Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Luba Pascoe and Version 2 by Beatrix Zheng.
Climatic and environmental factors may influence the arbovirus disease outbreak, transmission, and surveillance. Thus, there is a call for further research on the area. To benefit from arbovirus modeling, it is crucial to consider the influence of climatic and environmental factors, especially in Africa, where there are limited studies exploring this phenomenon.
- dengue fever
- arbovirus
- climatic variables
- environmental variables
- agent-based modeling
- agent-based modeling and simulation
1. Introduction
Infectious diseases such as dengue, malaria, chikungunya, Zika, and yellow fever, to mention a few, are emerging as a worldwide challenge in public health. They occur rapidly and spread to large areas in a relatively short space of time, thus leading to an increase in mortality rates globally as well as in Sub-Saharan Africa (SSA) [1][2][3][4][5][6][7][1,2,3,4,5,6,7].
In addition, globally, mosquito-related diseases which include dengue fever, malaria, chikungunya, Zika, yellow fever, and others have turned out to be a major public health concern, with estimates that half the approximated world population of 9 billion is in danger of contracting an arbovirus infection by 2050 [7][8][9][7,8,9].
Arthropods are abundant in tropical and subtropical regions, resulting in a high proportion of arboviruses in these regions [6]. The global distribution of viruses has caused some arboviruses to be endemic in specific regions of the world. Global warming, deforestation, and urbanization have resulted in a dramatic increase in vector-borne diseases in the world due to the rapid expansion of vector habitats [10]. The transportation of infected mosquitos and their eggs to various new ecological niches is increased through international travel, while shipping and industrialization can also facilitate virus-vector–human host interactions, causing outbreaks owing to lower herd immunity. Herd immunity in a community is acquired when a high percentage of the community is immune to disease through vaccination or previous infection. Lack of enough approved dengue vaccination strategies, different dengue strains, and lack of efficient and sustainable vector control strategies contribute to lower herd immunity. Furthermore, the active circulation of multi-serotypes of dengue make it hyperendemic in many countries. It is posited that a decrease in cross-immunity is among the contributory factor to large dengue outbreaks [11]. Outbreaks happening in new areas for the first time mostly tend to involve immunologically naïve populations resulting in high rates of attack [11]. Marchi, Trombetta, and Montomoli [10] report that “the greatest health risk of arbovirus emergence comes from extensive tropical urbanization and colonization of this expanding habitat by the highly anthropophilic mosquito, Aedes Aegypti, together with the recent invasion into the Americas, Europe and Africa of Aedes albopictus that could enhance transmission of these viruses in temperate regions”. Figure 1 shows the distribution of dengue, chikungunya, yellow fever and Zika infections in Africa [12].
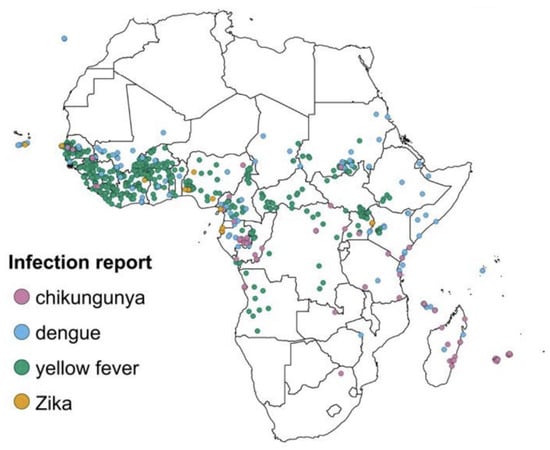
Figure 1.
Dengue virus (DENV) has spread throughout the world and is regarded as the most menacing arbovirus disease [6][7][13][14][6,7,13,14]. A study by Marchi, Trombetta, and Montomoli [10] reports on the existence of high levels of dengue endemic transmission in Americas, the western Pacific, and south-east Asia with around 4 billion people at risk of being infected. Additionally, DENV has been identified in Europe since 2010 and in 2012 an outbreak was recorded in Madeira. It has been stated that DENV’s four serotypes are spreading across Africa, although the most frequently reported serotype is DENV-2 [15]. A study by Mordecai et al. [16] reports the possible shift in the disease burden from malaria cases to arboviruses such as dengue and chikungunya in SSA countries due to climate change and urbanization. Climate change provides suitable environment that favors Ae. Aegypti while providing unfavorable environments for Anopheles gambiae [16][17][16,17]. The shift into vector-borne diseases burden is already witnessed in Sudan in which chikungunya, Rift Valley fever and dengue cases have been reported in Red Sea state, Kassala state and Darfur region [17]. It is alarming when this shift in disease burden is happening in Africa because most of the communities are poor, the governments and health systems do not have control program, health policies or local capacity for early detection and response to arboviral diseases as soon as they emerge [17]. In Tanzania, DENV has also been reported since 2010 [18] with a substantial rise in dengue fever cases in April and May of 2019 [19]. Dar es Salaam was declared an epicenter of the dengue outbreak that occurred in 2019 and the preceding outbreaks even though there have been occurrences in different regions [19].
Rweyemamu et al. [20] and Mahmood et al. [14] outline that, it is crucial to establish good monitoring, investigation, and reporting systems for infectious disease incidences which will play a major role in the management of existing diseases as well as contribute to an adaptive and flexible response to new and emerging diseases. Every year, millions of individuals are at risk of serious illness as a result of new infections. A lack of infrastructure for timely collecting, reporting, and analyzing epidemic data has posed a significant threat to public health security at the local, regional, and national levels [14][20][14,20]. In addition, it has been noted that policymakers, epidemiologists, and other related stakeholders depend highly on accurate and timely information to make informed decisions that will improve the well-being of the nation as well as the national healthcare system [14][21][22][14,21,22]. Furthermore, real-time data provide decision-makers with the knowledge required to respond effectively to the population’s health necessities; as a result, the value of timely and accurate data can be observed [23].
2. Dengue Fever
Dengue fever is a serious disease that affects tropical and subtropical countries worldwide [24][25][24,25]. The disease is a major public health concern with economic implications. Mahmood et al. [14] reported that 3.98 billion people in 128 countries are at risk of contracting dengue [13][26][27][13,26,27]. An annual estimate of over 105 million dengue cases is reported [11][28][11,28]. A study by Cattarino et al. [28] further estimates that most dengue disease burden was concentrated in South and Southeast Asia valued to about 58%, 26% occurring in SSA mostly concentrated in Central and Eastern Africa, and Latin America had an estimation of 16% of the global burden. Although the information about the prevalence of viral diseases is limited, it is noted that several studies have been carried out to determine the prevalence and spread of dengue infections and outbreaks in Tanzania [29]. Conversely, the literal roles of climatic, socio-environment and ecological variables in the spread of dengue have not been extensively investigated [30].
The health system in SSA lacks the capacity for adequate disease reporting and timely response due to unreliable data [22][31][22,31]. The untimeliness, incompleteness, inconsistency, and inaccuracy of the data, among other things, contribute to data unreliability [22][31][22,31].
Ae. Aegypti is the main vector for dengue, chikungunya, yellow fever and Zika [32][33][32,33]. Aedes albopictus which is a secondary vector for dengue, chikungunya, yellow fever and Zika is also present in some regions in Africa such as Cameroon, Gabon, Nigeria, Congo, Côte d’Ivoire, Central African Republic, Sudan and South Africa [16][34][35][16,34,35]. Ae. Aegypti is considered a domestic vector because it is dominant in urban areas, while Ae. Albopictus is mostly found in rural, peri-urban settings and forest areas in tropical, subtropical and temperate regions of the world [10][36][37][38][10,36,37,38]. The difference in distribution of these species is related to their behavior based on host preference, blood feeding, preference for vegetation, suitable conditions for resting and ovipositioning [35][36][37][35,36,37]. Ae. Albopictus can be quite competitively dominating when it coexists with Ae. Aegypti [16].
Some studies provide information on dengue transmission and risk factors in African countries such as Tanzania [29][34][39][40][29,34,39,40], Kenya [16][34][16,34], Uganda, Mozambique, [16] Sudan [17][35][41][17,35,41], Côte d’Ivoire, Cameroon, Gabon [16][34][42][16,34,42] Senegal, Nigeria, and Sierra Leone [16][34][16,34]. There is a growing evidence that in SSA epidemic and endemic transmissions of Aedes-transmitted arboviruses such as dengue, chikungunya and others have regularly occurred but have been undiagnosed or misdiagnosed as malaria [16]. The increase in climate suitability for Aedes-transmitted arboviruses pose an under-recognized public health burden to the African countries [16]. Among the factors that contribute to dengue infection transmission are climate change, urbanization, globalization of trade and travel, increased population density, and the unavailability of effective prevention control methods [16][43][16,43]. Furthermore, Dumont et al. [44] identified risk factors for dengue fever in which individual factors such as age, sex, and level of education were important in determining the risk of contracting DENV. Dumont et al. [44] indicated that household factors such as the number of people residing in a room as well as the size of the community increased the chance of contracting dengue fever. Socioeconomic and demographic factors included low income while living and traveling to endemic areas increased the chances to contact DENV. The existence of anthropogenic breeding areas for Ae. Aegypti (such as disposed of plastic containers and car tires that are not used), presence of vegetation density (such as leaf axils, fallen leaves, flower brats, and tree holes), height above sea level and existence of animals that are associated with dengue and chikungunya viruses’ transmission made up the environmental factors [7][44][7,44]. Thus, humidity, temperature, human migration, wind speed and sanitation contribute to the epidemic conditions in areas affected by dengue and chikungunya [6][44][45][46][6,44,45,46].
The World Health Organization (WHO) recommends integrated vector control strategies to prevent and control dengue infections that can lead to outbreaks [13][23][43][13,23,43]. These control strategies can be through environmental management control by eliminating the potential breeding sites for mosquitoes, namely stagnant water sites as well as chemical and biological controls. According to the literature, most of the control and prevention strategies are based on mosquito vector density management [23][43][23,43], which is influenced by different factors among them being climatic and environmental factors.
3. Agent-Based Modeling
Agent-based modeling (ABM) presents a useful approach for processing health data and producing simulations that provide meaningful information for decision-makers concerning different disease outbreaks, reporting, and containment. ABM describes the underlying social/epidemiological system as well as provides a versatile and powerful platform for modeling different healthcare interventions and answering a wide range of policy-making questions [5]. As a result, ABM is regarded as a relatively new simulation technique that is gaining popularity and supports many varied applications across different fields.
ABM exhibits several benefits that make it suitable for modeling real-world systems, including bounded rationality, emergent behavior, and the bottom-up approach in modeling which results in a macro-system based on sub-system interactions. Other benefits include the heterogeneity and discrete nature of agent-based models which make them suitable for modeling heterogeneous populations; networked interactions among agents, as well as the completeness of the agent-based models since they are well detailed as they provide both individual and aggregate level detail simultaneously. Furthermore, agent-based models are very flexible and allow the incorporation of randomness into the models with agents’ decisions being based on probability rather than being strictly deterministic [47][48][49][50][47,48,49,50].
ABM follows a bottom-up approach which makes it a flexible and powerful tool for modeling complicated systems that have many interacting components [51][52][51,52]. In epidemiology, due to the increased complexity of the systems that need to be analyzed and modeled based on their interdependencies, ABMs complement statistical and mathematical models making them more accurate and suitable for predictions [53][54][55][53,54,55]. Complex systems and complexity lead to the rise of unpredictable patterns or global structures as a direct outcome of local-level procedures [52]. Furthermore, the organization of data into databases at finer levels of granularity and the advancement in computation power makes ABM more favorable than system dynamics, spatial interaction, and diffusion models which cannot handle the heterogeneity of data although they have successfully predicted macro-level behavioral patterns. Furthermore, ABM is capable of simulating individuals, their interactions, and the resulting consequences [47][49][53][47,49,53].
Several studies have modeled different aspects of dengue epidemics. Jacintho et al. [56] observed the behavioral spread of dengue fever based on how the simulation agents interact with their environment. Jindal and Rao modeled mosquito-borne diseases including malaria, dengue, and chikungunya by considering the human mobility patterns as a major source of the spatial movement of infections [5]. Mahmood et al. [14] modeled the dynamics of the population together with how both humans and mosquitoes interact intending to assist epidemiologists to explore and predict infectious disease transmission and spread. In this researchtudy, the benefit of using ABM over the compartmental model has been explored and SEIR model is utilized through a proprietary AnyLogic framework. A network type based on distance is used in which messages are passed to accomplish the interaction of host and vector agents. The whole population of the host is initialized in a susceptible state rather than distributing the population with different initial states which can enhance realistic model configurations. Pathogen’s structure and behavior is restricted to serotype initialization that is DENV1, DENV2, DENV3 or DENV4 and the key parameters such as survivability, infectivity, incubation period plus transmissibility. The framework has not considered the cross immunity of the serotypes. The framework does not support a large population of a million or more agents and lacks a mobility layer that can incorporate the movements of both host and vector layers.
A study by Stiner and Chellamuthu [57] used ABM to model the complex agent mosquito with its spatial-temporal attributes; the life cycle of a mosquito is modeled to show the effect of temperature on the different stages of mosquito development depending on the different types of mosquitoes. Then, different control strategies are advised for the different diseases caused by the mosquitoes.
A study by Mniszewski et al. [58] used agent-based modeling to simulate the spread of infectious diseases through the population using EpiSims software. EpiSims uses three sets of information (population, location and movement of individuals between locations) to simulate a disease spread in a geographical area. The study leveraged the usefulness of various models by suggesting a hybrid network patch model to provide insights into the effect of variable probabilities in the infection model on agent-based modeling. However, this study does not capture the host–vector interactions [5].
4. Influence of Cclimatic and Eenvironmental Ffactors
Different studies have modeled how temperature, rainfall, and humidity affect the spread of arboviruses [59]. An increase in temperature favors the virus’ replication and a shorter extrinsic incubation period, which results in an increased density of infected vectors [59][60][59,60]. Extreme heat threatens adult mosquito survival rates and more quickly dries out breeding grounds, reducing mosquito numbers. Increased breeding sites and decreased mosquito mortality are also observed with an increase in precipitation and humidity levels [60], while higher precipitation may minimize mosquito numbers by washing out the immature stages [61].
Various temperature metrics have been used in different studies to establish their relationship with arbovirus incidences [59][60][59,60]. Metrics such as mean temperature, minimum temperature, maximum temperature, air temperature, and water temperature have been identified to influence mosquito development, especially during their aquatic stages that is egg, larvae, and pupae stages [14][24][25][32][62][63][64][65][66][14,24,25,32,68,75,76,78,79]. The range of Ae. Aegypti is tremendously constrained by yearly minimum low temperatures below which its eggs are inviable [67][69], while on the other hand, Ae. Aegypti abundance could increase or decrease depending on the rate of warming as well as the magnitude of temperature increase [6][7][67][6,7,69]. The optimal temperature range suitable for adult Aedes mosquitoes is established in different studies to be between 15 °◦C and 30 °◦C which is within the range of 10 °◦C to 35 °◦C.
A higher transmission of arboviral diseases can be observed during the rainy periods with higher temperatures and lower disease transmission during the dry season [24][68][24,67]. The abundance of Ae. Aegypti immatures in moderate and highly urbanized sites were found to be positively influenced by the size of the aquatic habitat and the amount of precipitation. Increased rainfall was determined as a risk factor for arbovirus outbreak during the study period by Rumisha et al. [68][67] when modeling malaria using Bayesian regression models and machine learning models. Low-lying regions and wetlands created suitable habitats for mosquitoes and increased the risk of disease transmission [67][69][69,72]. Flooding can be disadvantageous to vector populations, causing a reduction in the mosquito population by destroying breeding sites and aquatic stages of mosquitoes [70][66]. Consequently, an inverse relationship can exist between precipitation and mosquito populations where breeding sites have been washed away with flooding water [71][70]. Lack of rainfall and drought are also associated with lower disease incidence because drought usually results in a reduction in mosquito populations [68][67]. Ae. Aegypti breed in containers with clean water which are mostly located in human residences. Thus, changes in precipitation could affect the availability of these vector breeding sites depending on their location either indoors or outdoors rain filled objects and as a result, influence the vector abundance [73]. [72].
Humidity is governed by a combination of temperature and rainfall, it is potential for virus transmission because it influences the lifespan of the mosquito. Some studies have indicated that annual average water vapor pressure is a crucial climatic predictor of global dengue occurrence [72][73]. Humidity positively affected the number of female Ae. Aegypti were captured until an optimal of 80% was reached [63][64][71][70,75,76]. In slightly urbanized sites, the relative humidity was found to positively influence Ae. Aegypti immatures [24].
The vegetation index which was an indicator of the amount of green vegetation in an area was closely related to rainfall incidences. Vegetation created suitable habitats for mosquito breeding which as a result led to an increase in mosquito density [67][69][69,72]. The growth of vegetation is to some extent regulated by temperature and rainfall. It is associated with warmer temperatures and increased rainfall, as a result, an increase in vegetation indices is related to an increased number of eggs laid by mosquitoes. Vegetation cover provides suitable breeding sites which directly increases the vector abundance and the disease transmission rate [37][38][73][37,38,86]. Thus, the vegetation index has a positive relationship to egg prediction as well as oviposition activities leading to increased vector activity due to increases in habitat for mosquitoes [38]. Normalized difference vegetation index (NDVI) and normalized difference water index (NDWI) are the two variables that have been used to determine the vegetation cover and their influence on larval survival, breeding sites, oviposition activity, vector activity, and vector population growth [34][36][38][74][34,36,38,87].
5. Conclusion
More research needs to be conducted in the African context, incorporating the climatic factors, environmental factors, and their influence on dengue transmission using ABM and simulation.
