Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 3 by Beatrix Zheng and Version 4 by Beatrix Zheng.
The development of Phase Change Materials (PCMs) applications and products is closely related to the market penetration of the renewable energy technologies. With the initial aim of matching the phase shift between resource availability and demand in solar energy systems, the range of PCM applications expanded rapidly during the last decades, entering economic sectors where some form of passive thermal regulation was required.
- Phase Change Material
- Latent Heat Storage
- Thermal Energy Storage
- Thermal Regulation
1. Introduction
The development of renewable energy sources, a process which started during the 80s, received incentives from many sides. It is not entirely correct to say that the perspective of conventional fuel exhaustion caused exclusively the massive investment in renewable energy technology development; other technological developments such as solar cells based on polycrystalline technology reaching efficiency values in the range of 47% (a value considered impossible to reach a decade ago) as reported by Geisz [1], or fiber glass reinforced polyester or epoxy for wind turbine blades—Mishnaevsky [2], and generally speaking, the progress in the large area of physics and material science, led to a quantitative and qualitative development of all renewable energy technologies. Unlike conventional energy technology such as fossil fuel, most renewable energy technology has unpredictable availability and variable output. This turns out to be a major issue both in cases of integration into centralized grids and especially in cases of micro grids and distributed generation. In this last case, Energy Systems Integration becomes more complicated in terms of coordinating the operation and planning of sub-systems under reliability and cost-effectiveness principles.
Energy storage technologies have the key role of matching the demand with the supply, thus contributing to peak shaving and downsizing the rated capacity of the generation equipment. The energy storage concept is not new and it has many components. In order to reduce conversion losses (due to inherent irreversibility in the conversion chain) and capital cost with conversion equipment it is preferred to store energy in the form that is required for consumption. Thermal energy storage is just one of the many existing energy storage technologies.
In case of thermal energy storage systems, thermal energy is delivered to a storage system where it is stored for different periods of time and, when the consumer requires it, the stored energy is delivered in the same form (thermal energy). The absence of a conversion process into another form of energy makes the thermal energy storage process highly efficient, with losses incurred by the heat transfer from the storage system to the environment. Thermal storage can be achieved in three main ways:
Sensible heat storage. It consists of storing heat in a liquid or solid medium by means of varying its temperature. The choice of the storage medium depends on the process temperature and the desired capacity. Water is a good choice since it has high specific heat capacity and its vaporization temperature can be increased by increasing the pressure. Variation of the temperature in the case of sensible heat storage results in exergy loss, directly proportional to the temperature variation.
Latent heat storage. During the charging phase, the thermal energy is delivered to a medium that undergoes phase change from solid to liquid. The thermal energy is stored in this case mainly as latent heat with very small variation of the temperature. The exergy loss is minimized in this case and the storage capacity is significantly higher than in the case of sensible storage.
Chemical storage. Chemical thermal energy storage is achieved by means of reversible endotherm/exotherm chemical reactions using a thermochemical material such as silica gel/water, magnesium sulphate/water, lithium bromide/water, lithium chloride/water, and NaOH/water, Kalaiselvam and Parameshwaran [3].
2. PCM Systems Design Considerations
Phase change materials (PCMs) are materials and substances that need a form of containment and integration into the system they will be part of. The PCM systems design depends considerably on their application and PCM type.
The term PCM type covers a diverse classification which includes different classes of materials (paraffin waxes, fatty acids, hydrated salts, eutectics of organic and non-organic compounds and polymers), Pielichowska and Pielichowski [4]. In a very general sense, the term container will be used throughout this section in order to designate the physical system which holds the PCM and prevents the mixing with the other system component, leakage, physical and chemical interactions, and eventually, maintaining the design behaviour throughout the whole lifecycle. This section of the research discusses issues related to the containment forms and designs. Two main containment directions exist: (1) Encapsulation and (2) Form-Stable PCMs, as shown in Figure 1.
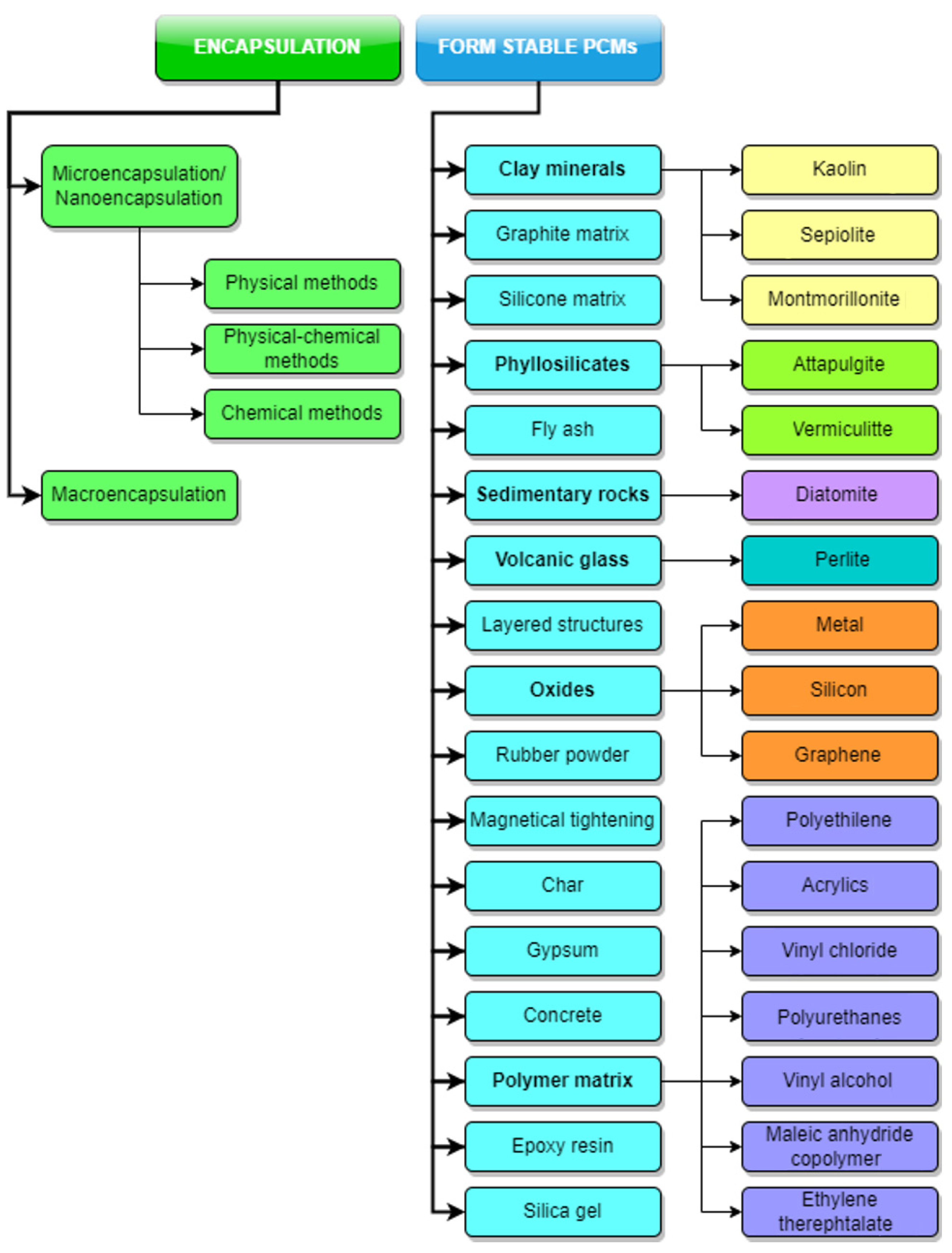
Figure 1. PCM containment methods. Kaolin [5][6]; Sepiolite [7]; Montmorillonite [8]; Attapulgite [9][10]; Vermiculitte [11][12]; Diatomite [11][13][14]; Perlite [15][16]; Metal [17]; Silicon [18]; Graphene [19][20]; Polyethilene [21]; Acrylics [22][23]; Vinyl chloride [24][25]; Polyurethanes [26][27]; Vinly alcohol [28][29]; Maleic anhydride copolymer [30][31][32][33]; Ethylene therephtalate [34].
2.1. PCM Encapsulation
The term encapsulation encompasses a wide range of techniques by which the PCM is contained within an enclosure that isolates it from the environment, preventing the mass exchanges and allowing only the heat exchange with the environment. PCM isolation from the environment has several advantages: the chemical and physical stability of the PCM is better preserved; depending on the geometry and material of the capsule, a higher heat transfer surface can be achieved;
On the other hand, several disadvantages must be mentioned: the capsule can contribute significantly to the overall heat storage system volume, especially in the case of macro-capsules; the capsule wall represents a thermal resistance; if the capsule geometry and material are not properly selected, the heat transfer between PCM and environment is mitigated.
2.2. Form-Stable PCMs
Unlike encapsulation, form-stable PCMs do not require a dedicated container to retain the PCM and prevent leakage and other types of undesired interaction with the environment. In the case of form-stable PCMs, the PCM is not literally contained but through physical and chemical interaction with a matrix material, a composite structure results, in which the PCM stores/releases heat through melting/solidification cycles while the integrity of the structure is preserved. The problem of volume variation during melting/solidification is less critical compared to the encapsulated PCMs. However, leakage can occur during the liquid phase of the PCM if the system is not properly designed.
The PCMs currently used in form-stable combinations are paraffins, fatty acids and their blends, polyethylene glycol, Kenisarin and Kenisarina [26]. Form-stable PCMs have several advantages over encapsulated PCMs: better dynamics of the charging/discharging process, better chemical and physical stability, higher heat transfer area-to-volume ratio. There is much more flexibility and variety in the choice of materials.
A recent review article Gao [35] identified five types of PCMs integration mechanisms into a matrix material: (1) layered packaging, (2) tubular packaging, (3) porous packaging, (4) core–shell packaging and (5) network packaging.
A reference review work reviewing the PCMs/structure material pairs to fabricate form-stable PCMs has been conducted by Kenisarin and Kenisarina [26]. A recent comprehensive review on the mechanisms that contribute to integration of PCMs into the matrix material has been conducted by Zhang et al. [36]. The three main types of mechanisms reported in [36] were covalent bonding, core–shell encapsulation and physical adsorption. Recent studies report the development of new form-stable PCMs: Yin et al. [37] developed a new kind of PCM micro topological structure—polyrotaxane. The structure of polyrotaxane was fully confirmed by 1H nuclear magnetic resonance, attenuated total reflection-Fourier transform infrared and X-ray diffraction. Then, the tensile properties, thermal stability in the air, phase change energy storage and shape memory properties of the films were systematically analysed. The results showed that all the mechanical performance, thermal stability in air and shape memory properties of polyrotaxane family were enhanced significantly compared to those of polyethylene oxide.
Form-stable PCMs are widely used in the building industry as passive elements to increase the heat storage capacity of the building envelope elements or internal partition walls and elements. Integration of various organic PCMs into plaster wallboards to obtain form-stable PCM structures was studied experimentally by Maleki et al. [38]. Nano-capsules containing phase change material n-dodecanol as core and polymethyl methacrylate and copper oxide nanoparticles as shell were synthesized by mini-emulsion polymerization. The physical properties of the form-stable PCM were characterized by using FTIR, SEM, TEM, DSC, TGA and laser particle diameter analyser. The plaster—nano-encapsulated PCM composites were produced using compression moulding. The thermo-physical properties investigated were effective thermal conductivity, latent heat and apparent specific heat. Soo et al. [39] prepared a silicone-octadecane PCM composite by using silicone rubber cured by a two-part system and n-octadecane with chemical purity 98%. Form stability, leakage and mechanical tests were carried out. Leakage tests showed minimal leakage for 50% octadecane loading samples (50Oct-Si) at 2.44%, and the composites eventually retained about 46–48% of the PCM.
The heat transfer process in the case of form-stable PCMs is more complicated than in the case of encapsulated PCMs. For this reason, most studies focus on experiments rather than modelling. Analytical/numerical modelling of the heat transfer in composite PCMs is usually based on simplifying assumptions. Dobri et al. [40] developed a semi-analytical model to describe the transient heat transfer in a PCM composite material consisting of micro-encapsulated paraffin as PCM and gypsum plaster as matrix material. The main simplifying assumptions were: (1) paraffin micro-capsules were spherical and (2) the paraffin micro-capsules are small enough relative to the thickness of the wall, and therefore at each time instant each particle is surrounded by a spatially uniform, time-dependent matrix temperature. Simulation results elucidated the impact of the particle radius and interfacial resistance on the transition at the end of the thermal management phase. Simulations of cyclic environmental temperatures more relevant to building applications demonstrated that PCM volume loadings as low as 5% can reduce the energy demand of a HVAC system by 15 to 20%.
The choice of the PCM impregnation into the support material method depends on the physical characteristics (pore size and pore density) of the support material and the PCM. A literature survey revealed several PCM impregnation methods, as presented in Table 1.
Table 1. PCM impregnation into the support material. Methods and materials.
| Reference | Method | Adsorption Rate | Support | PCM |
|---|---|---|---|---|
| [41] | Vacuum | 85% | Porous carbon | PEG |
| [42] | Sintering | NaCl, KCl, MgCl2 | Eutectic salt chloride | |
| [43] | Adsorption | 81% | Expanded graphite | K2HPO4⋅3H2O– NaH2PO4⋅2H2O–Na2S2O3⋅5H2O–H2O |
| [44] | Adsorption | - | Expanded graphite | KNO3-LiNO3-Ca(NO3)2 |
| [45] | Solution impregnation | 82% | Expanded graphite | LiNO3–KCl, LiNO3–NaNO3 LiNO3–NaCl |
| [18] | Vacuum-assisted melting infiltration | 97% | Graphene oxide | PEG-6000 |
| Hydrothermal reductio | 86% |
References
- Geisz, J.F.; France, R.M.; Schulte, K.L.; Steiner, M.A.; Norman, A.G.; Guthrey, H.L.; Young, M.R.; Song, T.; Moriarty, T. Six-junction III–V solar cells with 47.1% conversion efficiency under 143 Suns concentration. Nat. Energy 2020, 5, 326–335.
- Mishnaevsky, L., Jr.; Branner, K.; Petersen, H.N.; Beauson, J.; McGugan, M.; Sørensen, B.F. Materials for Wind Turbine Blades: An Overview. Materials 2017, 10, 1285.
- Kalaiselvam, S.; Parameshwaran, R. Thermal Energy Storage Technologies for Sustainability. In Systems Design, Assessment and Applications; Academic Press: Cambridge, MA, USA, 2014; ISBN 978-0-12-417291-3.
- Pielichowska, K.; Pielichowski, K. Phase change materials for thermal energy storage. Prog. Mater. Sci. 2014, 65, 67–123.
- Zhang, M.; Cheng, H.; Wang, C.; Zhou, Y. Kaolinite nanotube-stearic acid composite as a form-stable phase change material for thermal energy storage. Appl. Clay Sci. 2021, 201, 105930.
- Jafaripour, M.; Sadrameli, S.; Pahlavanzadeh, H.; Mousavi, S.S. Fabrication and optimization of kaolin/stearic acid composite as a form-stable phase change material for application in the thermal energy storage systems. J. Energy Storage 2021, 33, 102155.
- Xie, N.; Gao, X.; Zhong, Y.; Ye, R.; Chen, S.; Ding, L.; Zhong, T. Enhanced thermal performance of Na2HPO4·12H2O composite phase change material supported by sepiolite fiber for floor radiant heating system. J. Build. Eng. 2022, 56, 104747.
- Gao, N.; Tang, T.; Xiang, H.; Zhang, W.; Li, Y.; Yang, C.; Xia, T.; Liu, X. Preparation and structure-properties of crosslinking organic montmorillonite/polyurethane as solid-solid phase change materials for thermal energy storage. Sol. Energy Mater. Sol. Cells 2022, 244, 111831.
- Wen, R.; Zhu, X.; Yang, C.; Sun, Z.; Zhang, L.; Xu, Y.; Qiao, J.; Wu, X.; Min, X.; Huang, Z. A novel composite phase change material from lauric acid, nano-Cu and attapulgite: Preparation, characterization and thermal conductivity enhancement. J. Energy Storage 2022, 46, 103921.
- Shi, J.; Li, M. Surface modification effects in phase change material-infiltrated attapulgite. Mater. Chem. Phys. 2020, 254, 123521.
- Li, M.; Shi, J. Mechanical and thermal performance assessment of paraffin/expanded vermiculite-diatomite composite phase change materials integrated mortar: Experimental and numerical approach. Sol. Energy 2021, 227, 343–353.
- Gencel, O.; Sarı, A.; Ustaoglu, A.; Hekimoglu, G.; Erdogmus, E.; Yaras, A.; Sutcu, M.; Cay, V.V. Eco-friendly building materials containing micronized expanded vermiculite and phase change material for solar based thermo-regulation applications. Constr. Build. Mater. 2021, 308, 125062.
- Xu, T.; Wu, F.; Zou, T.; Li, J.; Yang, J.; Zhou, X.; Liu, D.; Bie, Y. Development of diatomite-based shape-stabilized composite phase change material for use in floor radiant heating. J. Mol. Liq. 2022, 348, 118372.
- Ren, M.; Zhao, H.; Gao, X. Effect of modified diatomite based shape-stabilized phase change materials on multiphysics characteristics of thermal storage mortar. Energy 2022, 241, 122823.
- Fan, Z.; Zhao, Y.; Ding, Y.; Shi, Y.; Liu, X.; Jiang, D. Fabrication and comprehensive analysis of expanded perlite impregnated with myristic acid-based phase change materials as composite materials for building thermal management. J. Energy Storage 2022, 55, 105710.
- Bian, Y.; Wang, K.; Wang, J.; Yu, Y.; Liu, M.; Lv, Y. Preparation and properties of capric acid: Stearic acid/hydrophobic expanded perlite-aerogel composite phase change materials. Renew. Energy 2021, 179, 1027–1035.
- Chen, X.; Tang, Z.; Liu, P.; Gao, H.; Chang, Y.; Wang, G. Smart Utilization of Multifunctional Metal Oxides in Phase Change Materials. Matter 2020, 3, 708–741.
- Kumar, P.M.; Mylsamy, K. Experimental investigation of solar water heater integrated with a nanocomposite phase change material. J. Therm. Anal. 2019, 136, 121–132.
- Ding, J.; Wu, X.; Shen, X.; Cui, S.; Chen, X. Form-stable phase change material embedded in three-dimensional reduced graphene aerogel with large latent heat for thermal energy management. Appl. Surf. Sci. 2020, 534, 147612.
- Zhao, Y.; Zhang, K.; Min, X.; Xiao, J.; Xu, Z.; Huang, Z.; Liu, Y.; Wu, X.; Fang, M. Graphene aerogel stabilized phase change material for thermal energy storage. Case Stud. Therm. Eng. 2022, 40, 102497.
- Rezaie, A.B.; Montazer, M. Shape-stable thermo-responsive nano Fe3O4/fatty acids/PET composite phase-change material for thermal energy management and saving applications. Appl. Energy 2020, 262.
- Li, Y.; Fu, Z.; Xue, M.; Shao, Z.; Li, Y.; Zhu, Q. Experimental study on preparation and thermal storage properties of expanded graphite/paraffin wax as a shape-stabilized phase change material. Energy Rep. 2022, 8, 324–331.
- Wang, T.; Qiu, X.; Chen, X.; Lu, L.; Zhou, B. Sponge-like form-stable phase change materials with embedded graphene oxide for enhancing the thermal storage efficiency and the temperature response in transport packaging applications. Appl. Energy 2022, 325, 119832.
- Amaral, C.; Gama, N.; Mohseni, F.; Amaral, J.; Marques, P.; Barros-Timmons, A.; Vicente, R. Development of structural layers PVC incorporating phase change materials for thermal energy storage. Appl. Therm. Eng. 2020, 179, 115707.
- Jin, X.; Li, J.; Xue, P.; Jia, M. Preparation and characterization of PVC-based form-stable phase change materials. Sol. Energy Mater. Sol. Cells 2014, 130, 435–441.
- Kenisarin, M.M.; Kenisarina, K.M. Form-stable phase change materials for thermal energy storage. Renew. Sustain. Energy Rev. 2012, 16, 1999–2040.
- Laza, J.M.; Veloso-Fernández, A.; Sanchez-Bodon, J.; Martín, A.; Goitandia, A.M.; Monteserín, C.; Mendibil, X.; Vidal, K.; Lambarri, J.; Aranzabe, E.; et al. Analysis of the influence of microencapsulated phase change materials on the behavior of a new generation of thermo-regulating shape memory polyurethane fibers. Polym. Test. 2022, 116, 107807.
- Zhou, L.; Shi, F.; Liu, G.; Ye, J.P.; Han, P.S.; Zhang, G. Fabrication and characterization of in situ cross-linked electrospun Poly(vinyl alcohol)/phase change material nanofibers. Sol. Energy 2021, 213, 339–349.
- Marske, F.; Dasler, J.; Haupt, C.; Bacia, K.; Hahn, T.; Enke, D. Influence of surfactants and organic polymers on monolithic shape-stabilized phase change materials synthesized via sol-gel route. J. Energy Storage 2022, 49, 104127.
- Sari, A.; Alkan, C.; Karaipekli, A.; Önal, A. Preparation, characterization and thermal properties of styrene maleic anhydride copolymer (SMA)/fatty acid composites as form stable phase change materials. Energy Convers. Manag. 2008, 49, 373–380.
- Liu, L.; Kong, L.; Wang, H.; Niu, R.; Shi, H. Effect of graphene oxide nanoplatelets on the thermal characteristics and shape-stabilized performance of poly(styrene-co-maleic anhydride)-g-octadecanol comb-like polymeric phase change materials. Sol. Energy Mater. Sol. Cells 2016, 149, 40–48.
- Wang, H.; Shi, H.; Qi, M.; Zhang, L.; Zhang, X.; Qi, L. Structure and thermal performance of poly(styrene-co-maleic anhydride)-g-alkyl alcohol comb-like copolymeric phase change materials. Thermochim. Acta 2013, 564, 34–38.
- Sarı, A.; Bicer, A.; Alkan, C. Thermal energy storage characteristics of poly(styrene-co-maleic anhydride)-graft-PEG as polymeric solid–solid phase change materials. Sol. Energy Mater. Sol. Cells 2017, 161, 219–225.
- Chen, C.; Wang, L.; Huang, Y. Ultrafine electrospun fibers based on stearyl stearate/polyethylene terephthalate composite as form stable phase change materials. Chem. Eng. J. 2009, 150, 269–274.
- Gao, D.-C.; Sun, Y.; Fong, A.M.; Gu, X. Mineral-based form-stable phase change materials for thermal energy storage: A state-of-the art review. Energy Storage Mater. 2022, 46, 100–128.
- Zhang, Y.; Jia, Z.; Hai, A.M.; Zhang, S.; Tang, B. Shape-stabilization micromechanisms of form-stable phase change materials-A review. Compos. Part A Appl. Sci. Manuf. 2022, 160, 107047.
- Yin, G.-Z.; Hobson, J.; Duan, Y.; Wang, D.-Y. Polyrotaxane: New generation of sustainable, ultra-flexible, form-stable and smart phase change materials. Energy Storage Mater. 2021, 40, 347–357.
- Maleki, B.; Khadang, A.; Maddah, H.; Alizadeh, M.; Ali Kazemian, A.; Muhamma Ali, H. Development and thermal performance of nano-encapsulated PCM/ plaster wallboard for thermal energy storage in buildings. J. Build. Eng. 2020, 32, 101727.
- Soo, X.Y.D.; Png, Z.M.; Chua, M.H.; Yeo, J.C.C.; Ong, P.J.; Wang, S.; Wang, X.; Suwardi, A.; Cao, J.; Chen, Y.; et al. A highly flexible form-stable silicone-octadecane PCM composite for heat harvesting. Mater. Today Adv. 2022, 14, 100227.
- Dobri, A.; Tsiantis, A.; Papathanasiou, T.; Wang, Y. Investigation of transient heat transfer in multi-scale PCM composites using a semi-analytical model. Int. J. Heat Mass Transf. 2021, 175, 121389.
- Zhao, Y.; Min, X.; Huang, Z.; Liu, Y.; Wu, X.; Fang, M. Honeycomb-like structured biological porous carbon encapsulating PEG: A shape-stable phase change material with enhanced thermal conductivity for thermal energy storage. Energy Build. 2018, 158, 1049–1062.
- Ran, X.; Wang, H.; Zhong, Y.; Zhang, F.; Lin, J.; Zou, H.; Dai, Z.; An, B. Thermal properties of eutectic salts/ceramics/expanded graphite composite phase change materials for high-temperature thermal energy storage. Sol. Energy Mater. Sol. Cells 2021, 225, 111047.
- Xie, N.; Li, Z.; Gao, X.; Fang, Y.; Zhang, Z. Preparation and performance of modified expanded graphite/eutectic salt composite phase change cold storage material. Int. J. Refrig. 2020, 110, 178–186.
- Lu, W.; Liu, G.; Xiong, Z.; Wu, Z.; Zhang, G. An experimental investigation of composite phase change materials of ternary nitrate and expanded graphite for medium-temperature thermal energy storage. Sol. Energy 2020, 195, 573–580.
- Zhong, L.; Zhang, X.; Luan, Y.; Wang, G.; Feng, Y.; Feng, D. Preparation and thermal properties of porous heterogeneous composite phase change materials based on molten salts/expanded graphite. Sol. Energy 2014, 107, 63–73.
More
