Rhein is a monomeric component of anthraquinone isolated from rhubarb, a traditional Chinese medicine. It has anti-inflflammation, anti-oxidation, anti-apoptosis, anti-bacterial and other pharmacological activities, as well as a renal protective effects. Rhein exerts its nephroprotective effects mainly through decreasing hypoglycemic and hypolipidemic, playing anti-inflflammatory, antioxidant and anti-fifibrotic effects and regulating drug-transporters. However, the latest studies show that rhein also has potential kidney toxicity in case of large dosages and long use times.
- traditional Chinese medicine
- rhein
- kidney protection
- nephrotoxicity
1. Introduction
Rhein (molecular formula C15H8O6), a lipophilic anthraquinone, is the main component of Senna alexandrina Mill., Rheum palmatum L., Aloe barbadensis Miller, and Polygonum multiflorum Thunb [1]. It contains two hydroxyl groups and one carboxyl group and has strong polarity and electrochemical REDOX properties [2]. Rhein has a lot of pharmacological effects, such as anti-inflammation [3], anti-cancer [4], anti-fibrosis [5], antioxidation [6], hepatoprotective [7], nephroprotective [8], lipid-lowering [9], and antimicrobial activities [10]. In spite of this, its poor solubility and low bioavailability limit its clinical applications. The study of rhein and its derivatives has been enriched by advances in drug separation and synthesis. Diacerein is one of the most common and representative derivatives of rhein, and it is used for the treatment of arthritis owing to its ability in reducing osteoclast formation and inhibiting the synthesis of resorptive factors [11]. Additionally, nanodrug delivery systems have been designed to overcome the poor solubility of rhein [12]. The pharmacological effects of these compounds lay the groundwork for the treatment of liver disease, osteoarthritis, diabetes, atherosclerosis, and a variety of cancers [13][14][15][16][17]. However, it has recently been reported that rhein also causes hepatotoxicity and nephrotoxicity [6][18].
2. Nephroprotective Effect
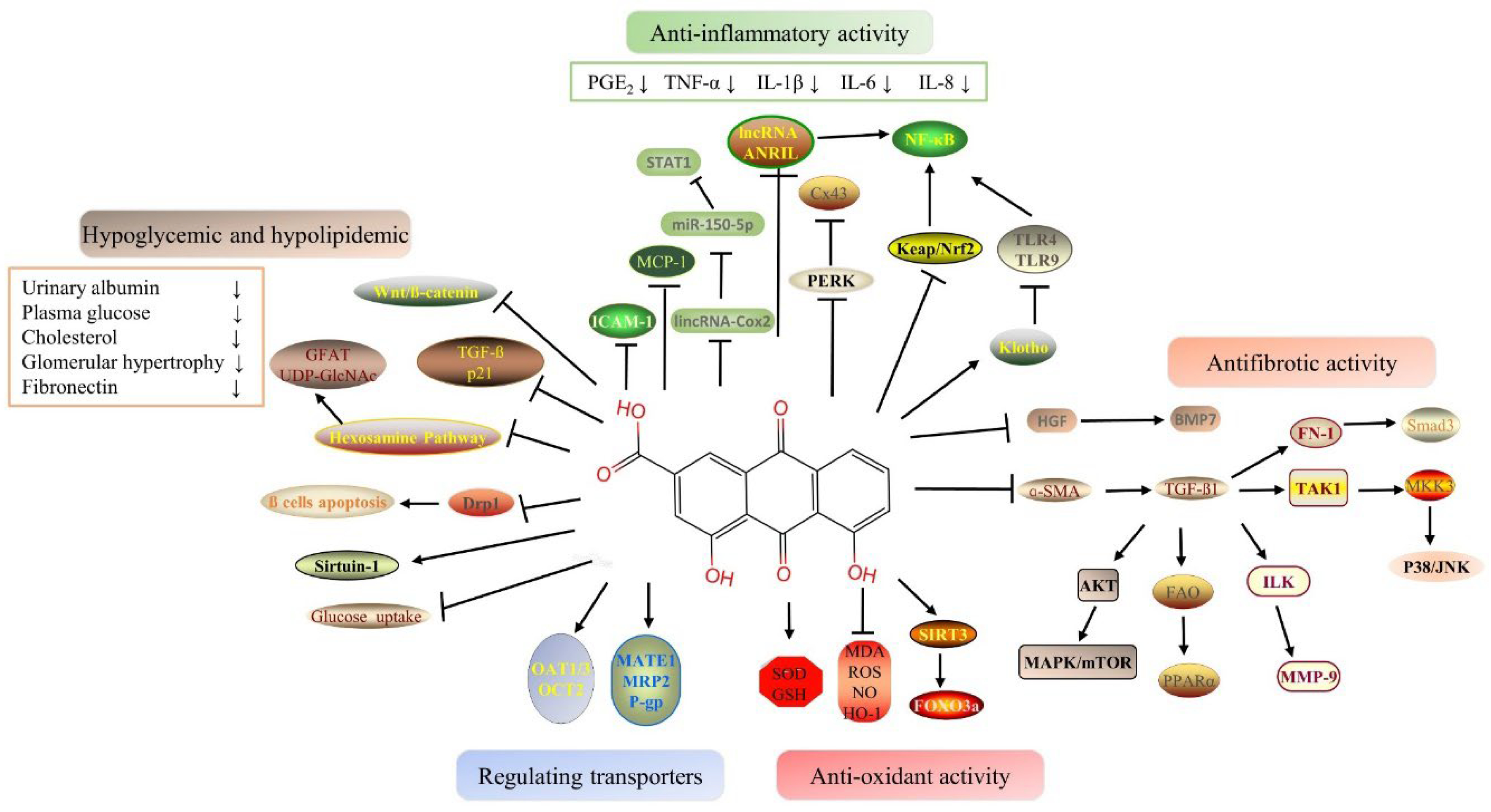
2.1. Hypoglycemic and Hypolipidemic Proprieties of Rhein
2.2. Anti-Inflammatory Proprieties of Rhein
As a result of hyperglycemia, advanced glycation end products (AGEs) damage the vascular endothelium, glomeruli, and tubules, which facilitates the emergence of DN eventually [33]. Diabetes is an entity of inflammation because its pathogenesis involves multiple inflammatory/proinflammatory factors, and it is characterized by chronic low-grade inflammatory disease [34]. The model of early diabetic nephropathy was established and showed increases in serum microalbuminuria, NADPH oxidase expression, and PKR-like eukaryotic initiation factor 2α kinase (PERK) level, and a decrease in connexin 43 (Cx43) in renal tissue. Upregulation of nuclear transcription factors, such as PERK, reveals the presence of endoplasmic reticulum stress (ER stress) [35]. Cx43, a family of connexins, may play a key role in exchanging small molecules within glomeruli and tubules in the kidney, which is essential for normal renal function [36]. Argirein, a derivative of rhein, can be produced by combining rhein with L-arginine by forming a hydrogen bond. It has anti-inflammatory activity derived from rhein. Argirein (200, 100, and 50 mg/kg) is more effective than aminoguanidine (AMG) (100 mg/kg), which has anti-inflammatory activity as a positive reference agent in reversing the above changes [36]. The mortality rate of AKI is about 70–80 % [37][38]. Currently, renal replacement therapy (RRT) is the only effective treatment for AKI [39]. Inflammatory response inherent to sepsis is thought to be the direct mechanism of AKI [40]. Based on rhein’s anti-inflammatory pharmacological activity, Yu C et al. (2015) explored the effects of rhein on sepsis-induced AKI by using lipopolysaccharide (LPS) and cecal ligation and puncture (CLP) models [41]. The potential mechanism of rhein may be related to its anti-inflammatory effects. Rhein inhibited the activation of NF-κB via restraining the expression and phosphorylation of the relevant proteins in the NF-κB signal pathway and hindering the transcription of NF-κB p65 [41]. It brings a new research direction for solving AKI related to endotoxemia. At the same time, the research of Liu M et al. (2021) also confirms this point [42]. The 5/6 nephrectomy model (5/6 Nx) in rats and LPS-induced HK-2 cells were used, and the results indicated that rhein inhibits inflammatory signaling pathways via decreasing the production of TNF-α, IL-6, and monocyte chemotactic protein (MCP-1) [42]. In addition, by inhibiting NF-κB phosphorylation, rhein diminished LPS-induced NF-κB activation [42]. The above results clearly indicate that rhein can be a promising therapeutic agent for renal disease by inhibiting multiple inflammatory mediators [41][42].2.3. Antioxidant Proprieties of Rhein
Rhein attenuated APAP-induced hepatotoxicity and nephrotoxicity in a dose-dependent manner [7]. The levels of serum glutamate-pyruvate transaminase (GPT), glutamate-oxaloacetic transaminase (GOT), urea nitrogen (UREA), creatinine (Crea), and reactive oxygen species (ROS) production were significantly decreased, and the contents of nitric oxide (NO), MDA, and GSH were recovered in the rhein treatment group [7]. Rhein relieved APAP-induced liver and kidney injury by ameliorating oxidative stress [7]. Diacerein (DIA) is used to treat osteoarthritis. DIA enters the body and is rapidly converted into its active metabolite rhein [43]. Furthermore, studies have been conducted one after another on whether DIA can alleviate AKI. The antioxidant effects of DIA were reported to protect renal function against doxorubicin-induced AKI [43].2.4. Antifibrotic Proprieties of Rhein
CKD and chronic kidney failure (CRF) develop as a result of renal fibrosis. The pathological mechanism of renal fibrosis is relatively complex. There is a variety of stimulating factors or mediators, such as growth factors, cytokines and toxins, which induce the occurrence of fibrosis through a variety of mechanisms and signal pathways [44][45]. TGF-β has been proven to be a major pathogenic factor for the progressive development of renal fibrosis [46]. TGF-β can induce renal tubular epithelial cells to transform into renal mesenchymal fibroblasts through the epithelial–mesenchymal transition (EMT) process [47]. In addition to the TGF-β pathway, the Notch, Wnt, and Hedgehog signaling pathways can also be activated in response to renal injury, thereby promoting renal fibrosis [48]. TGF-β regulates renal fibrosis progression through classic and non-classic pathways. The classical TGF-β pathway includes two signaling pathways, namely TGF-β/Smad and bone morphogenetic protein (BMP) [46]. However, they have opposite effects although they have similar downstream Smad signaling pathways. Smad3 is a key downstream mediator of TGF-β signal transduction [46]. BMP7 is a member of the TGF-β superfamily that antagonizes the effects of TGF-β [49]. Like liver growth factor (HGF), they both have anti-fibrotic effects [49]. Combined therapy of rhein and Danshensu (DSS) had a certain renal protective effect on chronic kidney damage. The mechanism may be related to anti-inflammatory and anti-fibrosis by downregulating the NF-κB-related pathway and inhibiting the TGF-β/Smad3 pathway, respectively [50]. Other studies have shown that rhein improved renal function and reduced renal fibrosis and interstitial inflammation by inducing HGF and BMP7 production [51]. In addition to the classical Smad signaling pathway, TGF-β can also regulate the downstream cellular response through other non-classical pathways and then adjust the pathological process of renal fibrosis [46]. p38 mitogen-activated protein kinase (MAPK) is one of the atypical signaling pathways of TGF-β1. TGF-β1 activates the downstream signaling pathway MKK3-p38 MAPK cascade through the activation of TAK1, ultimately leading to cell fibrosis [52]. Rhubarb and Astragalus capsules (RAC) that contain 2.25 mg/g rhein have been used in a clinical treatment for chronic kidney disease [53].2.5. Benefits of Rhein Via Drug-Transporter
Renal transporters transport endogenous substances, poisons, and drugs from the blood to the urine. As a result of renal injury, uptake transporters and efflux transporters are altered, which affects toxic excretion and aggravates renal injury [54]. Previous research has shown that the expressions of organic anion transporter 1 (OAT1), OAT3, and multidrug resistance related protein 2 (MRP2) were significantly decreased after cisplatin-induced AKI, which reduced the excretion of endotoxin and aggravated renal injury [54]. There are few studies on the relationship between the various pharmacological effects of rhein and transporters. Zhu Y et al. (2022) [8] showed that the gene levels and protein expressions of the renal transporters including Oat1, Oat3, Organic cation transporter 2 (OCT2), mammal multidrug, and toxin extrusion proteins 1 (Mate 1), Mrp2, and P-glycoprotein (P-gp) in vancomycin-induced nephrotoxicity (VIN) were significantly decreased. Plasma creatinine, BUN, and plasma indoxyl sulfate were not excreted efficiently. Rhein reversed the expressions of the above transporters, and thereby promoted the excretion of endotoxins and finally alleviated renal injury [8]. The discovery of VIN’s pathogenesis expands the field of study on the kidney protection effects of VIN.3. Toxicological Effects in Kidney
Total rhubarb anthraquinones (TRAs) include emodin, rhein, chrysophanol, aloe emodin, and other substances [55]. However, the clinical cases of liver injury and the progression of kidney disease caused by TCM contained TRAs are increasing. Rhein is an important ingredient in TRAs, which affects the kidneys in a positive and negative way. Studies on the nephrotoxicity effects of rhein and the related mechanisms are shown in Figure 2.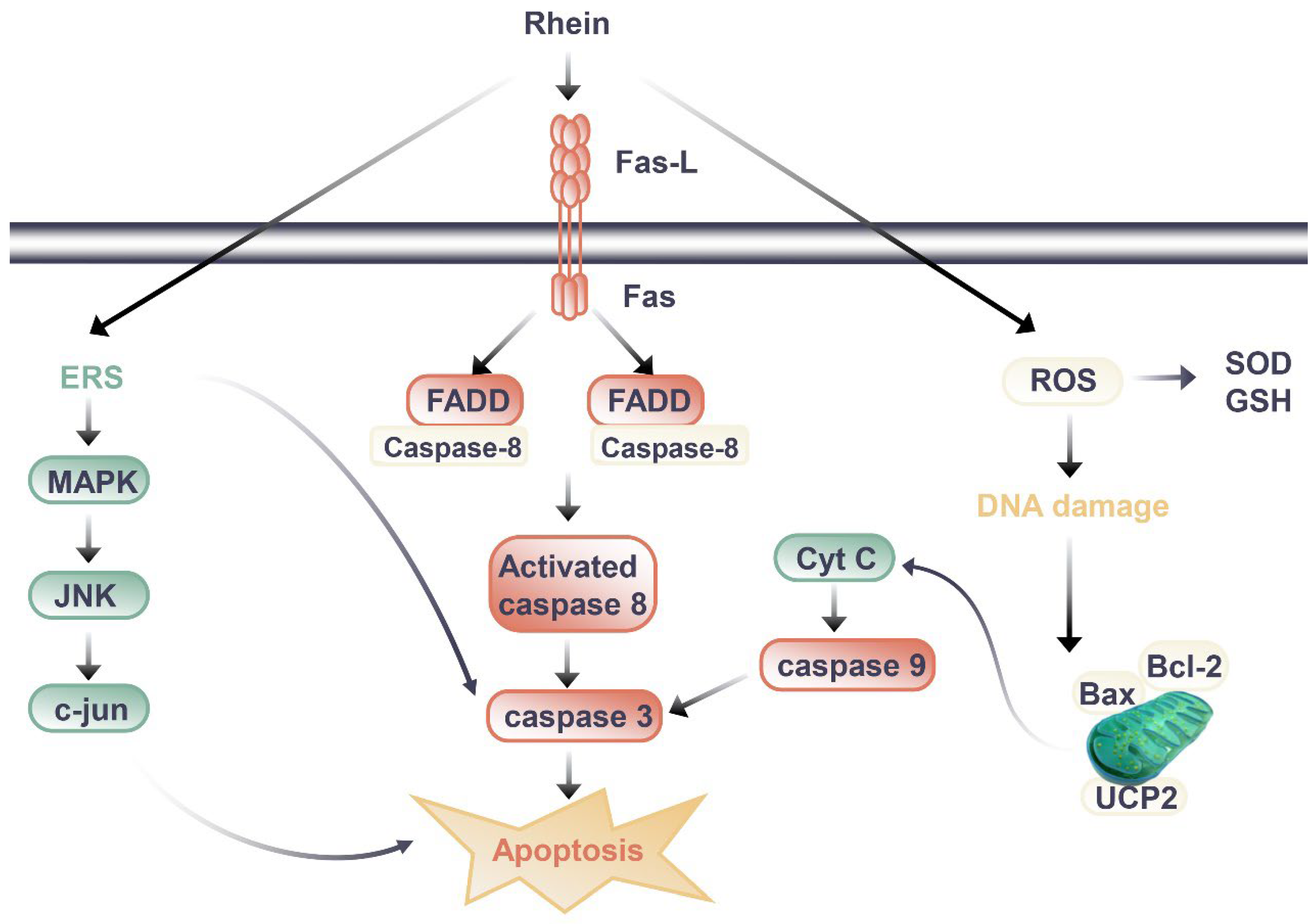
Figure 2. Signal pathway of nephrotoxic effect of rhein.
3.1. Rhein Nephrotoxicity: Mechanisms of Action and Possible Causes
Through the literature review, the difference between the kidney protection and nephrotoxicity effects of rhein may be related to the dosage and duration of rhein. When rhein exerted kidney protection effects, the dosage of rhein in animal experiments was mostly 20 to 150 mg/kg/day, and the duration of administration was mostly less than 14 days, with the longest time being 8 weeks when the rhein was at a slightly lower dosage. In mice, HU Y et al. (2019) observed renal toxicity after long-term administration of rhein [56]. Mice were randomly divided into three groups: blank group, low-dose rhein group (0.175g/kg) and high-dose rhein group (0.35g/kg). The drug was administered by gavage for 60 days [56]. Compared to the blank group of the same sex, BUN and SCr of mice in the administration group were increased, and the body weight of mice in the rhein high-dose group decreased [56]. The renal index of male mice in the administration group decreased significantly, and the content of GSH-Px decreased and the expression of TGF-β1 increased in male mice in the rhein high-dose group [56]. Its potential toxic mechanism may be caused by the imbalance of glutathione antioxidant system that can induce excessive oxidation, inflammatory reaction, and the apoptosis induced by the activation of caspase-3 [56].
In cell experiments, the transition between kidney protection and nephrotoxicity is more closely related to dose and duration of administration. Da H et al., (2009) evaluated the cytotoxic effects of emodin and rhein in HK-2 cells [57]. The results showed that both emodin and rhein could inhibit the growth of HK-2 cells, but the inhibitory effect of rhein was weaker than that of emodin [57]. From the experimental results of rhein on the survival rate of HK-2 cells, we found that rhein had obvious inhibitory effect on cell proliferation when it was treated with 40 μM for 24 hours, and the inhibitory effect gradually increased with the extension of incubation time; while the cell proliferation could be significantly inhibited after 12 hours of administration when the dosage of rhein was 100 μM [135]. It was preliminarily elucidated that rhein caused renal injury by inducing apoptosis. Researchers have also carried out a series of studies to further clarify the nephrotoxicity mechanisms of rhein. Most studies have shown that it is related to the induction of apoptosis (Figure 2). In addition to death-receptor signaling, the mitochondrial death pathway, oxidative stress, and endoplasmic reticulum stress all contribute to apoptosis [58]. It was found that rhein directly inhibited HK-2 cell growth and increased apoptosis in a dose- and time-dependent manner according to the study by Yang J et al. (2015) [59]. Rhein (50 and 100 μM, 24 h after administration) increased the mRNA levels of amino terminal kinase (c-Jun), activated transcription factor-2 (ATF-2), and caspase-3, and upregulated the expression of p38 MAPK and cleaved caspase-3. These results suggest that rhein may induce apoptosis in HK-2 cells through the MAPK signaling pathway [59]. Hao S et al. (2015) [60] also found rhein could dose-dependently inhibit the viability of HK-2 cells, increase the release of lactate dehydrogenase (LDH) and apoptosis rate, and significantly upregulate the mRNA or protein expressions of Fas, FasL, FADD, caspase-3, caspase-8, and Cytochrome C (Cyt-c). Apoptosis induced by the Fas pathway may be the mechanism behind rhein’s toxic effects on HK-2 cells in vitro. In another study [61], rhein reduced mitochondrial membrane potential and intracellular ATP level, released Cyt-c, and decreased Bcl-2 and Bax protein levels in HK-2 cells. Meanwhile, rhein increased the intracellular ROS level and inhibited mitochondrial uncoupling protein 2 (UCP2) expression, which regulates mitochondrial membrane potential, ROS generation, and ATP synthesis [61]. Rhein inhibited the expression of UCP2, significantly enhanced oxidative stress in cells, and thus promoted cell apoptosis, indicating the potential role of UCP2 in rhein nephrotoxicity [61].
3.2. Methods for Controlling Rhein Toxicity
In order to use rhein reasonably and safely, there may be some measures we should take. On the one hand, it is important to control the dosage and duration of rhein administration as described above; on the other hand, the compatibility of TCM can enhance its protective effects and reduce the toxicity. For instance, different doses of astragaloside IV (10, 20 and 40 μM) could reduce the occurrence of rhein-induced vacuolation, cell fusion, and the increase of necrotic cells in HK-2 cells [62]. After the combination of rhein and astragaloside IV in HK-2 cells for 48 hours, the cell inhibition rate and LDH leakage rate were significantly reduced [62]. The compatibility significantly increased the contents of SOD and GSH in cells and down-regulated the expression of MDA, which indicates that astragaloside IV could significantly inhibit the oxidative stress injury caused by rhein and then protect cells [62].
Abbreviations
References
- Ge, J.H.; Liu, X.H.; Xu, H.; Xu, D.Y.; Bai, F.P. Identification of different varieties of Rhei Radix et Rhizoma based on chemical analysis. China J. Chin. Mater. Med. 2015, 40, 2309–2313.
- Li, X.H.; Li, M.; Tao, Y.R. Development of pharmacological effects of rhein and its derivatives. Drugs Clinic. 2010, 25, 417–421.
- Hu, J.; Wang, D.; Wu, H.; Yang, Z.; Yang, N.; Dong, J. Long non-coding RNA ANRIL-mediated inflammation response is involved in protective effect of rhein in uric acid nephropathy rats. Cell Biosci. 2019, 9, 11.
- Henamayee, S.; Banik, K.; Sailo, B.L.; Shabnam, B.; Harsha, C.; Srilakshmi, S.; Vgm, N.; Baek, S.H.; Ahn, K.S.; Kunnumakkara, A.B. Therapeutic Emergence of Rhein as a Potential Anticancer Drug: A Review of Its Molecular Targets and Anticancer Properties. Molecules 2020, 25, 2278.
- Chen, Y.; Mu, L.; Xing, L.; Li, S.; Fu, S. Rhein alleviates renal interstitial fibrosis by inhibiting tubular cell apoptosis in rats. Biol. Res. 2019, 52, 50.
- Zhou, Y.X.; Xia, W.; Yue, W.; Peng, C.; Rahman, K.; Zhang, H. Rhein: A Review of Pharmacological Activities. Evid.-Based Complementary Altern. Med. eCAM 2015, 2015, 578107.
- Zhao, Y.L.; Zhou, G.D.; Yang, H.B.; Wang, J.B.; Shan, L.M.; Li, R.S.; Xiao, X.H. Rhein protects against acetaminophen-induced hepatic and renal toxicity. Food Chem. Toxicol. 2011, 49, 1705–1710.
- Zhu, Y.; Jin, H.; Huo, X.; Meng, Q.; Wang, C.; Sun, P.; Ma, X.; Sun, H.; Dong, D.; Wu, J.; et al. Protective effect of Rhein against vancomycin-induced nephrotoxicity through regulating renal transporters and Nrf2 pathway. Phytother. Res. PTR 2022, 1–19.
- Sheng, X.; Wang, M.; Lu, M.; Xi, B.; Sheng, H.; Zang, Y.Q. Rhein ameliorates fatty liver disease through negative energy balance, hepatic lipogenic regulation, and immunomodulation in diet-induced obese mice. Am. J. Physiol. Endocrinol. Metab. 2011, 300, E886–E893.
- Nguyen, A.T.; Kim, K.Y. Rhein inhibits the growth of Propionibacterium acnes by blocking NADH dehydrogenase-2 activity. J. Med. Microbiol. 2020, 69, 689–696.
- Boileau, C.; Tat, S.K.; Pelletier, J.P.; Cheng, S.; Martel-Pelletier, J. Diacerein inhibits the synthesis of resorptive enzymes and reduces osteoclastic differentiation/survival in osteoarthritic subchondral bone: A possible mechanism for a protective effect against subchondral bone remodelling. Arthritis Res. Ther. 2008, 10, R71.
- Wang, G.; Li, Q.; Chen, D.; Wu, B.; Wu, Y.; Tong, W.; Huang, P. Kidney-targeted rhein-loaded liponanoparticles for diabetic nephropathy therapy via size control and enhancement of renal cellular uptake. Theranostics 2019, 9, 6191–6208.
- Guo, M.Z.; Li, X.S.; Shen, D.M.; Guan, X.Q.; Xu, H.R.; Gao, J. Effect of Rhein on the development of hepatic fibrosis in rats. Chin. J. Hepatol. 2003, 11, 26–29.
- Moldovan, F.; Pelletier, J.P.; Jolicoeur, F.C.; Cloutier, J.M.; Martel-Pelletier, J. Diacerhein and rhein reduce the ICE-induced IL-1beta and IL-18 activation in human osteoarthritic cartilage. Osteoarthr. Cartil. 2000, 8, 186–196.
- Heo, S.K.; Yun, H.J.; Park, W.H.; Park, S.D. Rhein inhibits TNF-alpha-induced human aortic smooth muscle cell proliferation via mitochondrial-dependent apoptosis. J. Vasc. Res. 2009, 46, 375–386.
- Du, H.; Shao, J.Q.; Gu, P.; Wang, J.; Liu, Z.H. Effect of early intervention with rhein on islet function in db/db mice. J. South. Med. Univ. 2011, 31, 1526–1529.
- Chen, Y.Y.; Chiang, S.Y.; Lin, J.G.; Ma, Y.S.; Liao, C.L.; Weng, S.W.; Lai, T.Y.; Chung, J.G. Emodin, aloe-emodin and rhein inhibit migration and invasion in human tongue cancer SCC-4 cells through the inhibition of gene expression of matrix metalloproteinase-9. Int. J. Oncol. 2010, 36, 1113–1120.
- Sun, H.; Luo, G.; Chen, D.; Xiang, Z. A Comprehensive and System Review for the Pharmacological Mechanism of Action of Rhein, an Active Anthraquinone Ingredient. Front. Pharmacol. 2016, 7, 247.
- Brown, W.V. Microvascular complications of diabetes mellitus: Renal protection accompanies cardiovascular protection. Am. J. Cardiol. 2008, 102, 10l–13l.
- Skyler, J.S. Microvascular complications. Retinopathy and nephropathy. Endocrinol. Metab. Clin. N. Am. 2001, 30, 833–856.
- Leon, C.A.; Raij, L. Interaction of haemodynamic and metabolic pathways in the genesis of diabetic nephropathy. J. Hypertens. 2005, 23, 1931–1937.
- Reidy, K.; Kang, H.M.; Hostetter, T.; Susztak, K. Molecular mechanisms of diabetic kidney disease. J. Clin. Investig. 2014, 124, 2333–2340.
- Radi, Z.A. Kidney Pathophysiology, Toxicology, and Drug-Induced Injury in Drug Development. Int. J. Toxicol. 2019, 38, 215–227.
- Radi, Z.A.; Stewart, Z.S.; O’Neil, S.P. Accidental and Programmed Cell Death in Investigative and Toxicologic Pathology. Curr. Protoc. Toxicol. 2018, 76, e51.
- Kiritoshi, S.; Nishikawa, T.; Sonoda, K.; Kukidome, D.; Senokuchi, T.; Matsuo, T.; Matsumura, T.; Tokunaga, H.; Brownlee, M.; Araki, E. Reactive oxygen species from mitochondria induce cyclooxygenase-2 gene expression in human mesangial cells: Potential role in diabetic nephropathy. Diabetes 2003, 52, 2570–2577.
- Du, H.; Shao, J.; Gu, P.; Lu, B.; Ye, X.; Liu, Z. Improvement of glucose tolerance by rhein with restored early-phase insulin secretion in db/db mice. J. Endocrinol. Investig. 2012, 35, 607–612.
- Liu, J.; Chen, Z.; Zhang, Y.; Zhang, M.; Zhu, X.; Fan, Y.; Shi, S.; Zen, K.; Liu, Z. Rhein protects pancreatic β-cells from dynamin-related protein-1-mediated mitochondrial fission and cell apoptosis under hyperglycemia. Diabetes 2013, 62, 3927–3935.
- Dai, C.S.L.Z.; Chen, H.P. Effects of rhein on inhibiting the progression of diabetic nephropathy in STZ-induced diabetic rats. J. Nephrol. Dial. Transpl. 1998, 8, 413–505.
- Jia, Z.H.; Liu, Z.H.; Zheng, J.M.; Zeng, C.H.; Li, L.S. Combined therapy of rhein and benazepril on the treatment of diabetic nephropathy in db/db mice. Exp. Clin. Endocrinol. Diabetes 2007, 115, 571–576.
- Zheng, J.M.; Zhu, J.M.; Li, L.S.; Liu, Z.H. Rhein reverses the diabetic phenotype of mesangial cells over-expressing the glucose transporter (GLUT1) by inhibiting the hexosamine pathway. Br. J. Pharmacol. 2008, 153, 1456–1464.
- Masson, E.; Lagarde, M.; Wiernsperger, N.; El Bawab, S. Hyperglycemia and glucosamine-induced mesangial cell cycle arrest and hypertrophy: Common or independent mechanisms? IUBMB Life 2006, 58, 381–388.
- Inoki, K.; Haneda, M.; Maeda, S.; Koya, D.; Kikkawa, R. TGF-beta 1 stimulates glucose uptake by enhancing GLUT1 expression in mesangial cells. Kidney Int. 1999, 55, 1704–1712.
- Okada, T.; Nakao, T.; Matsumoto, H.; Shino, T.; Nagaoka, Y.; Tomaru, R.; Wada, T. Association between markers of glycemic control, cardiovascular complications and survival in type 2 diabetic patients with end-stage renal disease. Int. Med. 2007, 46, 807–814.
- Li, J.; Wang, J.J.; Yu, Q.; Wang, M.; Zhang, S.X. Endoplasmic reticulum stress is implicated in retinal inflammation and diabetic retinopathy. FEBS Lett. 2009, 583, 1521–1527.
- Hotamisligil, G.S. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell 2010, 140, 900–917.
- Hu, C.; Cong, X.D.; Dai, D.Z.; Zhang, Y.; Zhang, G.L.; Dai, Y. Argirein alleviates diabetic nephropathy through attenuating NADPH oxidase, Cx43, and PERK in renal tissue. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2011, 383, 309–319.
- Hoste, E.A.; Lameire, N.H.; Vanholder, R.C.; Benoit, D.D.; Decruyenaere, J.M.; Colardyn, F.A. Acute renal failure in patients with sepsis in a surgical ICU: Predictive factors, incidence, comorbidity, and outcome. J. Am. Soc. Nephrol. JASN 2003, 14, 1022–1030.
- Lafrance, J.P.; Miller, D.R. Acute kidney injury associates with increased long-term mortality. J. Am. Soc. Nephrol. JASN 2010, 21, 345–352.
- Wald, R.; Shariff, S.Z.; Adhikari, N.K.; Bagshaw, S.M.; Burns, K.E.; Friedrich, J.O.; Garg, A.X.; Harel, Z.; Kitchlu, A.; Ray, J.G. The association between renal replacement therapy modality and long-term outcomes among critically ill adults with acute kidney injury: A retrospective cohort study. Crit. Care Med. 2014, 42, 868–877.
- Kinsey, G.R.; Li, L.; Okusa, M.D. Inflammation in acute kidney injury. Nephron. Exp. Nephrol. 2008, 109, e102–e107.
- Yu, C.; Qi, D.; Sun, J.F.; Li, P.; Fan, H.Y. Rhein prevents endotoxin-induced acute kidney injury by inhibiting NF-κB activities. Sci. Rep. 2015, 5, 11822.
- Liu, M.; Wang, L.; Wu, X.; Gao, K.; Wang, F.; Cui, J.; Zhao, J.; Peng, L.; Wang, J.; Jia, Y.; et al. Rhein protects 5/6 nephrectomized rat against renal injury by reducing inflammation via NF-κB signaling. Int. Urol. Nephrol. 2021, 53, 1473–1482.
- Refaie, M.M.; Amin, E.F.; El-Tahawy, N.F.; Abdelrahman, A.M. Possible Protective Effect of Diacerein on Doxorubicin-Induced Nephrotoxicity in Rats. J. Toxicol. 2016, 2016, 9507563.
- Fattah, H.; Vallon, V. Tubular Recovery after Acute Kidney Injury. Nephron 2018, 140, 140–143.
- Falke, L.L.; Gholizadeh, S.; Goldschmeding, R.; Kok, R.J.; Nguyen, T.Q. Diverse origins of the myofibroblast—implications for kidney fibrosis. Nat. Rev. Nephrol. 2015, 11, 233–244.
- Tzavlaki, K.; Moustakas, A. TGF-β Signaling. Biomolecules 2020, 10, 487.
- Frangogiannis, N. Transforming growth factor-β in tissue fibrosis. J. Exp. Med. 2020, 217, e20190103.
- Edeling, M.; Ragi, G.; Huang, S.; Pavenstädt, H.; Susztak, K. Developmental signalling pathways in renal fibrosis: The roles of Notch, Wnt and Hedgehog. Nat. Rev. Nephrol. 2016, 12, 426–439.
- Meng, X.M.; Chung, A.C.; Lan, H.Y. Role of the TGF-β/BMP-7/Smad pathways in renal diseases. Clin. Sci. 2013, 124, 243–254.
- Guan, Y.; Wu, X.X.; Duan, J.L.; Yin, Y.; Guo, C.; Wei, G.; Wang, Y.H.; Zhu, Y.R.; Weng, Y.; Xi, M.M.; et al. Effects and Mechanism of Combination of Rhein and Danshensu in the Treatment of Chronic Kidney Disease. Am. J. Chin. Med. 2015, 43, 1381–1400.
- Su, J.; Yin, L.P.; Zhang, X.; Li, B.B.; Liu, L.; Li, H. Chronic allograft nephropathy in rats is improved by the intervention of rhein. Transplant. Proc. 2013, 45, 2546–2552.
- Kim, S.I.; Kwak, J.H.; Zachariah, M.; He, Y.; Wang, L.; Choi, M.E. TGF-beta-activated kinase 1 and TAK1-binding protein 1 cooperate to mediate TGF-beta1-induced MKK3-p38 MAPK activation and stimulation of type I collagen. Am. J. Physiol. Ren. Physiol. 2007, 292, F1471–F1478.
- Zeng, X.; Cai, G.; Liang, T.; Li, Q.; Yang, Y.; Zhong, X.; Zou, X.; Qin, M.; Mi, Z. Rhubarb and Astragalus Capsule Attenuates Renal Interstitial Fibrosis in Rats with Unilateral Ureteral Obstruction by Alleviating Apoptosis through Regulating Transforming Growth Factor beta1 (TGF-β1)/p38 Mitogen-Activated Protein Kinases (p38 MAPK) Pathway. Med. Sci. Monit. 2020, 26, e920720.
- Liu, T.; Meng, Q.; Wang, C.; Liu, Q.; Guo, X.; Sun, H.; Peng, J.; Ma, X.; Kaku, T.; Liu, K. Changes in expression of renal Oat1, Oat3 and Mrp2 in cisplatin-induced acute renal failure after treatment of JBP485 in rats. Toxicol. Appl. Pharmacol. 2012, 264, 423–430.
- Feng, S.X.; Li, J.S.; Qu, L.B.; Shi, Y.M.; Zhao, D. Comparative pharmacokinetics of five rhubarb anthraquinones in normal and thrombotic focal cerebral ischemia-induced rats. Phytother. Res. PTR 2013, 27, 1489–1494.
- Hu, Y.F.; Huang, W.Y.; Li, Y.Q.; Luo, Y.; Jiang, Q.; Liang, Y.S.; Zhu, Z.W.; Wang, P.; Meng, X.L. Mechanism of Rhein on Renal Toxicity of Mice. Chin. J. Exp. Tradit. Med. Formulae 2019, 25, 54–59.
- Da, H.Y.; Jiang, Z.Z.; Wang, C.F.; Zhang, L.Y.; Liu, G.Q. The toxic effects of rhein and emodin on human renal tubular epithelial cells in vitro. Chin. Tradit. Herb. Drug 2009, 40, 102–105.
- Faitova, J.; Krekac, D.; Hrstka, R.; Vojtesek, B. Endoplasmic reticulum stress and apoptosis. Cell. Mol. Biol. Lett. 2006, 11, 488–505.
- Yang, J.P.; Sun, H.; Wang, D.D.; Mao, Y.Y.F. MAPK Signal Transduction Pathway involves in Rhein-induced Apoptosis in HK-2 Cells. Chi. J. Exp. Tradit. Med. Formulae 2015, 21, 147–151.
- Sun, H.; Yang, J.P.; Mao, Y.; Wang, D.D.; Yu, F. Involvement of Fas-dependent pathway in rhein-induced apoptosis of HK- 2 cells. J. China Pharm. Univ. 2015, 46, 469–475.
- Mao, Y.; Zhang, M.; Yang, J.; Sun, H.; Wang, D.; Zhang, X.; Yu, F.; Li, J. The UCP2-related mitochondrial pathway participates in rhein-induced apoptosis in HK-2 cells. Toxicol. Res. 2017, 6, 297–304.
- Lai, Y. Effects of Compatibility of Rhubarb and Huangqi on the Toxicity and Oxidative Stress of Rhubarb On HK-2 Cells. Ph.D. Thesis, Guangzhou University of Chinese Medicine, Guangzhou, China, May 2018.
