-Derived Growth Factor-BB Enhances Alveolar Bone Defect Repair. Stem Cells Dev. 2019, 28, 1620–1631.
- Safi, I.N.; Al-Shammari, A.M.; Ul-Jabbar, M.A.; Hussein, B.M.A. Preparing polycaprolactone scaffolds using electrospinning technique for construction of artificial periodontal ligament tissue. J. Taibah. Univ. Med. Sci. 2020, 15, 363–373.
- Duan, X.; Ji, M.; Deng, F.; Sun, Z.; Lin, Z. Effects of connective tissue growth factor on human periodontal ligament fibroblasts. Arch. Oral Biol. 2017, 84, 37–44.
- Thomadakis, G.; Ramoshebi, L.N.; Crooks, J.; Rueger, D.C.; Ripamonti, U. Immunolocalization of Bone Morphogenetic Protein-2 and -3 and Osteogenic Protein-1 during murine tooth root morphogenesis and in other craniofacial structures. Eur. J. Oral Sci. 1999, 107, 368–377.
- Pitaru, S.; Pritzki, A.; Bar-Kana, I.; Grosskopf, A.; Savion, N.; Narayanan, A.S. Bone morphogenetic protein 2 induces the expression of cementum attachment protein in human periodontal ligament clones. Connect. Tissue Res. 2002, 43, 257–264.
- Kono, K.; Maeda, H.; Fujii, S.; Tomokiyo, A.; Yamamoto, N.; Wada, N.; Monnouchi, S.; Teramatsu, Y.; Hamano, S.; Koori, K.; et al. Exposure to transforming growth factor-beta1 after basic fibroblast growth factor promotes the fibroblastic differentiation of human periodontal ligament stem/progenitor cell lines. Cell Tissue Res. 2013, 352, 249–263.
- Kang, W.; Liang, Q.; Du, L.; Shang, L.; Wang, T.; Ge, S. Sequential application of bFGF and BMP-2 facilitates osteogenic differentiation of human periodontal ligament stem cells. J. Periodontal Res. 2019, 54, 424–434.
- Ding, T.; Kang, W.; Li, J.; Yu, L.; Ge, S. An in situ tissue engineering scaffold with growth factors combining angiogenesis and osteoimmunomodulatory functions for advanced periodontal bone regeneration. J. Nanobiotechnology 2021, 19, 247.
- James, A.W.; LaChaud, G.; Shen, J.; Asatrian, G.; Nguyen, V.; Zhang, X.; Ting, K.; Soo, C. A Review of the Clinical Side Effects of Bone Morphogenetic Protein-2. Tissue Eng. Part B Rev. 2016, 22, 284–297.
- Qu, M.; Jiang, X.; Zhou, X.; Wang, C.; Wu, Q.; Ren, L.; Zhu, J.; Zhu, S.; Tebon, P.; Sun, W.; et al. Stimuli-Responsive Delivery of Growth Factors for Tissue Engineering. Adv. Healthc. Mater. 2020, 9, e1901714.
More
