A copper tailing is a residue, product of the flotation of sulfide minerals, which contain a variety of elements that can be valorized. The extraction of metals from copper tailings consist of applying metallurgical techniques, such as acid leaching or magnetic concentration, to obtain a valuable product. Currently, this is an important objective, given that mining operations have increased the generation of tailings. Acid leaching is a process that consists of dissolving a solid material, such as a tailing, by applying an acid solution. This process forms two final products: an insoluble solid, rich in aluminosilicates, and an acid liquid solution with different metal ions. Both products may have different characteristics and can be used for subsequent applications.
Adapted from: https://doi.org/10.3390/met12111924
- copper tailing
- leaching
- valorization
1. Introduction
2. Collection and Characterization of the Tailing Sample
The tailiey obtained the tailing sample was obtained from a tailing dam placed in the north of Chile (Figure 1. Specifica).lly, Tthe sample (Figure 1b) was taken from the wall of the tailing dam using the trial pits technique. Then, this sample passed through a homogenization method considering rolling and quartering to obtain a representative sample. Subsequently, sieving was performed to analyze the size distribution and it was compared with a Laser Diffraction analysis. In addition, SEM-EDX and XRD analyses were performed to determine the tailing composition. Elements were determined with an AAS analysis.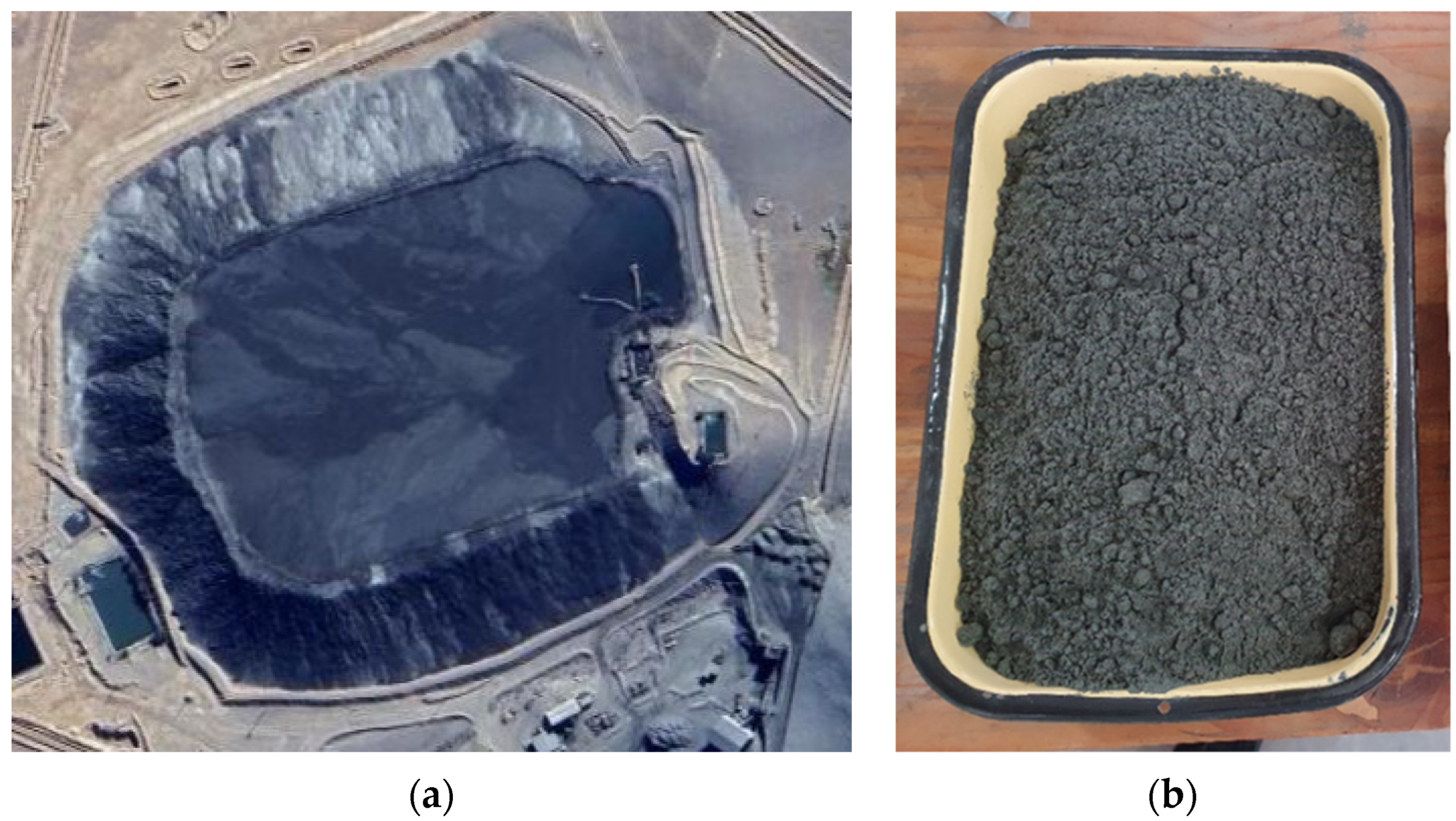
3. Leaching Procedure
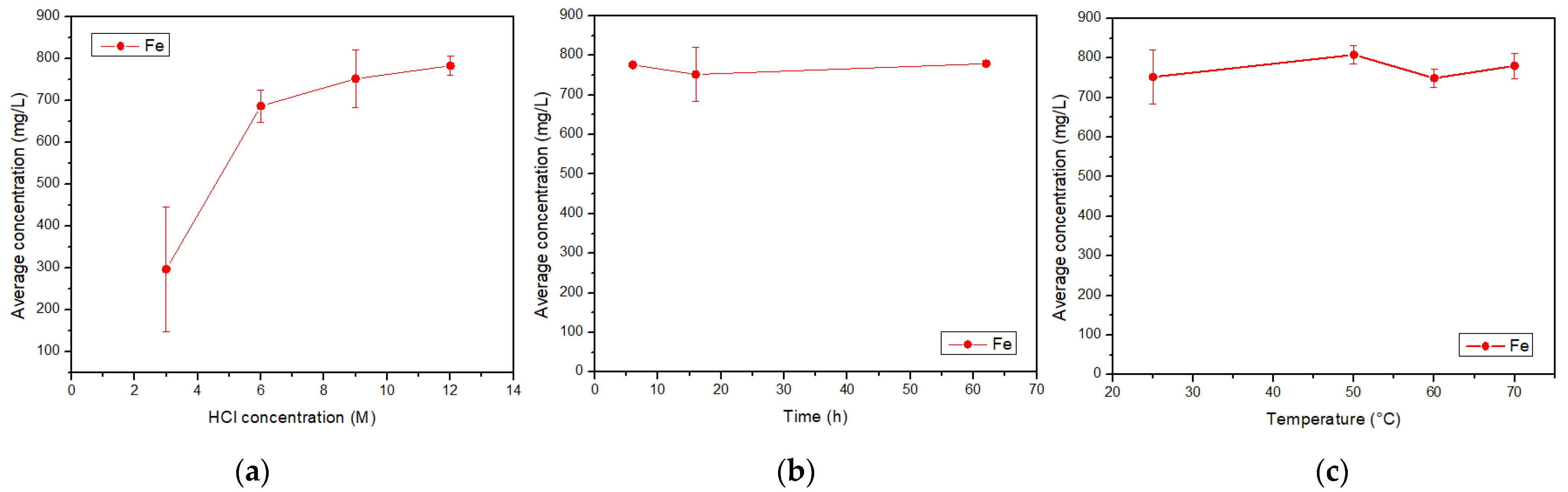
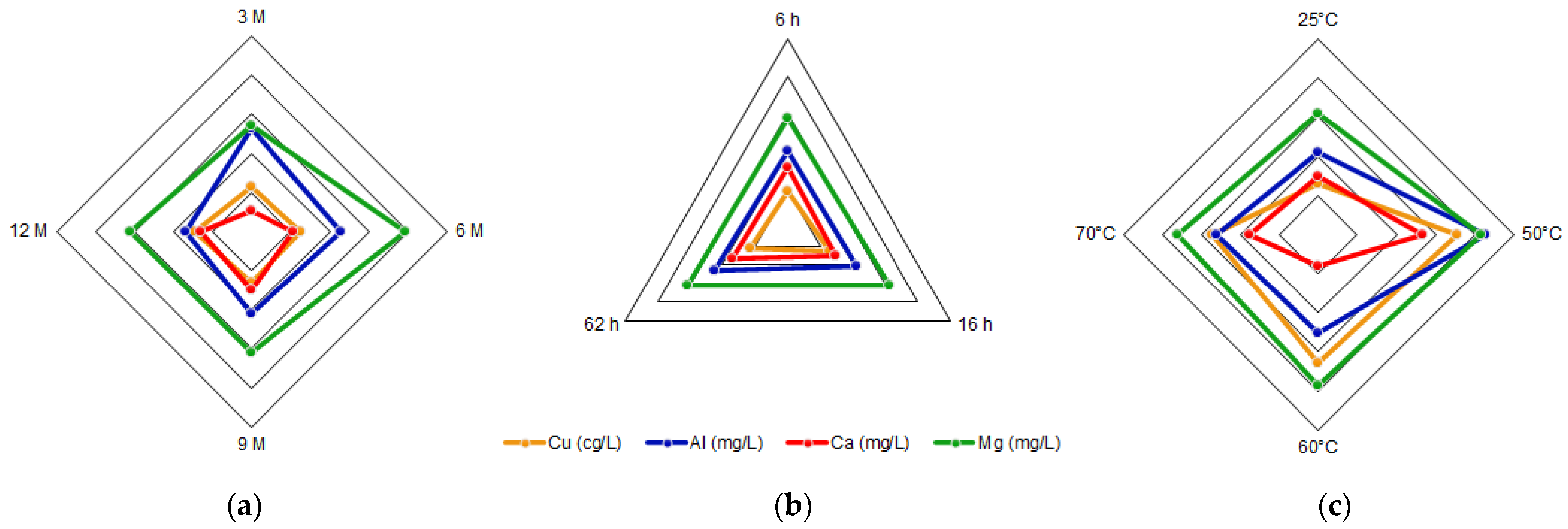
The initial tailing sample was leached using a HCl sr results showed that 80% olution. To perform the leaching, 1 g the samples were weighed and deposited in round-bottom flasks, each of them with 250 mL of the prepared acid solution (3 M, 6 M, 9 M or 12 M). The mixture was stirred with a magnetic stirrer at 550 rpm under a controlled temperature, coupling the flask with a laboratory condenser tube. After the leaching time, the solution was vacuum filtered using a porous glass filter. The filtered liquid solution and the insoluble solid left in the filter were reserved for later analysis. This procedure was repeated changing the acid concentration, the reaction time, and the reaction temperature as shown in Figure 2, where “C” is the acid concentration, “t” is time, and “T” is tem is approximately below an ASTM 60 mesh, corresperature. In addition, all combinations shown in Figure 2 were performed twice. The dissolution times were 6 h, 16 h, and 62 h, a long enough time. The temperatures were 25 °C (considered as room temperature), 50 °C, 60 °C, and 70 °C. After finishing the experiments, the total iron, copper, aluminum, calcium, and magnesium concentrations in the filtered acid solution were determined by AAS. The insoluble solids were dried, at 65 °C, for 24 h to be analyzed with SEM-EDX and XRD.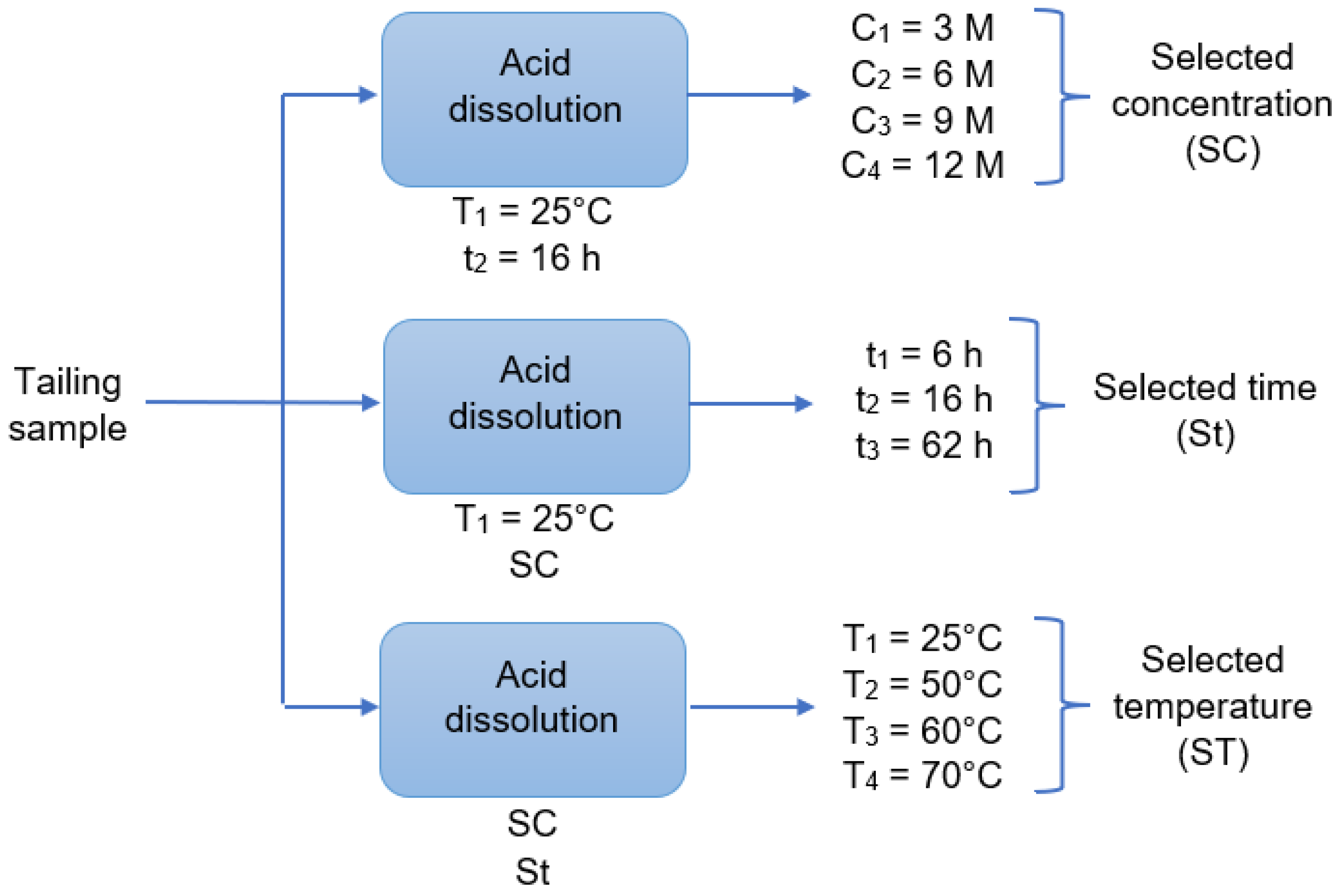
4. Results
4.1. Tailing Sample Characterization
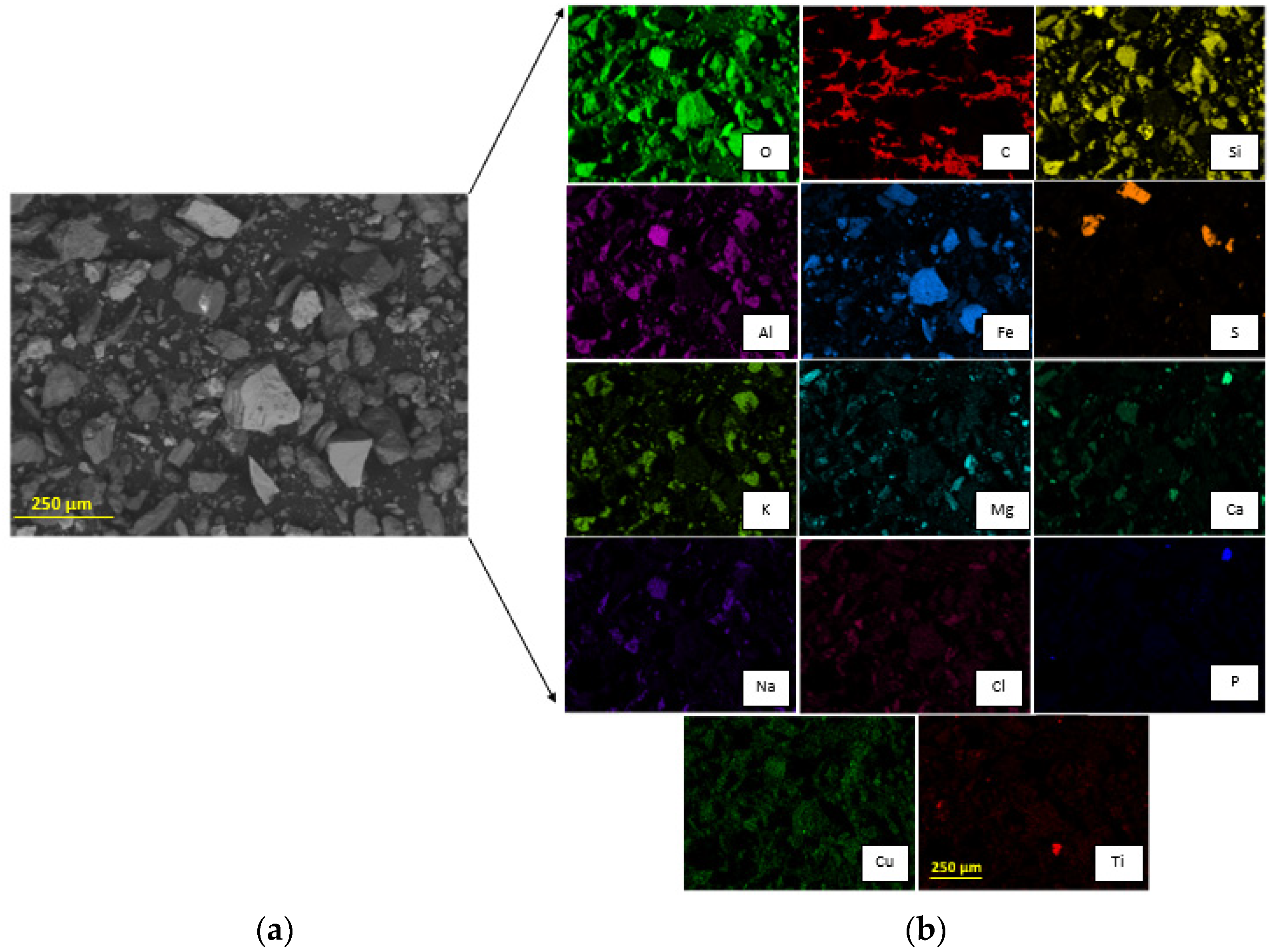
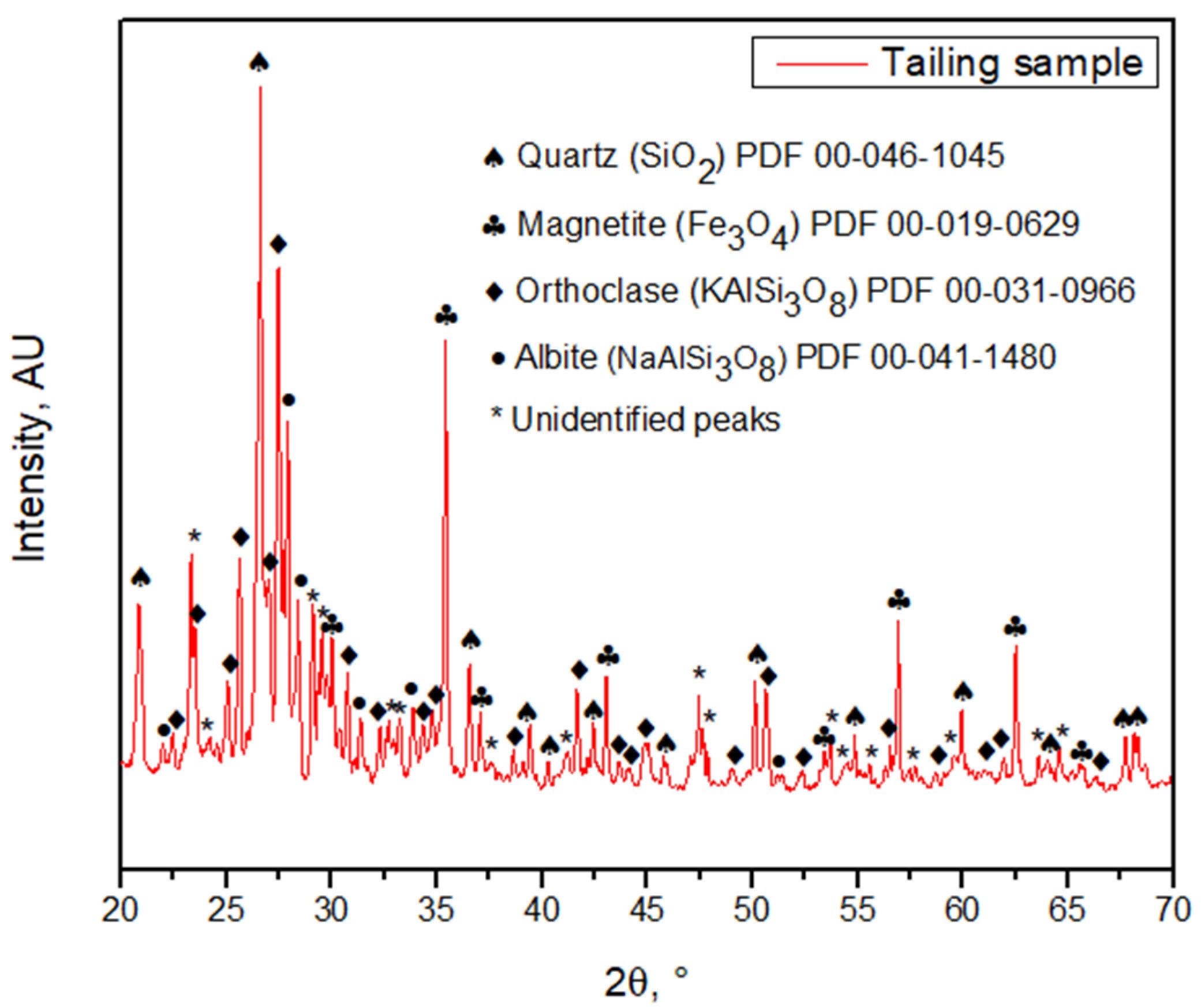
The ng to 250 µm, meaning that the particle size P80 was 221 ± 23 µm according to the granulometric analysis by sieving, and 216 ± 0.652 µm according to the Laser Diffraction analysis, meaning that 80% of the sample is approximately below an ASTM 60 mesh, corresponding to 250 µm (Figure 3)stribution is fine. The morphology and general composition of the tailing sample were identified by SEM-EDX, as seen in Figure 4. At 100×, the tailing paparticles are irregular, and the composition is based on silicates and iron oxides. The XRD analysis, shown in Figure 5, was applied to the tailing sample, revealing that the composition was mainly quartz (SiO2), magnetite (Fe3O4), orthoclase (KAlSi3O8), and albite. (NaAlSi3O8), confirming tThe SEM-EDX analysis. Through AAS, it was determined that the tailing contains a total iron grade of 19%.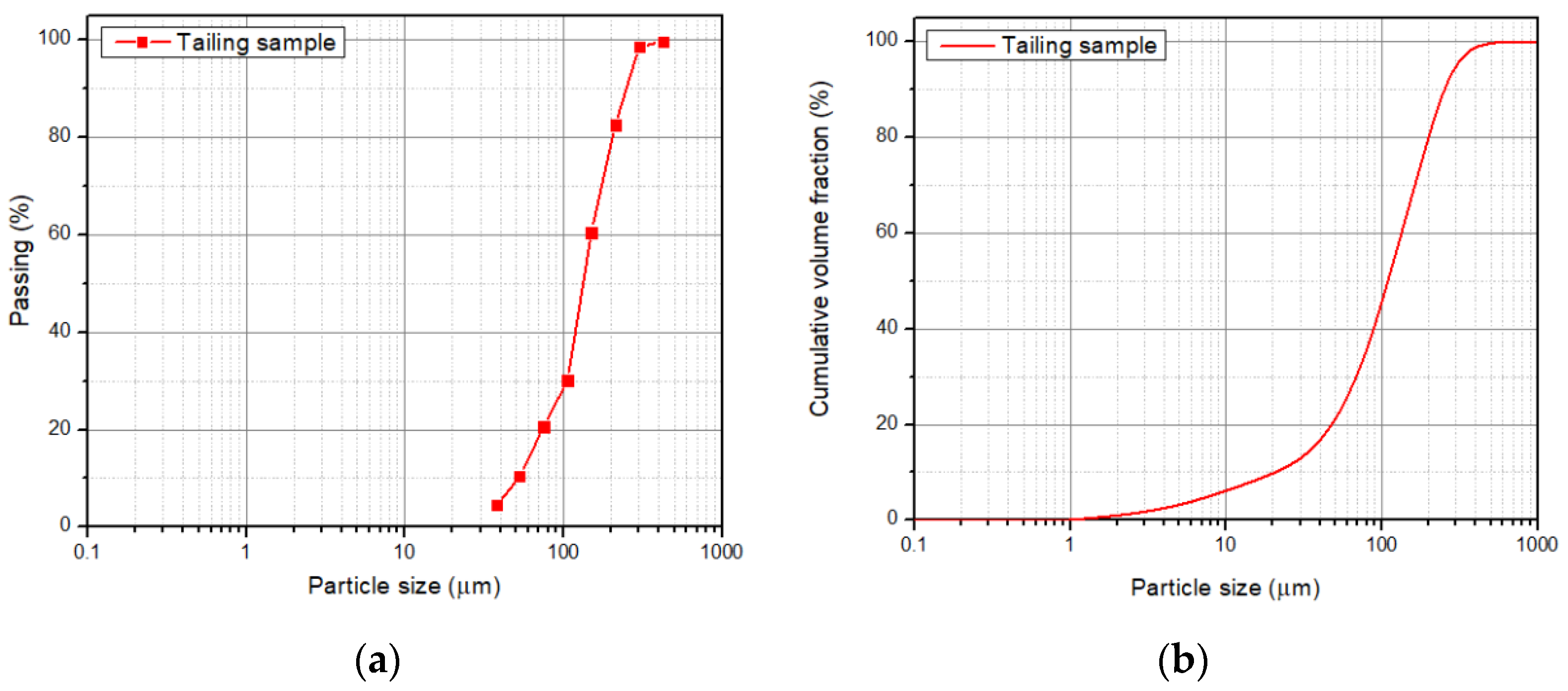
4.2. Acid Solution Characterization
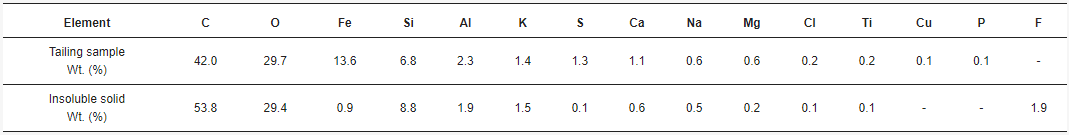
The, acid solutions obtained twhrough leaching and posterior filtration were analyzed by AAS, giving the concentrations of total Fe, Cu, Al, Ca, and Mg. The combinations of acid concentration (C), time (t), and temperature (T) for each experiment, along with their results, are shown in Table 1. The ich is a high concentrations for each element of interest were calculated as the average between the doubled experiments. Therefore, the standard error was calculated.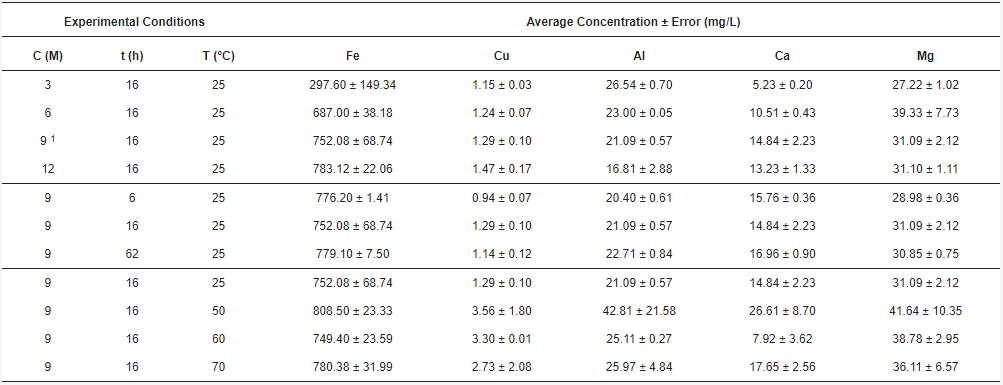
4.3. Insoluble Solid Characterization
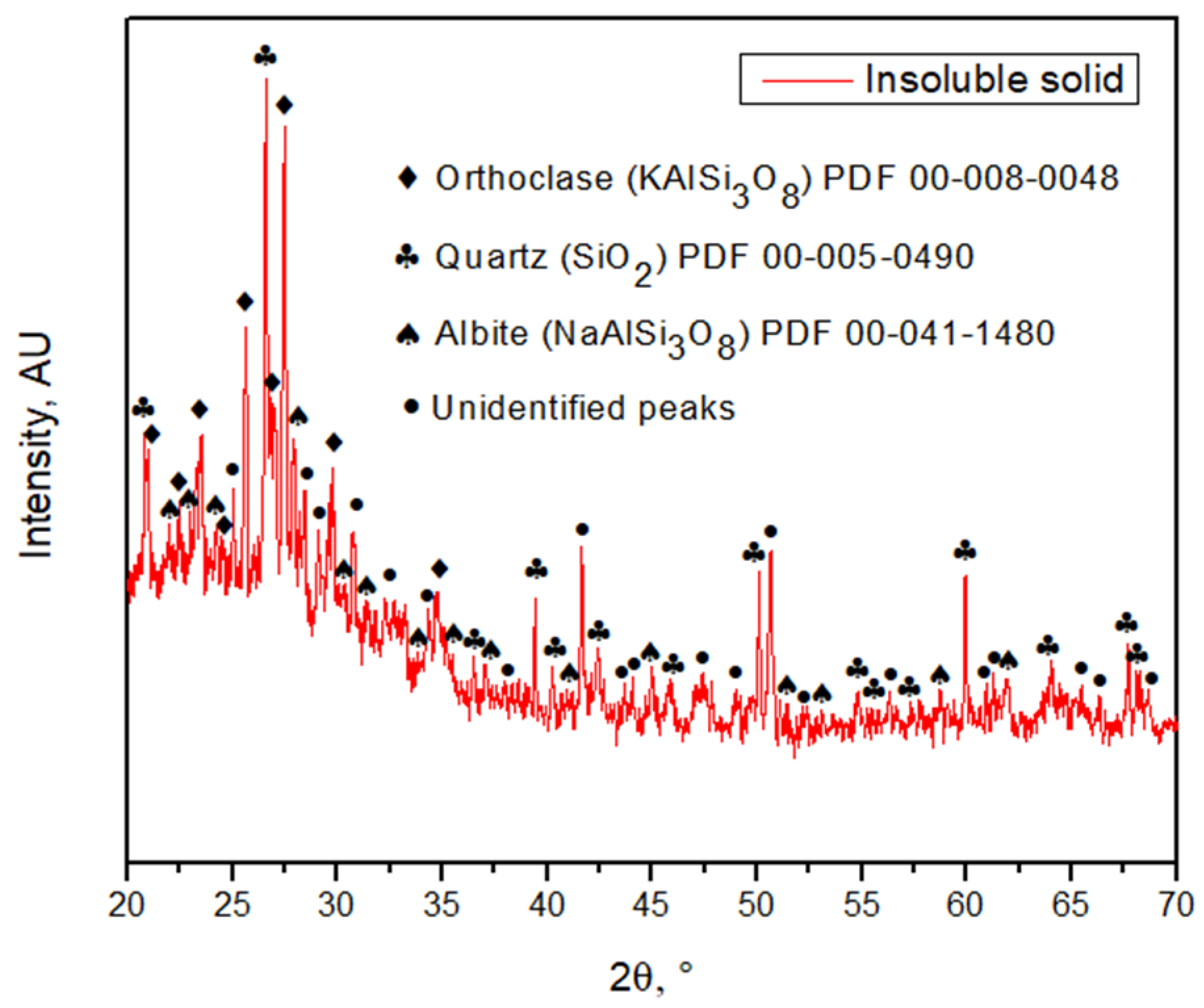
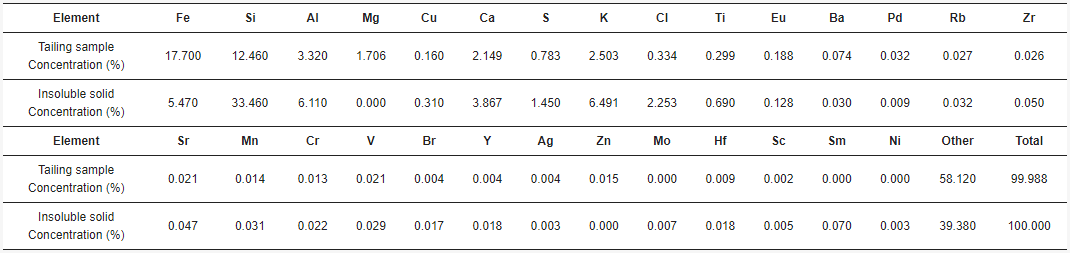
Bof iron. They SEM-EDX analysis, it was found that the solid samples had an irregular morphology composed mainly of silica with presence of several elements.cused on some Figure 6 shows one of the insoluble solids obtained from a solution leached with HCl 9 M, at 25 °C, for 16 h. The grains are irregularly shaped and the eleeresting elements are mostly homogeneously distributed in the sample. Table 2 such ashows the elemental composition of the tailing sample and the insoluble solid sample obtained under the conditions of HCl 9 M, 25 °C, and 16 h of leaching, by EDX. Results show the weight percent of each element found. Figure 7 shows the XRD of the scopper, aluminum, calcium ample obtained at 9 M, at 25 °C, for 16 h. The main phases in the sample are orthoclase (KAlSi3O8), qd magnesiuartz (SiO2), and albite (NaAlSi3O8), matching the SEM-EDX analysis. In the same analysis, it is possible to observe peaks corresponding to other unidentified crystalline species marked with black circles. For further comparison between tThey found that if the tailing sample and the insoluble solid, an X-ray Fluorescence (XRF) analysis was performed on both samples. The results are shown in Table 3.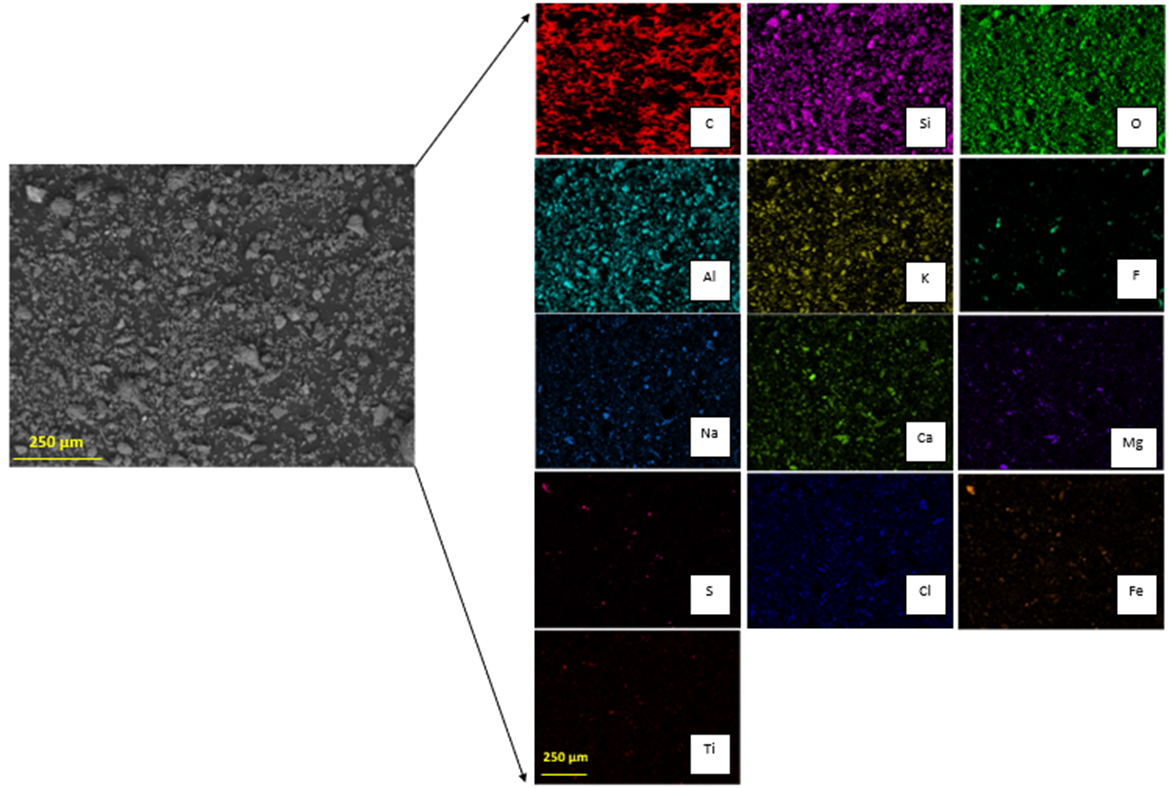
5. Discussion
6. Conclusions
The tailings of northern Chile contain an important amount of iron and other valuable species, such as copper and aluminum, which can be extracted through leaching with hydrochloric acid. The most relevant condition in this process is the acid concentration, due to its effect on the extraction of metals. Conditions can be selected according to the element that wants to be obtained, e.g., if a high iron extraction is wanted, a high acid concentration should be applied, while on the contrary, if a high extraction of aluminum is wanted, a low acid concentration should be used. The variables time and temperature do not show a relevant influence on the iron concentration, so it can be considered that it presents a constant behavior. Within the ranges studied, the conditions of 9 M acid concentration, 16 h reaction time, and a temperature of 25 °C were selected as the optimum leaching parameters. This process differs from traditional leaching by using hydrochloric acid instead of sulfuric acid; in addition, the raw material is a residue from a metallurgical process instead of mined ore. This proves that valuable elements such as iron, copper, and aluminum, along with calcium and magnesium, can be extracted from copper tailings for further valorization. The silicates obtained in the insoluble portion show low iron concentrations and could be used in other applications such as additives in the construction industry. It is important that these processes are circular, finding uses for waste such as tailings and also for the by-products of the tailing leaching. Environmental care is fundamental, so the production of by-products that are harmful to ecosystems must be avoided.References
- Aznar-Sánchez, J.A.; García-Gómez, J.J.; Velasco-Muñoz, J.F. Mining Waste and Its Sustainable Management: Advances in Worldwide Research. Minerals 2018, 8, 284.
- Reichl, C.; Schatz, M.; Zsak, G. World Mining Data. Miner. Prod. 2017, 32, 1–261.
- Cortés, S.; Soto, E.E.; Ordóñez, J.I. Recovery of Copper from Leached Tailing Solutions by Biosorption. Minerals 2020, 10, 158.
- Upadhyay, A.; Laing, T.; Kumar, V.; Dora, M. Exploring barriers and drivers to the implementation of circular economy practices in the mining industry. Resour. Policy 2021, 72, 102037.
- Kinnunen, P.; Kaksonen, A.H. Towards circular economy in mining: Opportunities and bottlenecks for tailings valorization. J. Clean. Prod. 2019, 228, 153–160.
- Araya, N.; Kraslawski, A.; Cisternas, L.A. Towards mine tailings valorization: Recovery of critical materials from Chilean mine tailings. J. Clean. Prod. 2020, 263, 121555.
- Valderrama, L.; Santander, M.; Zazzali, B.; Carmona, M. Concentración Magnética Aplicada a Relaves de cobre. HOLOS 2014, 6, 37–44.
- Lacassie, J.P.; Vivallo, W.; Díaz, A.; Ruiz-del-Solar, J. Geoquímica de yacimientos metálicos y de sedimentos, de las regiones de Atacama y Coquimbo, norte de Chile. In Proceedings of the XIV Congreso Geológico Chileno, La Serena, Chile, 4–8 October 2015; pp. 429–432.
- Ristović, I.; Štyriaková, D.; Štyriaková, I.; Šuba, J.; Širadović, E. Bioleaching Process for Copper Extraction from Waste in Alkaline and Acid Medium. Minerals 2022, 12, 100.
- Vargas, F. Copper Tailings as Supplementary Cementitious Material: Activation, Leaching and Environmental Behaviour; Pontificia Universidad Católica de Chile: Santiago de Chile, Chile, 2020.
- Godirilwe, L.L.; Haga, K.; Altansukh, B.; Takasaki, Y.; Ishiyama, D.; Trifunovic, V.; Avramovic, L.; Jonovic, R.; Stevanovic, Z.; Shibayama, A. Copper Recovery and Reduction of Environmental Loading from Mine Tailings by High-Pressure Leaching and SX-EW Process. Metals 2021, 11, 1335.
- Wang, J.; Zhang, Y.; Yu, L.; Cui, K.; Fu, T.; Mao, H. Effective separation and recovery of valuable metals from waste Ni-based batteries: A comprehensive review. Chem. Eng. J. 2022, 439, 135767.
- Chen, M.; Han, Z.; Wang, L. Recovery of valuable metals from copper slag by hydrometallurgy. Adv. Mater. Res. 2011, 402, 35–40.
- Goryachev, A.; Svetlov, A.; Kompanchenko, A.; Makarov, D. Sulfuric Acid Granulation of Copper—Nickel Ore Tailings: Leaching of Copper and Nickel in the Presence of Sulfide Oxidation Activators. Minerals 2022, 12, 129.
- Dimitrijević, M.; Urošević, D.; Milić, S.; Sokić, M.; Marković, R. Dissolution of copper from smelting slag by leaching in chloride media. J. Min. Metall. Sect. B Metall. 2017, 53, 407–412.
- Adrianto, L.R.; Pfister, S. Prospective environmental assessment of reprocessing and valorization alternatives for sulfidic copper tailings. Resour. Conserv. Recycl. 2022, 186, 106567. Santibáñez-Velásquez, L.E.; Guzmán, A.; Morel, M.J.; Extraction of Iron and Other Metals from Copper Tailings through Leaching. Metals 2022, 12, 1-11, https://doi.org/10.3390/met12111924.
- Leiva, E.; Cayazzo, M.; Dávila, L.; Torres, M.; Ledezma, C. Acid Mine Drainage Dynamics from a Paste Tailing Deposit: Effect of Sulfate Content on the Consistency and Chemical Stability after Storage. Metals 2021, 11, 860. Adrianto, L.R.; Pfister, S. Prospective environmental assessment of reprocessing and valorization alternatives for sulfidic copper tailings. Resour. Conserv. Recycl. 2022, 186, 106567.
- Google Maps. Tailing Location. Available online: https://www.google.com/maps/@-28.548177,-70.736858,450m/data=!3m1!1e3?hl=en-US (accessed on 19 October 2022).Leiva, E.; Cayazzo, M.; Dávila, L.; Torres, M.; Ledezma, C. Acid Mine Drainage Dynamics from a Paste Tailing Deposit: Effect of Sulfate Content on the Consistency and Chemical Stability after Storage. Metals 2021, 11, 860.
- Tang, H.; Zhao, L.; Yang, Y.; Han, H.; Wang, L.; Sun, W. Dissolution Kinetics of Chlorine from Iron Ore Sintering Dust. Metals 2021, 11, 1185.
- Šajn, R.; Ristovic, I.; Ceplak, B. Mining and Metallurgical Waste as Potential Secondary Sources of Metals—A Case Study for the West Balkan Region. Minerals 2022, 12, 547.
- Tao, L.; Wang, L.; Yang, K.; Wang, X.; Chen, L.; Ning, P. Leaching of iron from copper tailings by sulfuric acid: Behavior, kinetics and mechanism. R. Soc. Chem. 2021, 11, 5741–5752.
- Mubarak, Y. Leaching of Copper Ores: Effects of Operating Variables. Int. J. Emerg. Trends Eng. Res. 2020, 8, 4226–4235.
- Tayebi-Khorami, M.; Edraki, M.; Corder, G.; Golev, A. Re-Thinking Mining Waste through an Integrative Approach Led by Circular Economy Aspirations. Minerals 2019, 9, 286.
