Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Alisa Gisina and Version 3 by Conner Chen.
Cancer stem cells (CSCs) present in many tumors are an example of intratumoral heterogeneity of great importance. The original concept of CSCs emerged on the basis of the stem cell theory in its original form and on the reports about the exclusive ability of tumor cells expressing stem cell markers to induce tumor growth.The modern concept of CSCs has been updated by introducing the concept of the cells-of-origin of tumors and according to data obtained by genetic analysis combined with CSC-associated marker profiling and lineage tracing analysis.
- intratumoral heterogeneity
- cancer stem cell
- cancer-initiating cell
- cancer stem-like cell
- clonal evolution
- hierarchical model of carcinogenesis
- stochastic model of carcinogenesis
1. Introduction. Intratumoral Heterogeneity as the Reason for Failures in the Development of Effective Treatments.
Introduction. Intratumoral heterogeneity as the reason for failures in the development of effective treatments. At present time, along with optimizing the treatment protocols based on well-known therapeutics, the development and clinical trials of targeted drugs and various types of cancer immunotherapy are underway. However, for a large number of patients, the effectiveness of the applied treatment still remains low. Tumors are characterized by the inter-tumoral and intratumoral heterogeneity, which may be the reason for failures in the development of effective treatments. Malignant tumors comprise different cell types, such as cancer cells, stromal cells, endothelial cells and immune cells [1][25]. In addition, cancer cells actively proliferate, accumulate mutations and form genetically different clones contributing to the enhancement of tumor cell diversity [2][26]. Moreover, under the influence of stimuli coming from the microenvironment, epigenetic changes occur in individual cancer cells [3][4][5][27,28,29]. All this produces a vast heterogeneity of tumor cells, specific for each patient and even for each site of tumor growth [6][7][8][30,31,32]. This is an important practical aspect, since it causes the difference in the clinical manifestation of cancer in different patients. Obviously, the prognosis of the disease progression depends on the presence or absence of drug-resistant or metastasizing tumor cells [9][10][11][12][33,34,35,36]. The actuality of cancer stem cells (CSCs) is one of the manifestations of the intratumoral heterogeneity of great importance. These cells exhibit some phenotypic and functional properties of normal stem cells of the same tissue. It has been hypothesized that CSCs are responsible for tumor formation and progression [13][14][19,20].2. History of the Tterm and Mmodern Vview of the Ccancer Stem Cell Cstem cell concept.
In experiments using cell-sorting technologies (flow cytometry, magnetic separation) in combination with the in vivo cell transplantation to the immunodeficient animals, it was found that cells isolated from the same tumor differ by their tumor-initiating capacity [15][16][17][18][19][20][21][22][23][24][25][37,38,39,40,41,42,43,44,45,46,47]. The most tumorigenic were subpopulations of tumor cells expressing the markers of normal resident stem cells. The term “cancer stem cells” came into use to designate this kind of cell. Alternatively, some researchers use the term “cancer stem-like cells”. The presence of cancer stem cells (CSCs) was shown initially in hematological [15][17][37,39], and later in solid malignancies [14][26][20,21]. According to the hierarchical theory of oncogenesis, the tumor originates from a mutated stem cell and has a hierarchical cell composition [13][16][19,38]. Cancer stem cells are at the top of the hierarchy, and all other tumor cells are their differentiated progeny (see Figure 1A). Over time, evidence supporting the existence of a subpopulation of cancer cells with stem cell features has accumulated . However, the hierarchical theory did not find determinate confirmation and has been reconsidered [27][54]. It is likely that tumors combine the following features: genetic mosaicism manifested by the presence of clones with the ability to invade, metastasize and/or resist to drugs, epigenetic changes in cancer cells under the influence of the tumor microenvironment and the existence of subpopulations within separate subclones that have functional and molecular similarities to stem cells [27][28][54,55]. Moreover, one should distinguish the terms “cancer stem cell” and the “cell-of-origin” [29][56]. The proper definition for the cell-of-origin of cancer is “cancer-initiating cell”. Cancer stem cells, on the other hand, are the cells that maintain tumor propagation. According to the current views, different transformed cells, including stem cells, progenitors or even differentiated cells may be implicated as the cells-of-origin in tumor initiation in different cancers [30][31][57,58]. Experiments using genetic barcoding in vivo [32][59] and lineage tracing by means of single-cell RNA sequencing analysis [33][60] showed that the stem-like subpopulation of cancer cells consists of rare quiescent cells and a large population of frequently proliferating cells which give rise to non-cycling cells without a “stem-like signature”. For example, in a study of the clonal evolution of barcoded glioblastoma cells during serial xenotransplantation [32][59], it was demonstrated that slowly proliferating stem-like cells give rise to more frequently dividing progenitor cells with an extensive self-maintenance capacity, which proceed to the formation of non-proliferative cells. The authors also found rare “outlier” clones that deviated from this proliferative hierarchy and showed that chemotherapy enhances the expansion of pre-existing drug-resistant glioblastoma stem cells [32][59]. The scheme demonstrating the contemporary view of the cancer stem cell concept is shown in Figure 1B.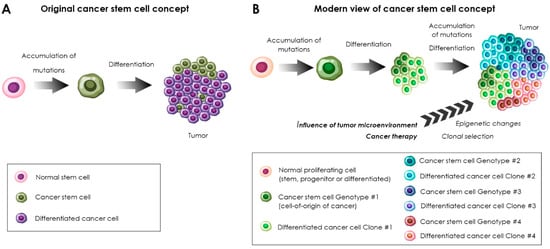
Figure 1. Evolution of the cancer stem cell concept. The original concept (A) emerged on the basis of the stem cell theory in its original form [13][19] and on the reports about the exclusive ability of tumor cells expressing stem cell markers to induce tumor growth [16][21][38,43]. The modern concept (B) has been updated by introducing the concept of the cells-of-origin of tumors [31][34][58,61] and according to data obtained by genetic analysis combined with CSC-associated marker profiling [35][24] and lineage tracing analysis [32][33][36][37][59,60,62,63]. Notably, the relationship between the cells-of-origin of cancer and the CSCs is yet not well understood and the characteristics of both cell types may dynamically change. The process of the CSC differentiation involves the activation of the rare quiescent stem cell-like subpopulation which gives rise to the progenitor-like actively proliferating cells and the subsequent generation of non-stem cell-like, non-cycling, non-tumorigenic “differentiated” cancer cells.
3. Promising Ddirections for Ffuture Rresearch of Ccancer Stem Cell Bstem cell biology
Unfortunately, no consensus on the markers of cancer stem cells was found because the results of different studies were controversial. Moreover, there is evidence of genetic heterogeneity in the subpopulation of cancer cells expressing common cancer stem cell markers, for example, in glioblastoma [35][24]. Thus, it is not clear whether CSCs represent a separate tumor population or whether they are just a manifestation of a certain phenotypic state that many cancer cells can assume under certain conditions. Genomic/transcriptomic/proteomic analysis of tumor tissue or bulk cell population provides the values of gene expression, the number of specific proteins and other indicators, averaged over all cells of the sample. As a result, information about minor subpopulations playing significant roles in oncogenesis and with the potential to be targets of prospective anti-cancer therapies is lost. Studies of the intratumoral cell heterogeneity at the single-cell level are rapidly gaining momentum and look quite promising with regard to the research of cancer stem cell biology and advancement of more effective knowledge-based therapies [38][18]. At present, a number of high-throughput technologies for studying tumor tissue at the single-cell level, including flow cytometry, mass cytometry and RNA and DNA single-cell sequencing, are already widely used. The use of single-cell omics approach for studying cell heterogeneity makes it possible to obtain a more detailed characterization of the tumor cell composition. High hopes are also associated with the advances in single-cell multimodal omics technologies, since it will provide data on the relationship between phenotype, genotype, transcriptional state and epigenetic changes in each single cell of the tumor and specifically in putative CSCs [39][137].
