ThisDNA entry is based on our recently published review paper: "Programmed DNA Damage and Physiological DSBs:
Mdouble-strand breaks (DSBs) are well known for their deleterious effects. Improper repair of these breaks can result in mutations, translocations appind eveng, Biological Significance and Perturbations in Disease States" (doi:10.3390/cells9081870)
by: loss of genetic material, which can later lead to tumor formation and cancer progression. There Sara Oster 1 and Rami I. Aqeilan 1,2,*
1e many exogenous agents that can cause DSBs. DSBs can also Themerge Concern Founddue to replication Laboratories, The Lautenberg Center for Immunology and Cancer Research,
Depstress activated by inhibition of DNA synthesis and/or activartment of Immunology and Cancer Research-IMRIC, Hebrew University-Hadassah Medical School,
Jerion of oncogenes. This review aims to summarize what is known aboust DNA damalem 9112001, Israel; sara.oster@mail.huji.ac.ige in its physiological
2 Deparcontment of Cancer Biology and Genetics, Wexner Medical Center, The Ohio State University,
Cext. In addition, we will examine the advancements of the past several years, which have made an impact on the study olumbus,f OH 43210, USA
* Cgenorrmespondence: ramiaq@ekmd.huji.ac.il landscape and its organization.
- physiological DSBs
- DNA repair
- meiosis
- transcription
- BCR
- chromosomal translocations
- NGS
1. Introduction
2. Programmed DSBs and Mechanisms of Repair
2.1. Meiosis
1.1. Meiosis
A process that requires DSBs for its execution is meiosis, in which gametes undergo two cell divisions, forming haploid cells. During the prophase I stage of meiosis, homologous chromosomes undergo recombination, allowing genes to ‘cross-over’ and exchange in order to achieve accurate segregation of homologs and gene diversification in the next generation. In this section, we discuss the mechanism of programmed breaks occurring via SPO11, and repair executed using Homologous Recombination (HR).
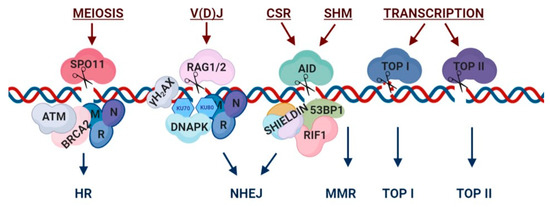
Programmed DNA breaks: nucleases, participating factors and repair pathways. Representative mechanistic view of programmed DNA breaks. During meiosis, SPO11 induces double-strand breaks (DSBs), leading to the recruitment of homologous recombination (HR) repair factors such as ATM, BRCA2 and the MRN complex. DSBs for V(D)J recombination are induced by RAG and are repaired via NHEJ, following γH2AX signaling and the recruitment of the MRN complex and DNA-PKc complex factors. The process of B-Cell Receptor (BCR) diversification including class switch recombination (CSR) and somatic hypermutation (SHM) are initiated by Activation-Induced Cytidine Deaminase (AID). CSR breaks lead to the recruitment of 53BP1, RIF1 and the shieldin complex, which drive repair via NHEJ. SHM breaks are repaired through mismatch-repair (MMR). Transcriptionally induced breaks can be either single- or double-strand breaks and are activated via topoisomerase I (TOP I) or topoisomerase II (TOP II), respectively. This illustration is a simplified version of highly complex break and repair mechanisms. Figure was generated using BioRender tool.
2.2. V(D)J Recombination
1.2. V(D)J Recombination
During lymphocyte development, T and B lymphocytes undergo a process called V(D)J recombination. The purpose of this process is to create diversity in the antigen receptor (TCR or BCR) genes, by creating breaks at specific sequences, which contain V, D or J coding segments. By utilizing DSBs and repair at the signal sites of the receptor genes, V(D)J recombination diversifies the repertoire of T cell receptors (TCR) in T lymphocytes as well as immunoglobulins (Ig) in B lymphocytes and thus allows for an enhanced ability to recognize a large range of pathogens and antigens. This section explains the process of RAG-induced breaks, as well as repair via non-homologous end joining (NHEJ).
2.3. Class-Switch Recombination (CSR)
1.3. Class-Switch Recombination (CSR)
Class-switch recombination (CSR) is a process that occurs in mature stimulated B-cells. During CSR, the constant immunoglobulin (Ig) heavy chain genes are broken and then recombined to allow deletion and exchange of the effector Ig gene. At the end of the process, only one of the Ig heavy-chain genes will be expressed in the antibody, which will define the function and capabilities of the antibodies the B-cell produces. In addition, mature B cells undergo somatic hypermutation (SHM). This process allows for variability of the Ig at the antigen binding area named ‘affinity maturation’, creating a large variety of antibodies. This section demonstrates the mechanism of AID-induced breaks and subsequent repair using NHEJ for CSR or mismatch repair (MMR) for SHM.
2.4. Replication and Transcription
1.4. Replication and Transcription
Processes that require the opening and separation of the double-stranded DNA, such as replication and transcription, face a significant amount of torsional tension due to the supercoiled state of the DNA. In order to overcome this obstacle, cells express several topoisomerase genes with the purpose of breaking the DNA and subsequently looping it around itself to release the tension. In this section, we describe how TOP1 and TOP2 each induce breakage and repair in differing mechanisms. In addition, we examine the roles of topoisomerase function in transcription, gene expression, formation of R-loops and maintenance of genome integrity during replication.
32.Mapping of DSBs by Next Generation Sequencing
The growing functional significance in physiological DSBs and programmed DNA damage has been further developed and improved due to the development of new methods and technologies, which utilize sequencing capabilities allowing, for the first time, a glimpse at the break pattern of cells, as it appears across the genome. Due to advancement in next-generation sequencing (NGS) this has recently become possible. NGS has revolutionized the landscape of genetic research by allowing for millions of strands to be simultaneously sequenced by the means of cell-free library preparation, making the process more effective and comprehensive. The range of applications for NGS in research and in diagnostics is boundless. In this section, we comprehensively introduce many of the methods that have allowed for the mapping of DNA break sites across the genome: ChIP-seq, BLESS, BLISS, DSBCapture, END-seq, HTGTS, GRO-seq, OxiDIP-seq, GLOE-seq and Break-seq.
43. New Insights on Misrepair of Physiological DSBs in Cancer Cells
Although DSBs and repair exist as part of the cell’s internal programming, aberrations in many of the factors involved can lead to tumor initiation and progression. It is not surprising, if so, to learn that many of these abnormalities arise due to changes in the programmed processes mentioned in earlier sections, leading to incorrect breakage and repair of the DNA. Programmed DNA damage and breaks that are incorrectly repaired or fused to the wrong break site can give rise to translocations, losses and inversions. All of these are potential drivers of many malignancies, as exemplified in this section.
