1. Introduction
The high incidence and mortality rate of cancer pose grave risks to the lives and well-being of all humans. It has long been a focus of research in life science to improve the accuracy of the early detection of malignant tumors and to address the dearth of effective tumor treatments
[1]. With the rapid development of nanotechnology, the diversity of structures and functions of biological nanomaterials has been further enriched and spread at an alarming rate to life sciences and clinical medicine, especially new nanomaterials that integrate multiple modes of diagnostic and therapeutic strategies in one, making precise diagnosis and treatment integration and synergistic treatment possible, and this is eagerly anticipated around the globe
[2][3][2,3].
Gold nanoparticles (AuNPs) are a type of colloidal or agglomerated particle with diameters between a few and hundreds of nanometers, composed of gold cores and surface shell layers. Due to their unique optical properties (surface plasmon resonance, surface-enhanced Raman scattering, etc.) and excellent catalytic properties, they hold great promise in a variety of applications, including biosensing, bioimaging, disease diagnosis, and treatment
[4][5][6][7][4,5,6,7].
Gold nanoclusters (AuNCs) are gold nanomaterials with significantly smaller dimensions (≤3 nm) and typically comprise a few to tens of atoms
[8]. Due to the quantum-limited effect, AuNCs have superior fluorescence properties and are utilized in a variety of scientific fields, including environmental detection, molecular labeling, and bioimaging
[9][10][11][12][13][9,10,11,12,13]. In addition, because AuNCs are smaller than the renal threshold, they are easier to eliminate from the body than AuNPs, resulting in greater biosafety and in vivo application potential
[14]. Physical, chemical, and biological techniques are now used by production enterprises and lab researchers to create AuNCs. In situ synthesis employing biomolecules (DNA, proteins, peptides, etc.) as templates are one of the chemical techniques that is gaining popularity among researchers
[15][16][17][15,16,17]. The principal causes are as follows. Firstly, the biomolecular template contains numerous active functional groups, such as -SH, -COOH, -NH
2, and -OH, which can bind gold atoms and improve their stability
[18][19][18,19]. Secondly, some reducing amino acids (e.g., tryptophan, tyrosine) can reduce Au
3+ ions to Au atoms in the presence of an appropriate pH environment, avoiding the use of strong reducing agents (e.g., NaBH4, CTAB) and have an improved biocompatibility
[20]. Thirdly, the physical and chemical properties of AuNCs, such as the number of atoms, particle size, and optical properties, can be rapidly modified by adjusting the template amino acid or nucleotide sequences
[21][22][23][21,22,23]. Lastly, the biological activities and functional binding sites of biomolecules provide a rich platform for further multi-functionalization of AuNCs
[24][25][24,25].
Meanwhile, for tumor tissue enrichment, small AuNCs with high permeability and long retention are preferable. Surface-modified AuNCs can reduce the reticuloendothelial system (RES) and non-specific uptake, as well as specifically bind to overexpressed tumor cell receptors to enhance tumor cell accumulation, resulting in an enhanced cytotoxic effect against tumor cells
[26][27][26,27]. The AuNCs can be rapidly excreted via the kidney, thereby minimizing damage to healthy tissues
[14]. In comparison to large AuNPs, AuNCs possess a larger specific surface area and, consequently, greater surface energy. Due to this surface effect, the surface atoms of AuNCs are reactive and readily bondable with other atoms. Large payloads of drugs, genes, and other therapeutic molecules can be effectively trapped and protected from enzymatic degradation in complex physiological microenvironments
[28][29][30][28,29,30]. Various internal and external stimuli may be used to regulate the release of drug-carrying molecules from functionalized AuNCs (e.g., pH, glutathione, light)
[31][32][31,32]. As a result, they can be used as carriers for efficient targeted transport of therapeutic molecules, to enhance drug aqueous solubility, to prevent drug leakage in healthy tissues prematurely, and mitigate potential side effects.
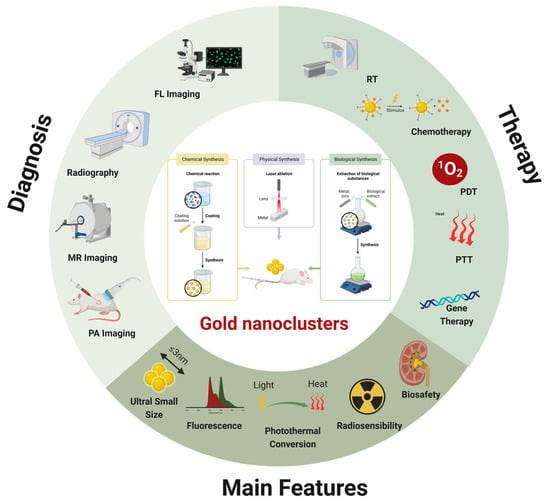
Indeed, numerous reviews have been conducted on the design and application of AuNCs, particularly in terms of fluorescence imaging. Nonetheless, an increasing number of studies are currently attempting to fully integrate the various properties of AuNCs (Scheme 1)..
Scheme 1.
Primary preparation strategies, distinctive properties, and combined applications in diagnosis and therapies of AuNCs(created with BioRender.com).
2. AuNCs as Imaging Agents in Tumor Theranostic
Since the successful construction of ultra-small AuNCs, the unique photoelectric effect resulting from their quantum size effect has been valued by researchers and utilized in a variety of sensing, detection, and bioimaging fields
[33][34][35][36][33,34,35,36]. AuNCs are ideally suited for integrated medical applications in diagnostics and treatment due to their superior biocompatibility and functional versatility
[37][38][37,38]. The atomic-level investigation of AuNCs has accelerated recently. Due to their precise size and composition, researchers have discovered that AuNCs have outstanding self-assembly and crystallization properties which endow them with more unique and diverse fluorescence properties
[39][40][39,40]. To start, the researchers summarize the recent studies on the integration and visualization of AuNCs for diagnosis and treatment based on different imaging modalities of AuNCs, respectively (
Table 1).
Table 1.
Application of AuNCs.
|
Multifunctional Nanoplatform
|
Role of AuNCs
|
Therapeutic Agent
|
Size (nm)
|
Imaging Mode
|
Cancer Types
|
Therapy Method
|
Activity
|
Ref
|
|
AuNCs-Ag@Keratin-Gd
|
Imaging
|
NM
|
5
|
FL, MRI
|
Breast cancer
|
Chemotherapy
|
In vivo and in vitro
|
[37]
|
|
CDGM NPs
|
Imaging, drug delivery
|
CAD, Ce6
|
2
|
FL
|
Lung cancer
|
PDT
|
In vivo and in vitro
|
[41][54]
|
|
AuS-U11
|
PTT-carrier
|
U11 peptide, cyanine dye Cy5.5, 5-ALA
|
10
|
FL
|
Pancreatic carcinoma
|
PTT, PDT
|
In vivo and in vitro
|
[42][55]
|
|
Au NBPs@PDA/AuNCs
|
Imaging
|
Au NBPs@PDA
|
2.1, 3.3
|
FL
|
Breast cancer, hepatocarcinoma
|
PTT
|
In vitro
|
[43][56]
|
|
Dox@HG-CAHs
|
Imaging
|
HA-ALD, Dox
|
2.8
|
FL, CT
|
Osteosarcoma
|
PTT, chemotherapy
|
In vivo and in vitro
|
[44][61]
|
|
AuNCs–LHRHa
|
Imaging, PTT
|
LHRH analogues
|
2.4
|
FL, CT
|
Prostatic cancer
|
PTT
|
In vitro
|
[45][67]
|
|
GTSL-CYC-HER2
|
Changed the zeta potential of liposomes, superior photothermal effect
|
HER2-modified thermosensitive liposome, cyclopamine
|
NA
|
CT, PTI
|
Breast cancer
|
Chemotherapy, PTT
|
In vivo and in vitro
|
[46][68]
|
|
Ce6&AuNCs/Gd-LDH
|
Imaging
|
Ce6
|
~2
|
MRI, FL
|
Hepatocarcinoma
|
PDT
|
In vivo and in vitro
|
[47][70]
|
|
AuNCs-ICG
|
Imaging, radiosensitizing effects
|
ICG
|
~1
|
FL, PAI, CT
|
Breast cancer
|
PDT, RT
|
In vivo and in vitro
|
[48][72]
|
|
Qu-GNCs
|
Imaging
|
Qu
|
1–3
|
FL
|
Lung cancer
|
Chemotherapy
|
In vitro
|
[49][74]
|
|
Fe3O4@PAA/AuNCs/ZIF-8 NPs
|
Imaging
|
DOX
|
NA
|
MRI, CT, FL
|
Hepatocarcinoma
|
Chemotherapy
|
In vivo and in vitro
|
[50][75]
|
|
AuNCs@GTMS-FA
|
Imaging, phototherapeutic agents
|
FA
|
2.8
|
FL
|
Breast cancer
|
PTT, PDT
|
In vitro
|
[51][80]
|
|
AuNCs/Dzs-Dox
|
NSET effect, shelter therapeutic cargos
|
Dzs-Dox
|
~1.76
|
FL
|
Breast cancer
|
Gene therapy, chemotherapy
|
In vivo and in vitro
|
[52][87]
|
|
HG-GNCs/GO-5FU
|
Bioimaging, phototherapeutic
|
HA, 5FU
|
2
|
FL
|
Lung cancer, breast cancer
|
Chemotherapy, PDT, PTT
|
In vitro
|
[53][93]
|
|
AuNCs@mSiO2@MnO2
|
Photosensitizer
|
MnO2 nanozyme
|
NA
|
MRI
|
Breast cancer
|
PDT
|
In vivo and in vitro
|
[54][94]
|
|
Au8NC
|
Radiosensitizing effects
|
Levonorgestrel
|
~2
|
FL
|
Esophagus cancer
|
RT
|
In vivo and in vitro
|
[55][105]
|
|
Au4-IO NP-cRGD
|
Imaging, radiosensitizing effects
|
IO nanocluster
|
2
|
FL, MRI
|
Breast cancer
|
RT, chemotherapy
|
In vivo and in vitro
|
[56][113]
|
|
PML-MF nanocarrier
|
Imaging
|
IO@AuNPs
|
NA
|
FL
|
Cervical cancer
|
PPTT, chemotherapy
|
In vitro
|
[57][117]
|
|
WLPD-Au25
|
Photosensitizer, drug delivery
|
WS2 nanoparticles, Dex, Captopril
|
2.5
|
CT
|
Breast cancer
|
PTT, PDT
|
In vivo
|
[58][118]
|
|
AuNCs/Cas9–gRNA
|
Imaging, drug delivery
|
Cas9–sgRNA plasmid
|
~1.56
|
FL
|
Osteosarcoma
|
Gene therapy
|
In vitro
|
[59][119]
|
|
K-AuNCs
|
Imaging, drug delivery
|
K
|
1–3
|
FL
|
Lung cancer
|
Chemotherapy
|
In vitro
|
[60][120]
|
|
EA-AB
|
Imaging
|
EB
|
NM
|
FL, MSOT Imaging
|
Breast cancer
|
Chemotherapy, PTT
|
In vivo and in vitro
|
[61][121]
|
|
Ce6-GNCs-Ab-CIK
|
Drug delivery
|
Ce6, CD3 antibody
|
NA
|
FL
|
Gastric cancer
|
Chemotherapy, PDT
|
In vivo and in vitro
|
[62][122]
|
|
Au4Cu4/Au25@Lip
|
Photothermogenesis effect, photoluminescence performance
|
Au4Cu4 nanoclusters
|
~2
|
FL, PTI
|
Cervical cancer
|
PTT, PDT
|
In vivo and in vitro
|
[63][123]
|
|
MB-loaded Au NC-mucin NPs
|
Imaging
|
MB
|
1.9 ± 0.34
|
FL
|
Cervical cancer
|
PDT
|
In vitro
|
[64][124]
|
|
ISQ@BSA-AuNC@AuNR@DAC@DR5
|
SERS substrate
|
DAC, ISQ
|
NA
|
NM
|
Amelanotic Melanoma
|
PTT, PDT
|
In vivo and in vitro
|
[65][125]
|
Abs: PTT: photothermal therapy; NSET: nanosurface energy transfer; CAD: MMP2 polypeptidecis-aconitic anhydride-modified doxorubicin; Ce6: photosensitizer chlorin e6; Au NBPs@PDA: polydopamine-capped gold nanobipyramids; HA-ALD: oxidized hyaluronic acid; Dox: doxorubicin; Ce6: chlorin e6; ICG: indocyanine green; K: Kaempferol; Qu: Quercetin; EB: Erlotinib; HA: hyaluronic acid; 5FU: 5-fluorouracil; FA: Folic acid; MB: Methylene blue; DAC: Dacarbazine; Dzs-Dox: DNAzyme, Dox; IO: Iron oxide; Dex: Dexamethasone; FL: fluorescence imaging; MRI: magnetic resonance imaging; CT: computed tomography; PT: photothermal imaging; PAI: photoacoustic imaging, MSOT: multispectral optoacoustic tomography; PDT: photodynamic therapy; RT: radiation therapy; PPTT: plasmonic photothermal therapy; NM: not mentioned; NA: not applicable.
3. AuNCs as Transport Agents in Combined Therapy
AuNCs are widely used for drug delivery and controlled release in vivo and ex vivo as one of the metallic nanomaterials with the longest research history. As one of the special ultra-small size nanostructures, AuNCs have a greater potential for combinatorial applications
[66][73]. Initially, AuNCs have a stable and inert internal core that can shield encapsulated drug molecules. Further, AuNCs have a high surface to volume ratio and can be loaded with a substantial quantity of small-molecule drugs via reasonable surface modification
[49][74]. In addition, AuNCs can be targeted for in vivo tumor transport via passive accumulation (e.g., enhanced permeability and retention effect) or active targeting (e.g., modified target molecules), thereby enhancing the bioavailability of drugs
[50][67][75,76]. Moreover, the ultra-small nanostructures enable precise targeting of subcellular organelle structures, such as the nucleus and mitochondria, supplement selection, and therapeutic strategies. In combination with the unique optoelectronic and chemical properties of AuNCs, they can achieve controlled and precise strikes against the internal environmental response of tumors and external signal stimuli, consequently reducing the toxic side effects that accompany chemotherapy.
The covalent modification of the AuNP surface generally adopts sodium borohydride reduction and ligand replacement methods, and the non-covalent binding mainly includes electrostatic interaction and hydrophobic interaction to adsorb the surrounding molecules, thus reducing the surface free energy. Jiang et al. prepared AuNCs loaded with adriamycin by a “green chemistry” approach using green tea extract, in which adriamycin was co-polymerized with the nanoclusters by π-π superposition and electrostatic interactions. The drug delivery system has good stability and can significantly inhibit tumors through the synergetic effect of photothermal therapy and chemotherapy
[68][77]. The Au-Cys-MTX/DOX NCs system constructed by Wu et al. is more stable than the non-covalent drug delivery system and can choose different drug release mechanisms according to the microenvironment of different tumor tissues. And due to the solidity of the amide bond, only a small amount of drug can be released from Au-Cys-MTX/DOX NCs in the absence of protease
[69][78].
4. AuNCs as Therapeutic Agents in Combined Therapy
4.1. Radiosensitization
Radiation therapy is an effective oncology treatment that uses ionizing radiation to target cancer cells. A part of the high-energy radiation will nonetheless be transferred to the normal tissues around the tumor, inflicting irreparable damage
[70][71][101,102]. Gold has a high atomic number and much greater electron density than soft tissues, which may boost photoelectric absorption and secondary electron yield, improve local energy deposition in tumor tissues, and expedite the death of tumor cells
[72][73][103,104]. In addition to the previously reported physical methods, gold clusters may potentially exert their radiosensitizing effects via biological pathways, for example, controlling the cell cycle, boosting free radical generation in response to radiation, altering cell autophagy, and causing apoptosis
[55][74][105,106]. Using nanogold for the first time for in vivo tumor radiation sensitization, Herold et al. demonstrated that gold particles might have a dose-enhancing impact on cells and C.B17/Icr scid mice cultured with EMT-6 mouse tumor cells when exposed to 200 kVp X-rays both in vitro and in vivo tests
[75][107].
The enhanced permeability and retention (EPR) effect may enhance the aggregation of AuNCs in the tumor, since smaller nanoclusters (<5 nm) can more easily penetrate tumor tissues and cross blood vessels than larger nanoparticles (>10 nm). Additionally, the tumor tissue’s decreased lymphatic outflow makes it difficult for nanoparticles to be effectively cleared away, which increases their retention in the tumor tissue
[76][77][108,109], hence improving the radiation treatment impact and reducing the harm to the surrounding normal cells. Attaching trastuzumab and folic acid targeting human epidermal growth factor receptor 2 (HER2) to 4.2 nm AuNCs, Roghayeh et al. demonstrated that the targeted AuNCs may infiltrate breast cancer SK-BR3 cells through HER-2-mediated mechanisms
[78][110]. Luo et al. created therapeutic AuNCs that can function as prostate cancer (PCa)-targeted radiosensitizers and chemotherapy carriers. Using PSMA-MMAE as a template and the reduction of Au
3+ by the reactive group, PSMA-AuNC-MMAE couples were synthesized. The gold nanocluster-attached prostate-specific membrane antigen (PSMA) could improve the targeting of AuNCs; the bound monomethyl auristatin E (MMAE) was a chemotherapeutic prodrug that enhanced the chemotherapeutic effect after binding with AuNC, and MMAE could also boost the radiosensitizing impact by inhibiting the cells in the G2-M area. Due to PSMA receptor amplification, PC3pip tumor cells maintained considerably more gold nanocluster complexes in PC3pip tumor-bearing animals than in PC3flu tumor-bearing mice, as shown by in vivo tests
[79][111]. Wu et al. created transformable gold nanocluster (AuNC) aggregates (called AuNC-ASON) using antisense oligonucleotides (ASON) that target survivin mRNA. The acidic tumor microenvironment modifies the electrostatic interactions between the polyelectrolyte poly(allylamine) (PAH) and glutathione surface ligands that stabilize AuNC, causing gold nanocluster aggregates to separate into 2 nm AuNCs and triggering the release of loaded antisense oligonucleotides for gene silencing. The findings of in vivo tests analyzing the co-localization of AuNC-ASON with nucleosomes/lysosomes revealed that AuNC-ASON has an excellent nucleosome/lysosome escape capacity. Moreover, real-time polymerase chain reaction (real-time PCR) research revealed that the expression of survivin mRNA in 4T1 cells followed the same pattern as cell viability, validating the mechanism of tumor cell eradication based on survivin gene silencing. With the aid of survivin gene interference, this treatment approach may increase and enhance the radiosensitivity of cancer cells and enable the simultaneous use of tumor radiation and gene therapy
[80][112].
The effectiveness of radiotherapy is contingent upon radiosensitivity, and a hypoxic tumor microenvironment renders tumor cells more resistant to ionizing radiation. As radiosensitizers, AuNCs may be used with oxygen carriers to reduce tumor hypoxia by generating reactive oxygen species (ROS) generation and enhancing the effectiveness of radiation. In the cRGD multifunctional treatment system, Au
4-IO NP-cRGD triggered the death of 4T1 cells by producing substantial quantities of reactive oxygen species in response to X-ray exposure. Experiments in vivo have shown that this multifunctional treatment platform is capable of directing Fenton response-assisted improved radiotherapy using dual-mode imaging based on magnetic resonance imaging of iron oxide (IO) nanoclusters and fluorescence imaging of Au
4 clusters
[56][113].
Due to the combination of physical, chemical, and biological factors, radiosensitization is a complicated phenomenon
[81][114]. The processes by which AuNCs exhibit radiosensitizing effects, particularly the biological pathways involved, are not well understood. In addition, the efficacy of the functionalized modification of AuNCs targeted to tumor tissues when administered in vivo, as well as the harm to healthy tissues and non-specific accumulation of long-term damage to persons, need more research. In the meantime, the increase in the size of AuNCs after different surface modifications reduces the clearance rate in the organism and increases the accumulation in the liver. However, it has not been conclusively determined whether there is an influence on the gene expression of individuals.
In fact, tumor tissues grow at inconsistent rates in all directions with irregular edges. Whether AuNCs can be conformally distributed according to the different shapes of tumors needs to be further investigated. In conclusion, more animal experiments and preclinical trials are needed for the practical translation of AuNCs to ensure that the multifunctional system of AuNCs can be efficiently and safely applied in the clinic.
4.2. Photothermal Conversion
As was mentioned earlier, the excellent photothermal conversion efficiency of AuNCs allows them to be used as ideal photothermal agents for multimodal imaging and therapeutic implementation. Therefore, examples of combined treatment based on the photothermal action of AuNCs will not be repeated in this section.
Focusing on the optimization aspect of the photothermal effect of AuNCs, recently, Yin’s group developed AuNCs as highly efficient photothermal treatment agents and provided a semiquantitative technique for determining their resonant frequency and absorption efficiency by integrating practical medium approximation theory with full-wave electrodynamic simulations. Guided by this theory, they created a space-confined seeded growth approach to prepare AuNCs. Under optimum growth circumstances, they obtained a record photothermal conversion efficiency of 84% for gold-based nanoclusters, due to collective plasmon-coupling-induced near-unity absorption efficiency. They showed the improved exceptional photothermal treatment performance of AuNCs in vivo. Their study shows the potential and effectiveness of AuNCs as nanoscale photothermal treatment agents
[82][115].
Lately, a nanoarchitecture comprising a conjugated gold nanorod (AuNR) and gold cluster hybrid system was developed to optimize the photothermal conversion efficiency. Due to the target specificity of folate receptors for cancer cells, the hybrid material exhibited high in vitro therapeutic efficacy after folic acid conjugation. More importantly, the nanoarchitecture of the hybrid material had no significant influence on the optical and thermal properties of either AuNCs or AuNRs, but exerted enhanced photothermal effects
[83][116].