The rising incidence and severity of malignant tumors threaten human life and health, and the current lagged diagnosis and single treatment in clinical practice are inadequate for tumor management. Gold nanoclusters (AuNCs) are nanomaterials with small dimensions (≤3 nm) and few atoms exhibiting unique optoelectronic and physicochemical characteristics, such as fluorescence, photothermal effects, radiosensitization, and biocompatibility.
- gold nanoclusters
- cancer diagnosis
- combination therapy
1. Introduction
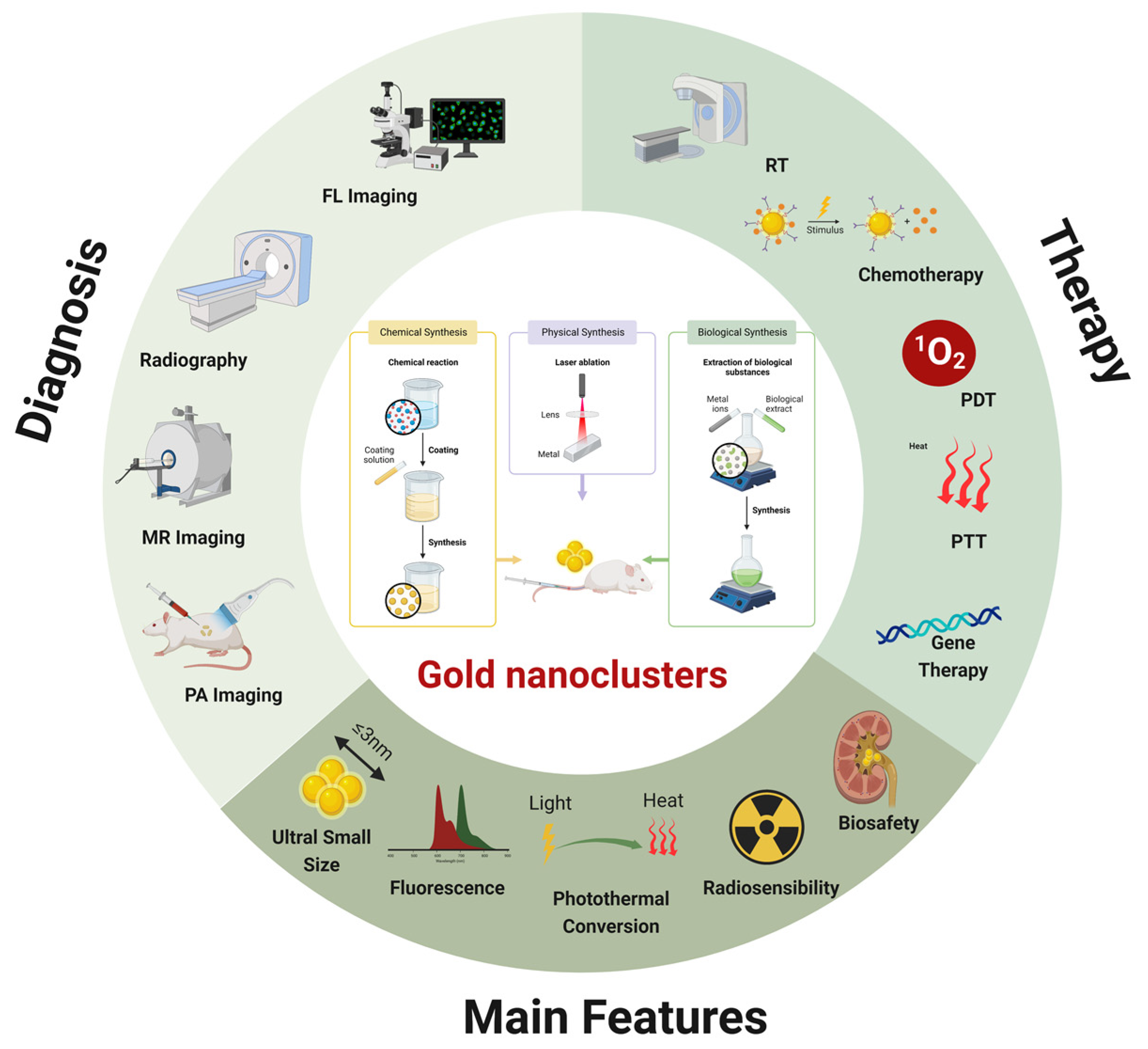
2. AuNCs as Imaging Agents in Tumor Theranostic
|
Multifunctional Nanoplatform |
Role of AuNCs |
Therapeutic Agent |
Size (nm) |
Imaging Mode |
Cancer Types |
Therapy Method |
Activity |
Ref |
|---|---|---|---|---|---|---|---|---|
|
AuNCs-Ag@Keratin-Gd |
Imaging |
NM |
5 |
FL, MRI |
Breast cancer |
Chemotherapy |
In vivo and in vitro |
[37] |
|
CDGM NPs |
Imaging, drug delivery |
CAD, Ce6 |
2 |
FL |
Lung cancer |
PDT |
In vivo and in vitro |
|
|
AuS-U11 |
PTT-carrier |
U11 peptide, cyanine dye Cy5.5, 5-ALA |
10 |
FL |
Pancreatic carcinoma |
PTT, PDT |
In vivo and in vitro |
|
|
Au NBPs@PDA/AuNCs |
Imaging |
Au NBPs@PDA |
2.1, 3.3 |
FL |
Breast cancer, hepatocarcinoma |
PTT |
In vitro |
|
|
Dox@HG-CAHs |
Imaging |
HA-ALD, Dox |
2.8 |
FL, CT |
Osteosarcoma |
PTT, chemotherapy |
In vivo and in vitro |
|
|
AuNCs–LHRHa |
Imaging, PTT |
LHRH analogues |
2.4 |
FL, CT |
Prostatic cancer |
PTT |
In vitro |
|
|
GTSL-CYC-HER2 |
Changed the zeta potential of liposomes, superior photothermal effect |
HER2-modified thermosensitive liposome, cyclopamine |
NA |
CT, PTI |
Breast cancer |
Chemotherapy, PTT |
In vivo and in vitro |
|
|
Ce6&AuNCs/Gd-LDH |
Imaging |
Ce6 |
~2 |
MRI, FL |
Hepatocarcinoma |
PDT |
In vivo and in vitro |
|
|
AuNCs-ICG |
Imaging, radiosensitizing effects |
ICG |
~1 |
FL, PAI, CT |
Breast cancer |
PDT, RT |
In vivo and in vitro |
|
|
Qu-GNCs |
Imaging |
Qu |
1–3 |
FL |
Lung cancer |
Chemotherapy |
In vitro |
|
|
Fe3O4@PAA/AuNCs/ZIF-8 NPs |
Imaging |
DOX |
NA |
MRI, CT, FL |
Hepatocarcinoma |
Chemotherapy |
In vivo and in vitro |
|
|
AuNCs@GTMS-FA |
Imaging, phototherapeutic agents |
FA |
2.8 |
FL |
Breast cancer |
PTT, PDT |
In vitro |
|
|
AuNCs/Dzs-Dox |
NSET effect, shelter therapeutic cargos |
Dzs-Dox |
~1.76 |
FL |
Breast cancer |
Gene therapy, chemotherapy |
In vivo and in vitro |
|
|
HG-GNCs/GO-5FU |
Bioimaging, phototherapeutic |
HA, 5FU |
2 |
FL |
Lung cancer, breast cancer |
Chemotherapy, PDT, PTT |
In vitro |
|
|
AuNCs@mSiO2@MnO2 |
Photosensitizer |
MnO2 nanozyme |
NA |
MRI |
Breast cancer |
PDT |
In vivo and in vitro |
|
|
Au8NC |
Radiosensitizing effects |
Levonorgestrel |
~2 |
FL |
Esophagus cancer |
RT |
In vivo and in vitro |
|
|
Au4-IO NP-cRGD |
Imaging, radiosensitizing effects |
IO nanocluster |
2 |
FL, MRI |
Breast cancer |
RT, chemotherapy |
In vivo and in vitro |
|
|
PML-MF nanocarrier |
Imaging |
IO@AuNPs |
NA |
FL |
Cervical cancer |
PPTT, chemotherapy |
In vitro |
|
|
WLPD-Au25 |
Photosensitizer, drug delivery |
WS2 nanoparticles, Dex, Captopril |
2.5 |
CT |
Breast cancer |
PTT, PDT |
In vivo |
|
|
AuNCs/Cas9–gRNA |
Imaging, drug delivery |
Cas9–sgRNA plasmid |
~1.56 |
FL |
Osteosarcoma |
Gene therapy |
In vitro |
|
|
K-AuNCs |
Imaging, drug delivery |
K |
1–3 |
FL |
Lung cancer |
Chemotherapy |
In vitro |
|
|
EA-AB |
Imaging |
EB |
NM |
FL, MSOT Imaging |
Breast cancer |
Chemotherapy, PTT |
In vivo and in vitro |
|
|
Ce6-GNCs-Ab-CIK |
Drug delivery |
Ce6, CD3 antibody |
NA |
FL |
Gastric cancer |
Chemotherapy, PDT |
In vivo and in vitro |
|
|
Au4Cu4/Au25@Lip |
Photothermogenesis effect, photoluminescence performance |
Au4Cu4 nanoclusters |
~2 |
FL, PTI |
Cervical cancer |
PTT, PDT |
In vivo and in vitro |
|
|
MB-loaded Au NC-mucin NPs |
Imaging |
MB |
1.9 ± 0.34 |
FL |
Cervical cancer |
PDT |
In vitro |
|
|
ISQ@BSA-AuNC@AuNR@DAC@DR5 |
SERS substrate |
DAC, ISQ |
NA |
NM |
Amelanotic Melanoma |
PTT, PDT |
In vivo and in vitro |
Abs: PTT: photothermal therapy; NSET: nanosurface energy transfer; CAD: MMP2 polypeptidecis-aconitic anhydride-modified doxorubicin; Ce6: photosensitizer chlorin e6; Au NBPs@PDA: polydopamine-capped gold nanobipyramids; HA-ALD: oxidized hyaluronic acid; Dox: doxorubicin; Ce6: chlorin e6; ICG: indocyanine green; K: Kaempferol; Qu: Quercetin; EB: Erlotinib; HA: hyaluronic acid; 5FU: 5-fluorouracil; FA: Folic acid; MB: Methylene blue; DAC: Dacarbazine; Dzs-Dox: DNAzyme, Dox; IO: Iron oxide; Dex: Dexamethasone; FL: fluorescence imaging; MRI: magnetic resonance imaging; CT: computed tomography; PT: photothermal imaging; PAI: photoacoustic imaging, MSOT: multispectral optoacoustic tomography; PDT: photodynamic therapy; RT: radiation therapy; PPTT: plasmonic photothermal therapy; NM: not mentioned; NA: not applicable.
3. AuNCs as Transport Agents in Combined Therapy
AuNCs are widely used for drug delivery and controlled release in vivo and ex vivo as one of the metallic nanomaterials with the longest research history. As one of the special ultra-small size nanostructures, AuNCs have a greater potential for combinatorial applications [73][66]. Initially, AuNCs have a stable and inert internal core that can shield encapsulated drug molecules. Further, AuNCs have a high surface to volume ratio and can be loaded with a substantial quantity of small-molecule drugs via reasonable surface modification [74][49]. In addition, AuNCs can be targeted for in vivo tumor transport via passive accumulation (e.g., enhanced permeability and retention effect) or active targeting (e.g., modified target molecules), thereby enhancing the bioavailability of drugs [75,76][50][67]. Moreover, the ultra-small nanostructures enable precise targeting of subcellular organelle structures, such as the nucleus and mitochondria, supplement selection, and therapeutic strategies. In combination with the unique optoelectronic and chemical properties of AuNCs, they can achieve controlled and precise strikes against the internal environmental response of tumors and external signal stimuli, consequently reducing the toxic side effects that accompany chemotherapy. The covalent modification of the AuNP surface generally adopts sodium borohydride reduction and ligand replacement methods, and the non-covalent binding mainly includes electrostatic interaction and hydrophobic interaction to adsorb the surrounding molecules, thus reducing the surface free energy. Jiang et al. prepared AuNCs loaded with adriamycin by a “green chemistry” approach using green tea extract, in which adriamycin was co-polymerized with the nanoclusters by π-π superposition and electrostatic interactions. The drug delivery system has good stability and can significantly inhibit tumors through the synergetic effect of photothermal therapy and chemotherapy [77][68]. The Au-Cys-MTX/DOX NCs system constructed by Wu et al. is more stable than the non-covalent drug delivery system and can choose different drug release mechanisms according to the microenvironment of different tumor tissues. And due to the solidity of the amide bond, only a small amount of drug can be released from Au-Cys-MTX/DOX NCs in the absence of protease [78][69].4. AuNCs as Therapeutic Agents in Combined Therapy
4.1. Radiosensitization
Radiation therapy is an effective oncology treatment that uses ionizing radiation to target cancer cells. A part of the high-energy radiation will nonetheless be transferred to the normal tissues around the tumor, inflicting irreparable damage [101,102][70][71]. Gold has a high atomic number and much greater electron density than soft tissues, which may boost photoelectric absorption and secondary electron yield, improve local energy deposition in tumor tissues, and expedite the death of tumor cells [103,104][72][73]. In addition to the previously reported physical methods, gold clusters may potentially exert their radiosensitizing effects via biological pathways, for example, controlling the cell cycle, boosting free radical generation in response to radiation, altering cell autophagy, and causing apoptosis [105,106][55][74]. Using nanogold for the first time for in vivo tumor radiation sensitization, Herold et al. demonstrated that gold particles might have a dose-enhancing impact on cells and C.B17/Icr scid mice cultured with EMT-6 mouse tumor cells when exposed to 200 kVp X-rays both in vitro and in vivo tests [107][75]. The enhanced permeability and retention (EPR) effect may enhance the aggregation of AuNCs in the tumor, since smaller nanoclusters (<5 nm) can more easily penetrate tumor tissues and cross blood vessels than larger nanoparticles (>10 nm). Additionally, the tumor tissue’s decreased lymphatic outflow makes it difficult for nanoparticles to be effectively cleared away, which increases their retention in the tumor tissue [108[76][77],109], hence improving the radiation treatment impact and reducing the harm to the surrounding normal cells. Attaching trastuzumab and folic acid targeting human epidermal growth factor receptor 2 (HER2) to 4.2 nm AuNCs, Roghayeh et al. demonstrated that the targeted AuNCs may infiltrate breast cancer SK-BR3 cells through HER-2-mediated mechanisms [110][78]. Luo et al. created therapeutic AuNCs that can function as prostate cancer (PCa)-targeted radiosensitizers and chemotherapy carriers. Using PSMA-MMAE as a template and the reduction of Au3+ by the reactive group, PSMA-AuNC-MMAE couples were synthesized. The gold nanocluster-attached prostate-specific membrane antigen (PSMA) could improve the targeting of AuNCs; the bound monomethyl auristatin E (MMAE) was a chemotherapeutic prodrug that enhanced the chemotherapeutic effect after binding with AuNC, and MMAE could also boost the radiosensitizing impact by inhibiting the cells in the G2-M area. Due to PSMA receptor amplification, PC3pip tumor cells maintained considerably more gold nanocluster complexes in PC3pip tumor-bearing animals than in PC3flu tumor-bearing mice, as shown by in vivo tests [111][79]. Wu et al. created transformable gold nanocluster (AuNC) aggregates (called AuNC-ASON) using antisense oligonucleotides (ASON) that target survivin mRNA. The acidic tumor microenvironment modifies the electrostatic interactions between the polyelectrolyte poly(allylamine) (PAH) and glutathione surface ligands that stabilize AuNC, causing gold nanocluster aggregates to separate into 2 nm AuNCs and triggering the release of loaded antisense oligonucleotides for gene silencing. The findings of in vivo tests analyzing the co-localization of AuNC-ASON with nucleosomes/lysosomes revealed that AuNC-ASON has an excellent nucleosome/lysosome escape capacity. Moreover, real-time polymerase chain reaction (real-time PCR) research revealed that the expression of survivin mRNA in 4T1 cells followed the same pattern as cell viability, validating the mechanism of tumor cell eradication based on survivin gene silencing. With the aid of survivin gene interference, this treatment approach may increase and enhance the radiosensitivity of cancer cells and enable the simultaneous use of tumor radiation and gene therapy [112][80]. The effectiveness of radiotherapy is contingent upon radiosensitivity, and a hypoxic tumor microenvironment renders tumor cells more resistant to ionizing radiation. As radiosensitizers, AuNCs may be used with oxygen carriers to reduce tumor hypoxia by generating reactive oxygen species (ROS) generation and enhancing the effectiveness of radiation. In the cRGD multifunctional treatment system, Au4-IO NP-cRGD triggered the death of 4T1 cells by producing substantial quantities of reactive oxygen species in response to X-ray exposure. Experiments in vivo have shown that this multifunctional treatment platform is capable of directing Fenton response-assisted improved radiotherapy using dual-mode imaging based on magnetic resonance imaging of iron oxide (IO) nanoclusters and fluorescence imaging of Au4 clusters [113][56]. Due to the combination of physical, chemical, and biological factors, radiosensitization is a complicated phenomenon [114][81]. The processes by which AuNCs exhibit radiosensitizing effects, particularly the biological pathways involved, are not well understood. In addition, the efficacy of the functionalized modification of AuNCs targeted to tumor tissues when administered in vivo, as well as the harm to healthy tissues and non-specific accumulation of long-term damage to persons, need more research. In the meantime, the increase in the size of AuNCs after different surface modifications reduces the clearance rate in the organism and increases the accumulation in the liver. However, it has not been conclusively determined whether there is an influence on the gene expression of individuals. In fact, tumor tissues grow at inconsistent rates in all directions with irregular edges. Whether AuNCs can be conformally distributed according to the different shapes of tumors needs to be further investigated. In conclusion, more animal experiments and preclinical trials are needed for the practical translation of AuNCs to ensure that the multifunctional system of AuNCs can be efficiently and safely applied in the clinic.4.2. Photothermal Conversion
As was mentioned earlier, the excellent photothermal conversion efficiency of AuNCs allows them to be used as ideal photothermal agents for multimodal imaging and therapeutic implementation. Therefore, examples of combined treatment based on the photothermal action of AuNCs will not be repeated in this section. Focusing on the optimization aspect of the photothermal effect of AuNCs, recently, Yin’s group developed AuNCs as highly efficient photothermal treatment agents and provided a semiquantitative technique for determining their resonant frequency and absorption efficiency by integrating practical medium approximation theory with full-wave electrodynamic simulations. Guided by this theory, they created a space-confined seeded growth approach to prepare AuNCs. Under optimum growth circumstances, they obtained a record photothermal conversion efficiency of 84% for gold-based nanoclusters, due to collective plasmon-coupling-induced near-unity absorption efficiency. They showed the improved exceptional photothermal treatment performance of AuNCs in vivo. Their study shows the potential and effectiveness of AuNCs as nanoscale photothermal treatment agents [115][82]. Lately, a nanoarchitecture comprising a conjugated gold nanorod (AuNR) and gold cluster hybrid system was developed to optimize the photothermal conversion efficiency. Due to the target specificity of folate receptors for cancer cells, the hybrid material exhibited high in vitro therapeutic efficacy after folic acid conjugation. More importantly, the nanoarchitecture of the hybrid material had no significant influence on the optical and thermal properties of either AuNCs or AuNRs, but exerted enhanced photothermal effects [116][83].References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424.
- Chen, X.; Zhu, H.; Huang, X.; Wang, P.; Zhang, F.; Li, W.; Chen, G.; Chen, B. Novel iodinated gold nanoclusters for precise diagnosis of thyroid cancer. Nanoscale 2017, 9, 2219–2231.
- Thambi, T.; Park, J.H.; Lee, D.S. Stimuli-responsive polymersomes for cancer therapy. Biomater. Sci. 2016, 4, 55–69.
- Akgonullu, S.; Yavuz, H.; Denizli, A. SPR nanosensor based on molecularly imprinted polymer film with gold nanoparticles for sensitive detection of aflatoxin B1. Talanta 2020, 219, 121219.
- Hong, L.; Lu, M.; Dinel, M.P.; Blain, P.; Peng, W.; Gu, H.; Masson, J.F. Hybridization conditions of oligonucleotide-capped gold nanoparticles for SPR sensing of microRNA. Biosens. Bioelectron. 2018, 109, 230–236.
- Chakraborty, A.; Das, A.; Raha, S.; Barui, A. Size-dependent apoptotic activity of gold nanoparticles on osteosarcoma cells correlated with SERS signal. J. Photochem. Photobiol. B Biol. 2020, 203, 111778.
- Chen, Z.; Lu, S.; Zhang, Z.; Huang, X.; Zhao, H.; Wei, J.; Li, F.; Yuan, K.; Su, L.; Xiong, Y. Green photoreduction synthesis of dispersible gold nanoparticles and their direct in situ assembling in multidimensional substrates for SERS detection. Mikrochim. Acta 2022, 189, 275.
- Broekgaarden, M.; Bulin, A.L.; Porret, E.; Musnier, B.; Chovelon, B.; Ravelet, C.; Sancey, L.; Elleaume, H.; Hainaut, P.; Coll, J.L.; et al. Surface functionalization of gold nanoclusters with arginine: A trade-off between microtumor uptake and radiotherapy enhancement. Nanoscale 2020, 12, 6959–6963.
- Luo, P.; Zheng, Y.; Qin, Z.; Li, C.; Jiang, H.; Wang, X. Fluorescence light up detection of aluminium ion and imaging in live cells based on the aggregation-induced emission enhancement of thiolated gold nanoclusters. Talanta 2019, 204, 548–554.
- Zhao, Y.; Zhou, H.; Zhang, S.; Xu, J. The synthesis of metal nanoclusters and their applications in bio-sensing and imaging. Methods Appl. Fluoresc. 2019, 8, 012001.
- Liu, H.; Zhu, N.; Li, M.; Huang, X.; Wu, P.; Hu, Z.; Shuai, J. Induced fluorescent enhancement of protein-directed synthesized gold nanoclusters for selective and sensitive detection of flame retardants. Sci. Total Environ. 2020, 713, 136488.
- Guo, T.; Li, W.; Qian, L.; Yan, X.; Cui, D.; Zhao, J.; Ni, H.; Zhao, X.; Zhang, Z.; Li, X.; et al. Highly-selective detection of EGFR mutation gene in lung cancer based on surface enhanced Raman spectroscopy and asymmetric PCR. J. Pharm. Biomed. Anal. 2020, 190, 113522.
- Nonappa, N. Luminescent gold nanoclusters for bioimaging applications. Beilstein. J. Nanotechnol. 2020, 11, 533–546.
- Zheng, B.; Wu, Q.; Jiang, Y.; Hou, M.; Zhang, P.; Liu, M.; Zhang, L.; Li, B.; Zhang, C. One-pot synthesis of (68)Ga-doped ultrasmall gold nanoclusters for PET/CT imaging of tumors. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 128, 112291.
- Liang, G.; Jin, X.; Zhang, S.; Xing, D. RGD peptide-modified fluorescent gold nanoclusters as highly efficient tumor-targeted radiotherapy sensitizers. Biomaterials 2017, 144, 95–104.
- Sha, Q.; Guan, R.; Su, H.; Zhang, L.; Liu, B.F.; Hu, Z.; Liu, X. Carbohydrate-protein template synthesized high mannose loading gold nanoclusters: A powerful fluorescence probe for sensitive Concanavalin A detection and specific breast cancer cell imaging. Talanta 2020, 218, 121130.
- Cui, H.; Shao, Z.S.; Song, Z.; Wang, Y.B.; Wang, H.S. Development of gold nanoclusters: From preparation to applications in the field of biomedicine. J. Mater. Chem. C 2020, 8, 14312–14333.
- Zhang, X.D.; Chen, J.; Luo, Z.; Wu, D.; Shen, X.; Song, S.S.; Sun, Y.M.; Liu, P.X.; Zhao, J.; Huo, S.; et al. Enhanced tumor accumulation of sub-2 nm gold nanoclusters for cancer radiation therapy. Adv. Healthc. Mater. 2014, 3, 133–141.
- Ramesh, B.S.; Giorgakis, E.; Lopez-Davila, V.; Dashtarzheneha, A.K.; Loizidou, M. Detection of cell surface calreticulin as a potential cancer biomarker using near-infrared emitting gold nanoclusters. Nanotechnology 2016, 27, 285101.
- Wu, H.; Qiao, J.; Hwang, Y.H.; Xu, C.; Yu, T.; Zhang, R.; Cai, H.; Kim, D.P.; Qi, L. Synthesis of ficin-protected AuNCs in a droplet-based microreactor for sensing serum ferric ions. Talanta 2019, 200, 547–552.
- Purohit, R.; Singh, S. Fluorescent gold nanoclusters for efficient cancer cell targeting. Int. J. Nanomed. 2018, 13, 15–17.
- El-Sayed, N.; Schneider, M. Advances in biomedical and pharmaceutical applications of protein-stabilized gold nanoclusters. J. Mater. Chem. B 2020, 8, 8952–8971.
- Liu, J.M.; Chen, J.T.; Yan, X.P. Near infrared fluorescent trypsin stabilized gold nanoclusters as surface plasmon enhanced energy transfer biosensor and in vivo cancer imaging bioprobe. Anal. Chem. 2013, 85, 3238–3245.
- Han, L.; Xia, J.M.; Hai, X.; Shu, Y.; Chen, X.W.; Wang, J.H. Protein-Stabilized Gadolinium Oxide-Gold Nanoclusters Hybrid for Multimodal Imaging and Drug Delivery. ACS Appl. Mater. Interfaces 2017, 9, 6941–6949.
- Hada, A.M.; Craciun, A.M.; Focsan, M.; Borlan, R.; Soritau, O.; Todea, M.; Astilean, S. Folic acid functionalized gold nanoclusters for enabling targeted fluorescence imaging of human ovarian cancer cells. Talanta 2021, 225, 121960.
- Pan, Y.; Li, Q.; Zhou, Q.; Zhang, W.; Yue, P.; Xu, C.; Qin, X.; Yu, H.; Zhu, M. Cancer cell specific fluorescent methionine protected gold nanoclusters for in-vitro cell imaging studies. Talanta 2018, 188, 259–265.
- Li, H.; Cheng, Y.; Liu, Y.; Chen, B. Fabrication of folic acid-sensitive gold nanoclusters for turn-on fluorescent imaging of overexpression of folate receptor in tumor cells. Talanta 2016, 158, 118–124.
- Xie, J.; Liang, R.; Li, Q.; Wang, K.; Hussain, M.; Dong, L.; Shen, C.; Li, H.; Shen, G.; Zhu, J.; et al. Photosensitizer-loaded gold nanocages for immunogenic phototherapy of aggressive melanoma. Acta Biomater. 2022, 142, 264–273.
- Peng, L.H.; Niu, J.; Zhang, C.Z.; Yu, W.; Wu, J.H.; Shan, Y.H.; Wang, X.R.; Shen, Y.Q.; Mao, Z.W.; Liang, W.Q.; et al. TAT conjugated cationic noble metal nanoparticles for gene delivery to epidermal stem cells. Biomaterials 2014, 35, 5605–5618.
- Lei, Y.; Tang, L.; Xie, Y.; Xianyu, Y.; Zhang, L.; Wang, P.; Hamada, Y.; Jiang, K.; Zheng, W.; Jiang, X. Gold nanoclusters-assisted delivery of NGF siRNA for effective treatment of pancreatic cancer. Nat. Commun. 2017, 8, 15130.
- Liu, R.; Xiao, W.; Hu, C.; Xie, R.; Gao, H. Theranostic size-reducible and no donor conjugated gold nanocluster fabricated hyaluronic acid nanoparticle with optimal size for combinational treatment of breast cancer and lung metastasis. J. Control. Release 2018, 278, 127–139.
- Liu, R.; Hu, C.; Yang, Y.; Zhang, J.; Gao, H. Theranostic nanoparticles with tumor-specific enzyme-triggered size reduction and drug release to perform photothermal therapy for breast cancer treatment. Acta Pharm. Sin. B 2019, 9, 410–420.
- Hong, G.; Zou, Z.; Huang, Z.; Deng, H.; Chen, W.; Peng, H. Split-type electrochemiluminescent gene assay platform based on gold nanocluster probe for human papillomavirus diagnosis. Biosens. Bioelectron. 2021, 178, 113044.
- Peng, H.P.; Jian, M.L.; Huang, Z.N.; Wang, W.J.; Deng, H.H.; Wu, W.H.; Liu, A.L.; Xia, X.H.; Chen, W. Facile electrochemiluminescence sensing platform based on high-quantum-yield gold nanocluster probe for ultrasensitive glutathione detection. Biosens. Bioelectron. 2018, 105, 71–76.
- Yu, Q.; Gao, P.; Zhang, K.Y.; Tong, X.; Yang, H.; Liu, S.; Du, J.; Zhao, Q.; Huang, W. Luminescent gold nanocluster-based sensing platform for accurate H2S detection in vitro and in vivo with improved anti-interference. Light Sci. Appl. 2017, 6, e17107.
- Yang, Y.; Xing, X.; Zou, T.; Wang, Z.; Zhao, R.; Hong, P.; Peng, S.; Zhang, X.; Wang, Y. A novel and sensitive ratiometric fluorescence assay for carbendazim based on N-doped carbon quantum dots and gold nanocluster nanohybrid. J. Hazard Mater. 2020, 386, 121958.
- Li, Y.; Cao, Y.; Wei, L.; Wang, J.; Zhang, M.; Yang, X.; Wang, W.; Yang, G. The assembly of protein-templated gold nanoclusters for enhanced fluorescence emission and multifunctional applications. Acta Biomater. 2020, 101, 436–443.
- Matus, M.F.; Hakkinen, H. Atomically Precise Gold Nanoclusters: Towards an Optimal Biocompatible System from a Theoretical-Experimental Strategy. Small 2021, 17, e2005499.
- Pigliacelli, C.; Acocella, A.; Diez, I.; Moretti, L.; Dichiarante, V.; Demitri, N.; Jiang, H.; Maiuri, M.; Ras, R.H.A.; Bombelli, F.B.; et al. High-resolution crystal structure of a 20 kDa superfluorinated gold nanocluster. Nat. Commun. 2022, 13, 2607.
- Linko, V.; Zhang, H.; Nonappa; Kostiainen, M.A.; Ikkala, O. From Precision Colloidal Hybrid Materials to Advanced Functional Assemblies. Acc. Chem. Res. 2022, 55, 1785–1795.
- Xia, F.; Hou, W.; Zhang, C.; Zhi, X.; Cheng, J.; de la Fuente, J.M.; Song, J.; Cui, D. pH-responsive gold nanoclusters-based nanoprobes for lung cancer targeted near-infrared fluorescence imaging and chemo-photodynamic therapy. Acta Biomater. 2018, 68, 308–319.
- Li, H.; Wang, P.; Deng, Y.; Zeng, M.; Tang, Y.; Zhu, W.H.; Cheng, Y. Combination of active targeting, enzyme-triggered release and fluorescent dye into gold nanoclusters for endomicroscopy-guided photothermal/photodynamic therapy to pancreatic ductal adenocarcinoma. Biomaterials 2017, 139, 30–38.
- Wang, J.; Gao, Y.; Liu, P.; Xu, S.; Luo, X. Core-Shell Multifunctional Nanomaterial-Based All-in-One Nanoplatform for Simultaneous Multilayer Imaging of Dual Types of Tumor Biomarkers and Photothermal Therapy. Anal. Chem. 2020, 92, 15169–15178.
- Yang, Z.; Li, Z.; Zhao, Y.; Zhao, Y.; Li, X.; He, L.; Zvyagin, A.V.; Yang, B.; Lin, Q.; Ma, X. Lotus Seedpod-Inspired Crosslinking-Assembled Hydrogels Based on Gold Nanoclusters for Synergistic Osteosarcoma Multimode Imaging and Therapy. ACS Appl. Mater. Interfaces 2022, 14, 34377–34387.
- Wang, Z.; He, L.; Che, S.; Xing, H.; Guan, L.; Yang, Z.; Li, X.; Zvyagin, A.V.; Lin, Q.; Qu, W. AuNCs-LHRHa nano-system for FL/CT dual-mode imaging and photothermal therapy of targeted prostate cancer. J. Mater. Chem. B 2022, 10, 5182–5190.
- Li, Y.; Song, W.; Hu, Y.; Xia, Y.; Li, Z.; Lu, Y.; Shen, Y. “Petal-like” size-tunable gold wrapped immunoliposome to enhance tumor deep penetration for multimodal guided two-step strategy. J. Nanobiotechnol. 2021, 19, 293.
- Mei, X.; Wang, W.; Yan, L.; Hu, T.; Liang, R.; Yan, D.; Wei, M.; Evans, D.G.; Duan, X. Hydrotalcite monolayer toward high performance synergistic dual-modal imaging and cancer therapy. Biomaterials 2018, 165, 14–24.
- Dan, Q.; Hu, D.; Ge, Y.; Zhang, S.; Li, S.; Gao, D.; Luo, W.; Ma, T.; Liu, X.; Zheng, H.; et al. Ultrasmall theranostic nanozymes to modulate tumor hypoxia for augmenting photodynamic therapy and radiotherapy. Biomater. Sci. 2020, 8, 973–987.
- Lakshmi, B.A.; Kim, S. Quercetin mediated gold nanoclusters explored as a dual functional nanomaterial in anticancer and bio-imaging disciplines. Colloids Surf. B Biointerfaces 2019, 178, 230–237.
- Bian, R.; Wang, T.; Zhang, L.; Li, L.; Wang, C. A combination of tri-modal cancer imaging and in vivo drug delivery by metal-organic framework based composite nanoparticles. Biomater. Sci. 2015, 3, 1270–1278.
- Ovais, M.; Mukherjee, S.; Pramanik, A.; Das, D.; Mukherjee, A.; Raza, A.; Chen, C. Designing Stimuli-Responsive Upconversion Nanoparticles that Exploit the Tumor Microenvironment. Adv. Mater. 2020, 32, e2000055.
- Sun, H.; Ma, W.; Duan, S.; Huang, J.; Jia, R.; Cheng, H.; Chen, B.; He, X.; Wang, K. An endogenous stimulus detonated nanocluster-bomb for contrast-enhanced cancer imaging and combination therapy. Chem. Sci. 2021, 12, 12118–12129.
- Yang, Y.; Wang, S.; Wang, C.; Tian, C.; Shen, Y.; Zhu, M. Engineered Targeted Hyaluronic Acid-Glutathione-Stabilized Gold Nanoclusters/Graphene Oxide-5-Fluorouracil as a Smart Theranostic Platform for Stimulus-Controlled Fluorescence Imaging-Assisted Synergetic Chemo/Phototherapy. Chem. Asian J. 2019, 14, 1418–1423.
- Yin, Z.; Ji, Q.; Wu, D.; Li, Z.; Fan, M.; Zhang, H.; Zhao, X.; Wu, A.; Cheng, L.; Zeng, L. H2O2-Responsive Gold Nanoclusters @ Mesoporous Silica @ Manganese Dioxide Nanozyme for “Off/On” Modulation and Enhancement of Magnetic Resonance Imaging and Photodynamic Therapy. ACS Appl. Mater. Interfaces 2021, 13, 14928–14937.
- Jia, T.T.; Yang, G.; Mo, S.J.; Wang, Z.Y.; Li, B.J.; Ma, W.; Guo, Y.X.; Chen, X.; Zhao, X.; Liu, J.Q.; et al. Atomically Precise Gold-Levonorgestrel Nanocluster as a Radiosensitizer for Enhanced Cancer Therapy. ACS Nano 2019, 13, 8320–8328.
- Hua, Y.; Wang, Y.; Kang, X.; Xu, F.; Han, Z.; Zhang, C.; Wang, Z.Y.; Liu, J.Q.; Zhao, X.; Chen, X.; et al. A multifunctional AIE gold cluster-based theranostic system: Tumor-targeted imaging and Fenton reaction-assisted enhanced radiotherapy. J. Nanobiotechnol. 2021, 19, 438.
- Pan, U.N.; Sanpui, P.; Paul, A.; Chattopadhyay, A. Protein-Nanoparticle Agglomerates as a Plasmonic Magneto-Luminescent Multifunctional Nanocarrier for Imaging and Combination Therapy. ACS Appl. Bio Mater. 2019, 2, 3144–3152.
- Zhou, J.; Wang, Q.; Geng, S.; Lou, R.; Yin, Q.; Ye, W. Construction and evaluation of tumor nucleus-targeting nanocomposite for cancer dual-mode imaging—Guiding photodynamic therapy/photothermal therapy. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 102, 541–551.
- Tao, Y.; Yi, K.; Hu, H.; Shao, D.; Li, M. Coassembly of nucleus-targeting gold nanoclusters with CRISPR/Cas9 for simultaneous bioimaging and therapeutic genome editing. J. Mater. Chem. B 2021, 9, 94–100.
- Govindaraju, S.; Roshini, A.; Lee, M.H.; Yun, K. Kaempferol conjugated gold nanoclusters enabled efficient for anticancer therapeutics to A549 lung cancer cells. Int. J. Nanomed. 2019, 14, 5147–5157.
- Zhan, C.; Huang, Y.; Lin, G.; Huang, S.; Zeng, F.; Wu, S. A Gold Nanocage/Cluster Hybrid Structure for Whole-Body Multispectral Optoacoustic Tomography Imaging, EGFR Inhibitor Delivery, and Photothermal Therapy. Small 2019, 15, e1900309.
- Xia, F.; Hou, W.; Liu, Y.; Wang, W.; Han, Y.; Yang, M.; Zhi, X.; Li, C.; Qi, D.; Li, T.; et al. Cytokine induced killer cells-assisted delivery of chlorin e6 mediated self-assembled gold nanoclusters to tumors for imaging and immuno-photodynamic therapy. Biomaterials 2018, 170, 1–11.
- Liu, X.; Yang, Y.; Wang, X.; Liu, X.; Cheng, H.; Wang, P.; Shen, Y.; Xie, A.; Zhu, M. Self-assembled Au4Cu4/Au25 tumor nanotheranostics with PT/fluorescence imaging-guided synergetic PTT/PDT. J. Mater. Chem. B 2021, 9, 6396–6405.
- Dutta, D.; Sailapu, S.K.; Simon, A.T.; Ghosh, S.S.; Chattopadhyay, A. Gold-Nanocluster-Embedded Mucin Nanoparticles for Photodynamic Therapy and Bioimaging. Langmuir 2019, 35, 10475–10483.
- Sujai, P.T.; Joseph, M.M.; Karunakaran, V.; Saranya, G.; Adukkadan, R.N.; Shamjith, S.; Thomas, R.; Nair, J.B.; Swathi, R.S.; Maiti, K.K. Biogenic Cluster-Encased Gold Nanorods as a Targeted Three-in-One Theranostic Nanoenvelope for SERS-Guided Photochemotherapy against Metastatic Melanoma. ACS Appl. Bio Mater. 2019, 2, 588–600.
- Li, H.; Li, H.; Wan, A. Luminescent gold nanoclusters for in vivo tumor imaging. Analyst 2020, 145, 348–363.
- Jiang, M.; Lin, Y.; Fang, X.; Liu, M.; Ma, L.; Liu, J.; Chen, M.; Yang, Y.; Wang, C. Enhancement of gold-nanocluster-mediated chemotherapeutic efficiency of cisplatin in lung cancer. J. Mater. Chem. B 2021, 9, 4895–4905.
- Jiang, X.; Sun, Y.; Shang, L.; Yang, C.; Kong, L.; Zhang, Z. Green tea extract-assembled nanoclusters for combinational photothermal and chemotherapy. J. Mater. Chem. B 2019, 7, 5972–5982.
- Wu, S.; Yang, X.; Luo, F.; Wu, T.; Xu, P.; Zou, M.; Yan, J. Biosynthesis of flower-shaped Au nanoclusters with EGCG and their application for drug delivery. J. Nanobiotechnol. 2018, 16, 90.
- Haume, K.; Rosa, S.; Grellet, S.; Smialek, M.A.; Butterworth, K.T.; Solov’yov, A.V.; Prise, K.M.; Golding, J.; Mason, N.J. Gold nanoparticles for cancer radiotherapy: A review. Cancer Nanotechnol. 2016, 7, 8.
- Citrin, D.E. Recent Developments in Radiotherapy. N. Engl. J. Med. 2017, 377, 1065–1075.
- Taggart, L.E.; McMahon, S.J.; Butterworth, K.T.; Currell, F.J.; Schettino, G.; Prise, K.M. Protein disulphide isomerase as a target for nanoparticle-mediated sensitisation of cancer cells to radiation. Nanotechnology 2016, 27, 215101.
- Chen, Y.; Yang, J.; Fu, S.; Wu, J. Gold Nanoparticles as Radiosensitizers in Cancer Radiotherapy. Int. J. Nanomed. 2020, 15, 9407–9430.
- Zhang, X.; Chen, X.; Jiang, Y.W.; Ma, N.; Xia, L.Y.; Cheng, X.; Jia, H.R.; Liu, P.; Gu, N.; Chen, Z.; et al. Glutathione-Depleting Gold Nanoclusters for Enhanced Cancer Radiotherapy through Synergistic External and Internal Regulations. ACS Appl. Mater. Interfaces 2018, 10, 10601–10606.
- Herold, D.M.; Das, I.J.; Stobbe, C.C.; Iyer, R.V.; Chapman, J.D. Gold microspheres: A selective technique for producing biologically effective dose enhancement. Int. J. Radiat. Biol. 2000, 76, 1357–1364.
- Ejigah, V.; Owoseni, O.; Bataille-Backer, P.; Ogundipe, O.D.; Fisusi, F.A.; Adesina, S.K. Approaches to Improve Macromolecule and Nanoparticle Accumulation in the Tumor Microenvironment by the Enhanced Permeability and Retention Effect. Polymers 2022, 14, 2601.
- Islam, R.; Maeda, H.; Fang, J. Factors affecting the dynamics and heterogeneity of the EPR effect: Pathophysiological and pathoanatomic features, drug formulations and physicochemical factors. Expert Opin. Drug Deliv. 2022, 19, 199–212.
- Samani, R.K.; Tavakoli, M.B.; Maghsoudinia, F.; Motaghi, H.; Hejazi, S.H.; Mehrgardi, M.A. Trastuzumab and folic acid functionalized gold nanoclusters as a dual-targeted radiosensitizer for megavoltage radiation therapy of human breast cancer. Eur. J. Pharm. Sci. 2020, 153, 105487.
- Luo, D.; Wang, X.; Walker, E.; Springer, S.; Ramamurthy, G.; Burda, C.; Basilion, J.P. Targeted Chemoradiotherapy of Prostate Cancer Using Gold Nanoclusters with Protease Activatable Monomethyl Auristatin E. ACS Appl. Mater. Interfaces 2022, 14, 14916–14927.
- Wu, C.; Du, X.; Jia, B.; Zhang, C.; Li, W.; Liu, T.C.; Li, Y.Q. A transformable gold nanocluster aggregate-based synergistic strategy for potentiated radiation/gene cancer therapy. J. Mater. Chem. B 2021, 9, 2314–2322.
- Ghahremani, F.; Kefayat, A.; Shahbazi-Gahrouei, D.; Motaghi, H.; Mehrgardi, M.A.; Haghjooy-Javanmard, S. AS1411 aptamer-targeted gold nanoclusters effect on the enhancement of radiation therapy efficacy in breast tumor-bearing mice. Nanomedicine 2018, 13, 2563–2578.
- Chen, J.; Gong, M.; Fan, Y.; Feng, J.; Han, L.; Xin, H.L.; Cao, M.; Zhang, Q.; Zhang, D.; Lei, D.; et al. Collective Plasmon Coupling in Gold Nanoparticle Clusters for Highly Efficient Photothermal Therapy. ACS Nano 2022, 16, 910–920.
- Nair, R.V.; Puthiyaparambath, M.F.; Chatanathodi, R.; Nair, L.V.; Jayasree, R.S. A nanoarchitecture of a gold cluster conjugated gold nanorod hybrid system and its application in fluorescence imaging and plasmonic photothermal therapy. Nanoscale 2022, 14, 13561–13569.
