Canine inflammatory bowel diseases (IBD) are of increasing interest in veterinary medicine. They refer to complex and debilitating conditions of dogs’ gastrointestinal tract. Although little evidence for causal inferences is currently available, it is believed that IBD pathophysiology entails intricate interactions between environmental factors, the intestinal immune system, and the microbial communities that colonize the gut. To better understand the mechanisms underlying these disorders, leveraging factors associated with the development of these diseases is imperative. Of these factors, emerging evidence supports the role of dietary patterns as key players influencing the composition and function of gut microbes, with subsequent effects on health and disease.
- canine inflammatory bowel disease (IBD)
- diet
- gut microbiota
- holobiont
1. Introduction
2. Gut Microbiota in Canine IBD
Growing evidence suggests that bacteria present in a dog’s gut may play an essential role in its health and disease [28][26]. The gut microbiota of healthy dogs is known to comprise three main phyla: Fusobacterium, Bacteroidetes, and Firmicutes [29][27]. Within this core bacterial community, several taxa are members of the phylum Firmicutes, including bacilli and clostridia, most of which are short-chain fatty acid (SCFA) producers, such as Faecalibacterium spp. [30,31][28][29]. Bacteroidetes is another prominent phylum and includes the genera Bacteroides and Prevotella [32][30]. Similarly, the phylum Fusobacterium has been commonly associated with health in dogs [32][30]. Key roles of the gut microbiota include protecting against pathogens, shaping the immune system, and providing beneficial metabolites to host epithelial cells through fermentative reactions [28][26]. Microbial metabolites may influence host health, gut microbes, and multiple interacting communities, thereby maintaining the holobiont symbiosis [33][31]. They provide other beneficial effects, notably, immunomodulatory, anti-diarrheal and regulatory effects of GI motility [34][32]. Gut microbiota is also involved in the metabolism of bile acids (BA) as potential mediators linking gut bacteria to metabolic and inflammatory disorders [28][26]. Links between gut microbiota composition/function and a myriad of diseases have been widely reported. In fact, it was demonstrated in mice that gut microbiota causes several pathologies, including obesity and dyslipidemia [35,36][33][34]. Evidence suggests that microbial ecosystem imbalance or dysbiosis has been correlated with several inflammatory diseases in dogs, such as IBD [37][35]. Intestinal dysbiosis in dogs with IBD is often characterized by a decrease in bacterial richness and diversity [30][28]. Metagenomic analyses have highlighted a lower abundance of Firmicutes, while Proteobacteria increases in dogs with IBD compared to dogs with a healthy status [38][36]. The abundance of Faecalibacterium spp. and Fusobacterium spp. were also significantly decreased in dogs with IBD relative to healthy controls [39][37]. In addition, higher abundances of adherent and invasive Escherichia coli (AIEC) were noted in colonic biopsies from dogs with granulomatous colitis, thus highlighting a potential link with gut inflammation [40][38]. Metabolic alterations have also been reported, including impaired short chain fatty acids (SCFAs) and tryptophan metabolites production, which may influence intestinal homeostasis and immunological tolerance [41,42][39][40]. SCFAs (i.e., acetate, propionate and butyrate) are the main end products of intestinal bacterial fermentation of non-digestible food components, such as dietary fiber. Lower levels of acetate and propionate were detected in fecal samples from dogs with IBD compared to healthy subjects [43][41]. These SCFAs are known to hold therapeutic promises in IBD as they improve epithelial barrier integrity and alleviate gut inflammation in vivo. In addition to SCFAs deficiency, an altered BA metabolism has been demonstrated in canine IBD [44][42]. The conversion of primary BA to secondary BA is largely known to be achieved by gut microbes. BA play key roles in the emulsification and absorption of dietary lipids and serve as potent signaling molecules that act through the farnesoid X receptor (FXR) and Takeda G-protein-coupled receptor 5 (TGR5). By activating FXR and TGR5, BA can influence a variety of processes, including inflammation and lipid, glucose and energy metabolism. Accordingly, changes in gut bacterial populations have been suggested to influence inflammatory parameters and pathways through changes in BA metabolism [28,45][26][43]. In dogs with IBD, the decrease in the abundance of Clostridium hiranonis, a potent BA converter, is correlated with the alteration of the BA metabolism. Conversely, treatment of intestinal inflammation is accompanied by an increase in the abundance of C. hiranonis and a normalization of the BA metabolism [39][37]. The links between dietary interventions, SCFAs, BA metabolism and canine inflammation are yet to be explored. Similarly, the relevance of BA as potential therapeutic targets in dogs would need to be thoroughly addressed as investigations related to this field are still in their infancy.3. Diet-Microbiota Interactions in Canine IBD
3.1. Dietary Proteins
Diets with high protein levels were associated with a modification of the gut microbiota composition in healthy beagles, mainly characterized by an increase in the genus Lactobacillus abundance. This change was linked to high concentrations of butyrate in dogs that were fed a high-protein diet [46][44]. Furthermore, a high protein diet has been shown to promote the growth of Clostridium perfringens and to reduce the abundance of Clostridium cluster XIVa (also known as the Clostridium coccoides group) in a similar population of healthy dogs [47][45]. Although the findings from these studies suggest that high-protein diets exert significant effects on the canine gut community, as they elicit the growth of select Clostridium species, a major limitation of such trials is the relatively small size of the studied cohort, limited to only twelve and nine Beagles, respectively [46,47][44][45]. Other significant differences were observed in the microbiota composition, with a higher Firmicutes:Bacteroidetes ratio in response to a high protein-low carbohydrate (HPLC) diet when compared with a low protein-high carbohydrate (LPHC) diet. Several taxa became detectable in response to diet, such as Lactobacillus ruminis, which was detected in 59% of LPHC-fed dogs [48][46]. In another study, the fecal microbiota of dogs fed a HPLC diet showed an increased abundance of Bacteroidetes in addition to an enrichment in the phylum Firmicutes [49][47].3.2. Dietary Tryptophan and Indole Derivatives
In humans with IBD, reduced availability of tryptophan or tryptophan metabolites has been suggested to contribute to the disease [51,52][48][49]. Tryptophan represents a precursor of several microbial and host metabolites, including serotonin and vitamin B3 [53][50]. Tryptophan metabolites are known as one of the most important endogenous ligands of the aryl hydrocarbon receptor (AhR), a nuclear protein involved in the regulation of gene expression and in maintaining intestinal homeostasis [54][51]. Microbial metabolites or dietary factors may influence this pathway. Dogs with IBD and dogs with protein losing enteropathy have also been shown to exhibit lower plasma tryptophan levels than healthy dogs [55,56][52][53]. While these studies highlight a potential role of tryptophan in dogs with IBD and protein losing enteropathy, the small cohort size (10 dogs) in the IBD study and the retrospective study design for the protein losing enteropathy pathology represent major limitations. Further prospective studies with larger cohorts are needed. Further analysis of dog’s gut microbiota would be beneficial to such studies as the link between the decrease in the plasma concentration of tryptophan and dysbiosis is not yet established. In addition to the functional abnormality of the intestinal microbiota, an absorption defect linked to intestinal inflammation could also be involved.3.3. Dietary Fibers and SCFAs
Fibers can be defined as non-digestible carbohydrates that come from plants. They can be classified according to their solubility or fermentability. Soluble or fermentable fibers, such as pectin, gum Arabic, and fructooligosaccharides, support normal GI microflora growth and provide fuel for colonocytes. Several human studies showed that they also delay gastric emptying and inhibit absorption in the small intestine [33][31]. Insoluble fibers, such as cellulose and oat fiber, were shown to increase the volume and water content of stools, to absorb toxins and to normalize colonic motility [33][31]. SCFAs, including butyrate, acetate and propionate, are well-studied microbial metabolites primarily produced by the bacterial fermentation of non-digestible dietary fibers. Thus far, most human clinical trials investigating the anti-inflammatory effects of dietary fibers have been linked with a higher luminal production of SCFAs following the intake of high-fiber foods [57,58][54][55]. It is well demonstrated that SCFAs not only contribute to the regulation of the mucosal barrier function but also provide immune regulatory functions [33][31]. In addition, their production provides an acidic luminal environment that inhibits the proliferation of pH-sensitive pathogenic bacteria such as Enterobacteriaceae [59][56]. Furthermore, in human studies, SCFAs are likely to modulate inflammation by increasing the production of anti-inflammatory cytokines, decreasing pro-inflammatory cytokines, and activating the transcription factor Foxp3 [60][57]. Studies applied to dogs in this regard are still in their infancy and few reports have explored the role of fiber-enriched diets in canine IBD. Interestingly, the intake of high-fiber diets has recently been shown to alleviate acute large-bowel diarrhea and to exhibit significant clinical benefits in dogs [61][58] (Figure 1). However, the use of antibiotic and antiparasitic treatments and the absence of microbiota analysis are important limitations in this sentudry. More controlled studies are therefore required to confirm these effects. Further metagenomic analysis of dogs’ gut microbiomes would shed light on the functional potential of this community, and provide mechanistic knowledge linking dietary fiber, gut microbiota, and the treatment of canine IBD.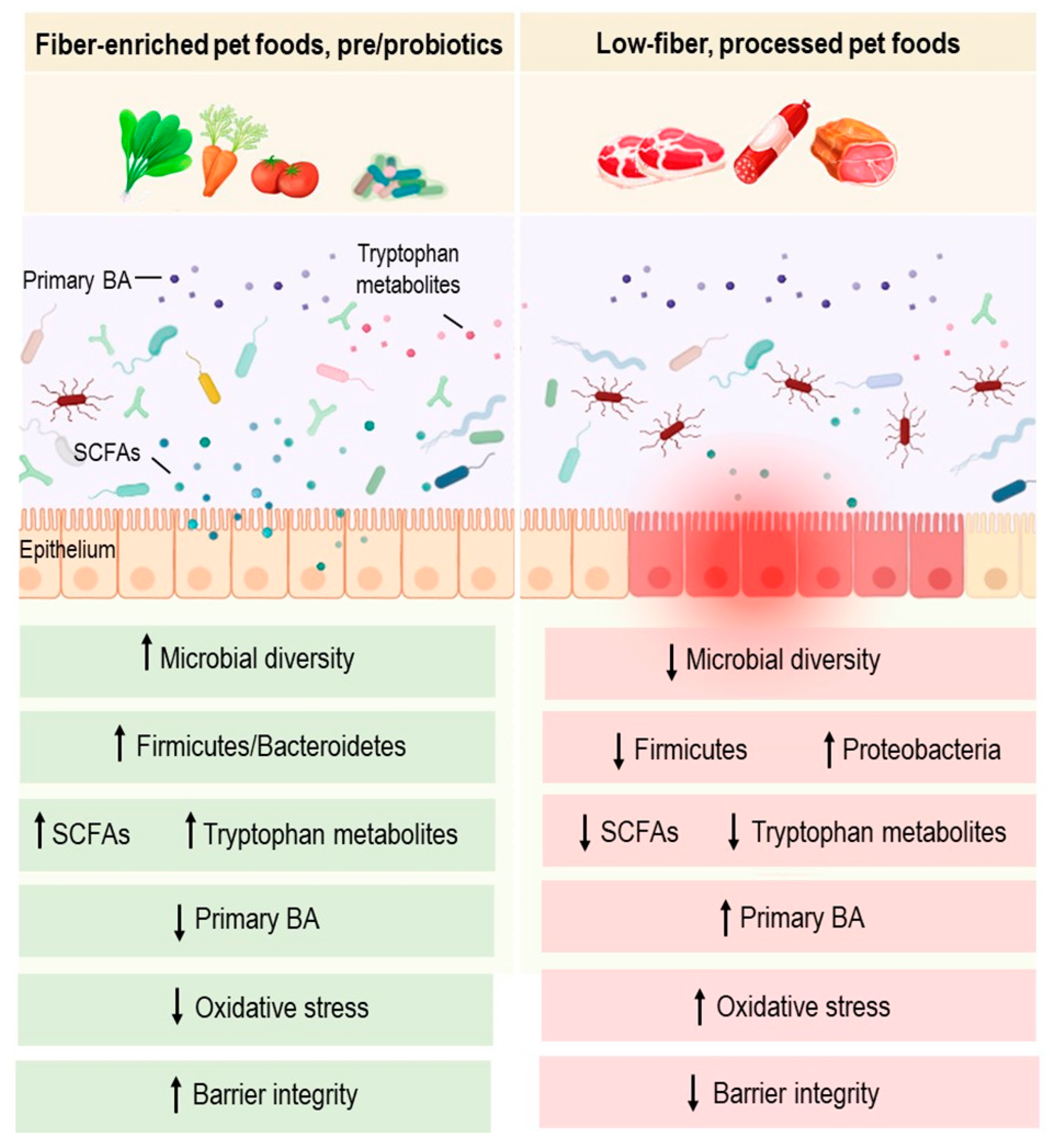
References
- Vindigni, S.M.; Zisman, T.L.; Suskind, D.L.; Damman, C.J. The intestinal microbiome, barrier function, and immune system in inflammatory bowel disease: A tripartite pathophysiological circuit with implications for new therapeutic directions. Therap. Adv. Gastroenterol. 2016, 9, 606–625.
- Nishida, A.; Inoue, R.; Inatomi, O.; Bamba, S.; Naito, Y.; Andoh, A. Gut microbiota in the pathogenesis of inflammatory bowel disease. Clin. J. Gastroenterol. 2018, 11, 1–10.
- Simpson, J.M.; Martineau, B.; Jones, W.E.; Ballam, J.M.; Mackie, R.I. Characterization of fecal bacterial populations in canines: Effects of age, breed and dietary fiber. Microb. Ecol. 2002, 44, 186–197.
- Vanhoutte, T.; Huys, G.; De Brandt, E.; Fahey, G.C., Jr.; Swings, J. Molecular monitoring and characterization of the faecal microbiota of healthy dogs during fructan supplementation. FEMS Microbiol. Lett. 2005, 249, 65–71.
- Biagi, G.; Cipollini, I.; Grandi, M.; Zaghini, G. Influence of some potential prebiotics and fibre-rich foodstuffs on composition and activity of canine intestinal microbiota. Anim. Feed Sci. Technol. 2010, 159, 50–58.
- Beloshapka, A.N.; Dowd, S.E.; Suchodolski, J.S.; Steiner, J.M.; Duclos, L.; Swanson, K.S. Fecal microbial communities of healthy adult dogs fed raw meat-based diets with or without inulin or yeast cell wall extracts as assessed by 454 pyrosequencing. FEMS Microbiol. Ecol. 2013, 84, 532–541.
- Panasevich, M.R.; Kerr, K.R.; Dilger, R.N.; Fahey, G.C., Jr.; Guérin-Deremaux, L.; Lynch, G.L.; Wils, D.; Suchodolski, J.S.; Steer, J.M.; Dowd, S.E.; et al. Modulation of the faecal microbiome of healthy adult dogs by inclusion of potato fibre in the diet. Br. J. Nutr. 2015, 113, 125–133.
- Suchodolski, J.S.; Markel, M.E.; Garcia-Mazcorro, J.F.; Unterer, S.; Heilmann, R.M.; Dowd, S.E.; Kachroo, P.; Ivanov, I.; Minamoto, Y.; Dillman, E.M.; et al. The fecal microbiome in dogs with acute diarrhea and idiopathic inflammatory bowel disease. PLoS ONE 2012, 7, e51907.
- Honneffer, J.B.; Minamoto, Y.; Suchodolski, J.S. Microbiota alterations in acute and chronic gastrointestinal inflammation of cats and dogs. World J. Gastroenterol. 2014, 20, 16489–16497.
- Minamoto, Y.; Otoni, C.C.; Steelman, S.M.; Büyükleblebici, O.; Steiner, J.M.; Jergens, A.E.; Suchodolski, J.S. Alteration of the fecal microbiota and serum metabolite profiles in dogs with idiopathic inflammatory bowel disease. Gut Microbes 2015, 6, 33–47.
- AlShawaqfeh, M.K.; Wajid, B.; Minamoto, Y.; Markel, M.; Lidbury, J.A.; Steiner, J.M.; Serpedin, E.; Suchodolski, J.S. A dysbiosis index to assess microbial changes in fecal samples of dogs with chronic inflammatory enteropathy. FEMS Microbiol. Ecol. 2017, 93, fix136.
- Marchesi, M.C.; Timpano, C.C.; Busechian, S.; Pieramati, C.; Rueca, F. The role of diet in managing inflamatory bowel disease affected dogs: A retrospective cohort study on 76 cases. Vet. Ital. 2017, 53, 297–302.
- Hou, J.K.; Abraham, B.; El-Serag, H. Dietary intake and risk of developing inflammatory bowel disease: A systematic review of the literature. Am. J. Gastroenterol. 2011, 106, 563–573.
- Chassaing, B.; Koren, O.; Goodrich, J.K.; Poole, A.C.; Srinivasan, S.; Ley, R.E.; Gewirtz, A.T. Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature 2015, 519, 92–96.
- Desai, M.S.; Seekatz, A.M.; Koropatkin, N.M.; Kamada, N.; Hickey, C.A.; Wolter, M.; Pudlo, N.A.; Kitamoto, S.; Terrapon, N.; Muller, A.; et al. A Dietary Fiber-Deprived Gut Microbiota Degrades the Colonic Mucus Barrier and Enhances Pathogen Susceptibility. Cell 2016, 167, 1339–1353.e21.
- Köhler, I.; Ballhausen, B.D.; Stockhaus, C.; Hartmann, K.; Wehner, A. Prevalence of and risk factors for feline hyperthyroidism among a clinic population in Southern Germany. Tierärztliche Prax. Kleintiere 2016, 44, 149–157.
- Alexander, P.; Berri, A.; Moran, D.; Reay, D.; Rounsevell, M.D.A. The global environmental paw print of pet food. Glob. Environ. Chang. 2020, 65, 102153.
- Valdés, F.; Villanueva, V.; Durán, E.; Campos, F.; Avendaño, C.; Sánchez, M.; Domingoz-Araujo, C.; Valenzuela, C. Insects as Feed for Companion and Exotic Pets: A Current Trend. Animals 2022, 12, 1450.
- Reilly, L.M.; He, F.; Rodriguez-Zas, S.L.; Southey, B.R.; Hoke, J.M.; Davenport, G.M.; de Godoy, M.R.C. Use of Legumes and Yeast as Novel Dietary Protein Sources in Extruded Canine Diets. Front. Vet. Sci. 2021, 8, 667642.
- Fritsch, D.A.; Jackson, M.I.; Wernimont, S.M.; Feld, G.K.; MacLeay, J.M.; Brejda, J.J.; Cochrane, C.Y.; Gross, K.L. Microbiome function underpins the efficacy of a fiber-supplemented dietary intervention in dogs with chronic large bowel diarrhea. BMC Vet. Res. 2022, 18, 245.
- Lee, A.H.; Lin, C.Y.; Do, S.; Oba, P.M.; Belchik, S.E.; Steelman, A.J.; Schauwecker, A.; Swanson, K.S. Dietary supplementation with fiber, “biotics,” and spray-dried plasma affects apparent total tract macronutrient digestibility and the fecal characteristics, fecal microbiota, and immune function of adult dogs. J. Anim. Sci. 2022, 100, skac048.
- Kc, D.; Sumner, R.; Lippmann, S. Gut microbiota and health. Postgrad. Med. 2020, 132, 274.
- Fan, Y.; Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2021, 19, 55–71.
- Muegge, B.D.; Kuczynski, J.; Knights, D.; Clemente, J.C.; González, A.; Fontana, L.; Henrissat, B.; Knight, R.; Gordon, J.I. Diet drives convergence in gut microbiome functions across mammalian phylogeny and within humans. Science 2011, 332, 970–974.
- Zmora, N.; Suez, J.; Elinav, E. You are what you eat: Diet, health and the gut microbiota. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 35–56.
- Blake, A.B.; Guard, B.C.; Honneffer, J.B.; Lidbury, J.A.; Steiner, J.M.; Suchodolski, J.S. Altered microbiota, fecal lactate, and fecal bile acids in dogs with gastrointestinal disease. PLoS ONE 2019, 14, e0224454.
- Hand, D.; Wallis, C.; Colyer, A.; Penn, C.W. Pyrosequencing the canine faecal microbiota: Breadth and depth of biodiversity. PLoS ONE 2013, 8, e53115.
- Garcia-Mazcorro, J.F.; Lanerie, D.J.; Dowd, S.E.; Paddock, C.G.; Grützner, N.; Steiner, J.M.; Ivanek, R.; Suchodolski, J.S. Effect of a multi-species synbiotic formulation on fecal bacterial microbiota of healthy cats and dogs as evaluated by pyrosequencing. FEMS Microbiol. Ecol. 2011, 78, 542–554.
- Garcia-Mazcorro, J.F.; Dowd, S.E.; Poulsen, J.; Steiner, J.M.; Suchodolski, J.S. Abundance and short-term temporal variability of fecal microbiota in healthy dogs. Microbiologyopen 2012, 1, 340–347.
- Vázquez-Baeza, Y.; Hyde, E.R.; Suchodolski, J.S.; Knight, R. Dog and human inflammatory bowel disease rely on overlapping yet distinct dysbiosis networks. Nat. Microbiol. 2016, 1, 16177.
- Postler, T.S.; Ghosh, S. Understanding the Holobiont: How Microbial Metabolites Affect Human Health and Shape the Immune System. Cell Metab. 2017, 26, 110–130.
- Minamoto, Y.; Minamoto, T.; Isaiah, A.; Sattasathuchana, P.; Buono, A.; Rangachari, V.R.; McNeely, I.H.; Lidbury, J.; Steiner, J.M.; Suchodolski, J.S. Fecal short-chain fatty acid concentrations and dysbiosis in dogs with chronic enteropathy. J. Vet. Intern. Med. 2019, 33, 1608–1618.
- Ridaura, V.K.; Faith, J.J.; Rey, F.E.; Cheng, J.; Duncan, A.E.; Kau, A.L.; Griffin, N.W.; Lombard, V.; Henrissat, B.; Bain, J.R.; et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science 2013, 341, 1241214.
- Le Roy, T.; Lécuyer, E.; Chassaing, B.; Rhimi, M.; Lhomme, M.; Boudebbouze, S.; Ichou, F.; Haro Barceló, J.; Huby, T.; Guerin, M.; et al. The intestinal microbiota regulates host cholesterol homeostasis. BMC Biol. 2019, 17, 94.
- Schmitz, S.; Glanemann, B.; Garden, O.A.; Brooks, H.; Chang, Y.M.; Werling, D.; Allenspach, K. A prospective, randomized, blinded, placebo-controlled pilot study on the effect of Enterococcus faecium on clinical activity and intestinal gene expression in canine food-responsive chronic enteropathy. J. Vet. Intern. Med. 2015, 29, 533–543.
- Suchodolski, J.S.; Ruaux, C.G.; Steiner, J.M.; Fetz, K.; Williams, D.A. Assessment of the qualitative variation in bacterial microflora among compartments of the intestinal tract of dogs by use of a molecular fingerprinting technique. Am. J. Vet. Res. 2005, 66, 1556–1562.
- Guard, B.C.; Honneffer, J.B.; Jergens, A.E.; Jonika, M.M.; Toresson, L.; Lawrence, Y.A.; Webb, C.B.; Hill, S.; Lidbury, J.A.; Steiner, J.M.; et al. Longitudinal assessment of microbial dysbiosis, fecal unconjugated bile acid concentrations, and disease activity in dogs with steroid-responsive chronic inflammatory enteropathy. J. Vet. Intern. Med. 2019, 33, 1295–1305.
- Simpson, K.W.; Dogan, B.; Rishniw, M.; Goldstein, R.E.; Klaessig, S.; McDonough, P.L.; German, A.J.; Yates, R.M.; Russell, D.G.; Johnson, S.E.; et al. Adherent and invasive Escherichia coli is associated with granulomatous colitis in boxer dogs. Infect. Immun. 2006, 74, 4778–4792.
- Suchodolski, J.S.; Camacho, J.; Steiner, J.M. Analysis of bacterial diversity in the canine duodenum, jejunum, ileum, and colon by comparative 16S rRNA gene analysis. FEMS Microbiol. Ecol. 2008, 66, 567–578.
- Vich Vila, A.; Imhann, F.; Collij, V.; Jankipersadsing, S.A.; Gurry, T.; Mujagic, Z.; Kurilshikov, A.; Bonder, M.J.; Jiang, X.; Tigchelaar, E.F.; et al. Gut microbiota composition and functional changes in inflammatory bowel disease and irritable bowel syndrome. Sci. Transl. Med. 2018, 10, eaap8914.
- Derrien, M.; Alvarez, A.S.; de Vos, W.M. The Gut Microbiota in the First Decade of Life. Trends Microbiol. 2019, 27, 997–1010.
- Xenoulis, P.G.; Palculict, B.; Allenspach, K.; Steiner, J.M.; Van House, A.M.; Suchodolski, J.S. Molecular-phylogenetic characterization of microbial communities imbalances in the small intestine of dogs with inflammatory bowel disease. FEMS Microbiol. Ecol. 2008, 66, 579–589.
- Suchodolski, J.S. Diagnosis and interpretation of intestinal dysbiosis in dogs and cats. Vet. J. 2016, 215, 30–37.
- Xu, J.; Verbrugghe, A.; Lourenço, M.; Cools, A.; Liu, D.J.X.; Van de Wiele, T.; Marzorati, M.; Eeckhaut, V.; Van Immerseel, F.; Vanhaecke, L.; et al. The response of canine faecal microbiota to increased dietary protein is influenced by body condition. BMC Vet. Res. 2017, 13, 374.
- Zentek, J.; Marquart, B.; Pietrzak, T.; Ballèvre, O.; Rochat, F. Dietary effects on bifidobacteria and Clostridium perfringens in the canine intestinal tract. J. Anim. Physiol. Anim. Nutr. 2003, 87, 397–407.
- Coelho, L.P.; Kultima, J.R.; Costea, P.I.; Fournier, C.; Pan, Y.; Czarnecki-Maulden, G.; Hayward, M.R.; Forslund, S.K.; Schmidt, T.S.B.; Descombes, P.; et al. Similarity of the dog and human gut microbiomes in gene content and response to diet. Microbiome 2018, 6, 72.
- Martínez-López, L.M.; Pepper, A.; Pilla, R.; Woodward, A.P.; Suchodolski, J.S.; Mansfield, C. Effect of sequentially fed high protein, hydrolyzed protein, and high fiber diets on the fecal microbiota of healthy dogs: A cross-over study. Anim. Microbiome 2021, 3, 42.
- Sugihara, K.; Morhardt, T.L.; Kamada, N. The Role of Dietary Nutrients in Inflammatory Bowel Disease. Front. Immunol. 2019, 9, 3183.
- Heinken, A.; Hertel, J.; Thiele, I. Metabolic modelling reveals broad changes in gut microbial metabolism in inflammatory bowel disease patients with dysbiosis. NPJ Syst. Biol. Appl. 2021, 7, 19.
- Alkhalaf, L.M.; Ryan, K.S. Biosynthetic manipulation of tryptophan in bacteria: Pathways and mechanisms. Chem. Biol. 2015, 22, 317–328.
- Ghiboub, M.; Verburgt, C.M.; Sovran, B.; Benninga, M.A.; de Jonge, W.J.; Van Limbergen, J.E. Nutritional Therapy to Modulate Tryptophan Metabolism and Aryl Hydrocarbon-Receptor Signaling Activation in Human Diseases. Nutrients 2020, 12, 2846.
- Kathrani, A.; Allenspach, K.; Fascetti, A.J.; Larsen, J.A.; Hall, E.J. Alterations in serum amino acid concentrations in dogs with protein-losing enteropathy. J. Vet. Intern. Med. 2018, 32, 1026–1032.
- Tamura, Y.; Ohta, H.; Kagawa, Y.; Osuga, T.; Morishita, K.; Sasaki, N.; Takiguchi, M. Plasma amino acid profiles in dogs with inflammatory bowel disease. J. Vet. Intern. Med. 2019, 33, 1602–1607.
- Joossens, M.; De Preter, V.; Ballet, V.; Verbeke, K.; Rutgeerts, P.; Vermeire, S. Effect of oligofructose-enriched inulin (OF-IN) on bacterial composition and disease activity of patients with Crohn’s disease: Results from a double-blinded randomised controlled trial. Gut 2012, 61, 958.
- Welters, C.F.; Heineman, E.; Thunnissen, F.B.; van den Bogaard, A.E.; Soeters, P.B.; Baeten, C.G. Effect of dietary inulin supplementation on inflammation of pouch mucosa in patients with an ileal pouch-anal anastomosis. Dis. Colon Rectum 2002, 45, 621–627.
- Sun, Y.; O’Riordan, M.X. Regulation of bacterial pathogenesis by intestinal short-chain Fatty acids. Adv. Appl. Microbiol. 2013, 85, 93–118.
- Smith, P.M.; Howitt, M.R.; Panikov, N.; Michaud, M.; Gallini, C.A.; Bohlooly-Y, M.; Glickman, J.N.; Garrett, W.S. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 2013, 341, 569–573.
- Lappin, M.R.; Zug, A.; Hovenga, C.; Gagne, J.; Cross, E. Efficacy of feeding a diet containing a high concentration of mixed fiber sources for management of acute large bowel diarrhea in dogs in shelters. J. Vet. Intern. Med. 2022, 36, 488–492.
