Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Matthias Widbiller and Version 2 by Dean Liu.
Efforts to heal damaged pulp tissue through tissue engineering have produced positive results in pilot trials. However, the differentiation between real regeneration and mere repair is not possible through clinical measures. Therefore, preclinical study models are still of great importance, both to gain insights into treatment outcomes on tissue and cell levels and to develop further concepts for dental pulp regeneration.
- regenerative endodontics
- study model
- dental pulp
1. Introduction
The dental pulp has important functions, and its loss can have serious consequences. A root-filled tooth may remain in the oral cavity without pulp, but it lacks the ability to react to sensory stimuli, issue an immune response, or form reparative dentin [1]. Additionally, remaining hard tissue is weakened, and as a result, root fractures occur more frequently than in vital teeth [2]. If immature teeth are affected, root development comes to a halt, leaving thin dentin walls and an open apex behind, which complicates further therapies [3][4][3,4]. To overcome the biological and mechanical drawbacks of traditional endodontic treatment, research focused on pulp regeneration has gained interest over the last years. Several approaches in the realm of endodontic tissue engineering are being explored, which can be categorized into primarily cell-free methods, where resident stem cells re-populate the root canal, and cell-based approaches, where cells are introduced by transplantation [5]. In both approaches, the three pillars of classical tissue engineering, i.e., stem cells, signaling molecules, and a scaffold material, are present [6].
Interestingly, endodontic tissue engineering has already been translated into randomized clinical trials. Patients with irreversible pulpitis have been treated with transplantation of autologous [7] or allogenic [8] mesenchymal stem cells into root canals. In these studies, all teeth that received treatment through tissue engineering have survived after 12 months, and even positive responses to sensitivity testing were evident in a considerable number of cases. Further observations, such as radiographic reduction in apical lesion size and root lengthening and thickening, demonstrated clinical success [9]. However, the question remains whether this success is associated with biological regeneration of the pulp or a repair process. Teeth that have undergone regenerative procedures and are later extracted for other reasons often show proof of repair by ectopically formed tissues instead of restitutio ad integrum [5]. Strengthening the tooth root by the apposition of any hard tissue may be of clinical value and could contribute to increased mechanical resistance, but functional issues, e.g., adequate biological response of the dental pulp to external stimuli, remain unresolved [3][10][3,10]. In this context, histological examination is the only way to determine the exact nature of newly generated tissues. However, this is, of course, impossible in a systematic way in clinical studies. For this reason, preclinical study models are still indispensable for the development of new pulp regeneration procedures and for the biological evaluation of outcomes.
2. In Vivo Ectopic and Semiorthotopic Models
The transplantation of biological samples into the subcutaneous space of experimental animals is another method to create a physiological environment. In this context, ectopic means that tissues or cells are transplanted into experimental animals at a nonphysiological location. Cells in scaffolds can be transplanted by themselves or with signaling molecules [11][12][13][14][15][12,80,81,82,83]. However, especially in the context of pulp biology, cells are often implanted together on dentin disks [16][17][84,85], in tooth slices [18][19][20][21][59,86,87,88], in dentin cylinders, or in tooth roots [22][23][24][25][26][27][28][46,50,89,90,91,92,93] in order to simulate their natural environment. Since the directly surrounding or adjoining tissue is not ectopic, but rather corresponds to the natural environment (orthotopic), the term semiorthotopic is often used [29][94]. Here, the proximity to blood vessels enables nutrient supply to cells and the removal of waste products, and the animals can, thus, be considered in vivo bioreactors [30][71]. Additionally, interactions with resident peripheral nerve cells, connective tissues, and the immune system can be studied. Immunodeficient animals are most often utilized to prevent unwanted immunogenic reactions. Implantation sites can vary. Small incisions through the skin can, for example, be made on the dorsum of mice, and subcutaneous pockets created by blunt dissection. After implant placement, wounds are closed by stapling or stitching [21][88]. Due to its abundant blood supply, the rat renal capsule is another location for ectopic transplantation; however, it is more difficult to access, and the mortality rate of experimental animals is higher than after subcutaneous implantation [31][32][95,96]. The subcutaneous implantation of autologous dental pulp cells or scaffold constructs into the dorsal surface of rabbits was also suggested as a valid ectopic model [33][97]. Ruangsawasdi et al. investigated the implantation of cell-free tooth roots filled with fibrin into the calvaria of rats and found that this placement produced more tissue ingrowth in the same time period than the dorsal location. This article suggested that rat calvaria could provide a microenvironment similar to the tooth socket [34][98]. Favorable outcomes can be achieved with ectopic and semiorthotopic transplantation, as they offer very translational features, are reproducible, and are well-described in the literature. Compared to other preclinical in vivo models, the utilization of smaller animals, such as mice, is preferred, as breeding and housing are less expensive and murine anatomy is well-understood. The surgical procedure of implant placement is easy to perform and results in minimal distress for the animals. Nevertheless, ethical concerns still need to be considered, and especially in the early stages of research, cell cultures should be preferred. The decision to use animals should never be taken lightly. It must also be noted that newly formed tissue, blood vessels, or nerve fibers can be of human or rodent origin. These ambiguities need to be kept in mind and reviewed in order to draw the correct conclusions regarding tissue formation (Table 1).Table 1. Strengths and weaknesses of in vitro and in vivo models.
| In Vitro | In Vivo | |||
|---|---|---|---|---|
| Monolayer | 3D Culture | Ectopic | Semiorthotopic | |
| high cost | ||||
Table 2. Selected references for applications of each study model, including both in vitro and in vivo. The asterisk indicates categories that are not conceivable in the present classification.
| Study Models | In Vitro | In Vivo | ||||||
|---|---|---|---|---|---|---|---|---|
| Ectopic | Semiorthotopic | |||||||
| + | ||||||||
| Scaffold culture | Wang et al., 2010 [13][81] Galler et al., 2012 [23][ | + | 50++ | ++ | ||||
| ] | Qu and Liu, 2013 [35][40] Widbiller et al., 2016 [36][52] Lin et al., 2021 [37][42] |
Buurma et al., 1999 [12][80] Gronthos et al., 2000 [11][12] Wang et al., 2010 [13][81] Lee et al., 2011 [14][82] De Almeidas et al., 2014 [15][83] |
* | ethical concerns | + | + | +++ | |
| Spheroid and organoid | Xiao and Tsutsui, 2013 [38][99] Dissanayaka et al., 2014 [18 | +++ | ||||||
| ] | [59] Jeong et al., 2020 [39][60] Zheng et al., 2021 [40][100] Chan et al., 2021 [41][101] |
* | literature experience | +++ | + | ++ | ++ | |
| Dentin disk | Sloan et al., 1998 [42][102] Huang et al., 2006 [43][19] Widbiller et al., 2019 [44][103] Atesci et al., 2020 [45][104] |
* | Batouli et al., 2003 [16][84] Goncalves et al., 2007 [17][85] |
difficult implementation | + | |||
| Tooth slice | ++ | Casagrande et al., 2010 [46][45] | ++ | * | Cordeiro et al., 2008 [19][86] Prescott et al., 2009 [20][87] Sakai et al., 2010 [47][105] Casagrande et al., 2010 [46][45] Sakai et al., 2011 [21++ |
|||
| ] | [ | 88 | ] Dissanayaka et al., 2014 [18][59] |
reproducibility | +++ | ++ | + | + |
| Dentin cylinder and tooth root | Rosa et al., 2013 [22][46] | * | Galler et al., 2011 [24][89] Galler et al., 2012 [23][50] Rosa et al., 2013 [22][46] Takeuchi et al., 2015 [25][90] Widbiller et al., 2018 [26][91] With coronal plug: Huang et al., 2010 [27][92] Zhu et al., 2018 [28][93] |
mimicry of natural situation | + | ++ | ++ | +++ |
2.1. Dentin Disk and Tooth Slice
Despite the fact that various research applications are based on the ectopic implantation of cells alone or cells encapsulated in a scaffold material [15][48][49][24,83,106], pulp cannot be restored without considering the pulp–dentin complex. The close mechanical and functional connections of cells and dentin are the reasons why many researchers choose to combine pulp-derived cells with dentin disks or tooth slices in vitro and implant them subcutaneously. Therefore, dentin disks or tooth slices are usually obtained in the area of solid coronal dentin or the pulp cavity from human molars respectively. The cells can then be seeded on top of solid dentin disks or cast within a scaffold into the former pulp chamber [20][21][46][45,87,88] (Figure 14A,B).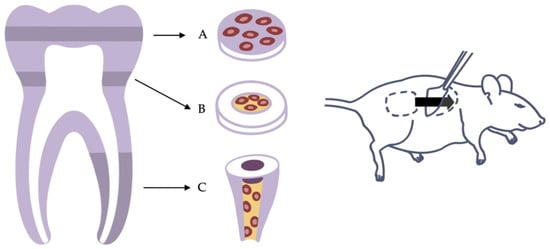
Figure 14. Variants of the ectopic transplantation model. (A) Dentin disk with cells seeded on top. (B) Tooth slice with cells and scaffold inserted into the pulp chamber. (C) Root fragment model with cells and scaffold inserted into the root canal.
2.2. Dentin Cylinder and Tooth Root
Sufficient vascularization is a prerequisite for cells to survive and generate new tissue [19][30][53][71,86,108]. In a tooth slice model, nutrients and oxygen may reach the cells easily by diffusion from neighboring tissues, as the diffusion distance is short. However, the anatomy of an actual tooth is different. Diffusion from the root tip all the way to the crown is not possible. Only the advancement of a functional vascular system allows cells to expand into the entire pulp cavity and tissue to develop even far from the apical entry [53][54][108,109]. As blood vessels have only restricted access to the root canal through the apical foramen, models using dentin cylinders or tooth roots mimic the difficulties of the clinical situation more accurately. Here, whole roots or parts of them are separated from extracted teeth, prepared, and filled with cells and a scaffold material. Sample constructs can then be implanted, for example, into a mouse dorsum to be accessed by blood vessels and nerve fibers (Figure 14C). Whereas leaving both ends of the dentin cylinder open may provide optimal blood supply from two directions, sealing of the coronal opening with a bioactive material corresponds to clinical situations, as the unilateral sprouting of vessels into the tooth root presents a challenge [22][26][27][46,91,92]. However, the decision of how to prepare the roots must be made depending on the application and the specific research question. This semiorthotopic model situation allows a variety of investigations and analyses. The focus can be on qualitative factors, such as the formation of odontoblast-like cells or the expressions of certain markers, as well as quantitative factors, such as the number of blood vessels or nerve fibers or the amount of newly formed tissue. Furthermore, the model has been continuously developed and modified over the past years to answer specific questions or to counteract limitations. For example, Widbiller et al. established a customized tooth root model to test cell-homing approaches for dental pulp regeneration [26][91]. Here, the root canal was filled with a growth-factor-laden hydrogel with the ability to promote chemotaxis. Stem cells were then placed only at the apical opening of the root to mimic the apical papilla as the stem cell source of immature teeth. After the recovery of the tooth roots from the mouse subcutaneous space, the samples can be processed histologically, and the newly formed tissue can be analyzed by various techniques (Figure 25) [55][110].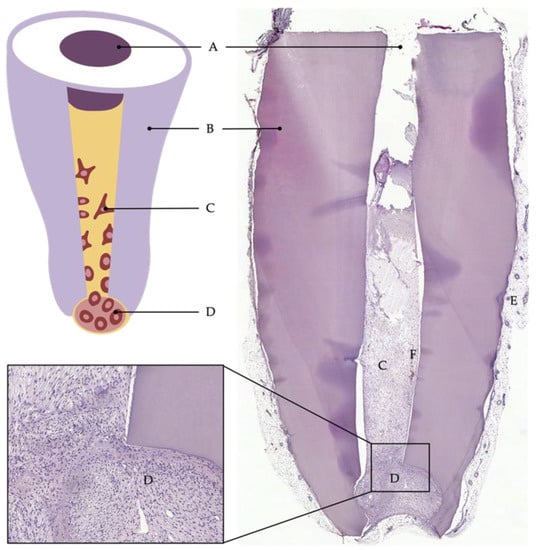
Figure 25. Cell-homing model. Tooth root recovered after 6 weeks of implantation into subcutaneous dorsal space of mice: (A) coronal plug, (B) dentin of root walls, (C) cells that migrated into the root canal, (D) apical reservoir of stem cells in collagen, (E) murine tissue, (F) blood vessel.
