Polymers and plastics are crucial materials in many sectors of our economy, due to their numerous advantages. They also have some disadvantages, among the most important are problems with the recycling and disposal of used plastics. The recovery of waste plastics is increasing every year, but over 27% of plastics are landfilled. The rest is recycled, where, unfortunately, incineration is still the most common management method. From an economic perspective, waste management methods that lead to added-value products are most preferred—as in the case of material and chemical recycling. Since chemical recycling can be used for difficult wastes (poorly selected, contaminated), it seems to be the most effective way of managing these materials. Moreover, as a result this of kind of recycling, it is possible to obtain commercially valuable products, such as fractions for fuel composition and monomers for the reproduction of polymers.
- plastic waste
- liquefaction
- fuels
- quality standards
1. Introduction
2. Plastic Waste Management
The problem with the management of plastics waste is already global, but the situation is improving every year. This is evidenced, inter alia, by the annual data published in the report of the Plastics Europe Foundation, which indicates that, in the European Union in 2020, compared to 2006, recycling of plastic waste increased by almost 118%, energy recovery through incineration increased by 77% and the amount of waste sent to landfills decreased by over 46% [11][10]. In 2020, over 29 million tonnes of post-consumer plastic waste were collected in European Union countries. Unfortunately, as much as 23.4% of them went to landfills, and only 34.6% was subjected to typical recycling. A total of 42% of collected waste was utilized through energy recovery through incineration (energy recycling) [11][10]. This form of recycling (in accordance with the principle of waste management hierarchy [17][11]) should only be used as a last resort for waste that is not suitable for other forms of recycling, such as depolymerization, material or chemical recycling. Sadly, the above data show that waste incineration is the most common and dominant form of PW management, which unfortunately leads to the complete elimination of plastic material from the economy and makes it impossible to use this material in any other way. In addition, large amounts of harmful and toxic compounds are generated during incineration [18][12]. The still relatively low level of PW recycling (depolymerization, material and chemical recycling) results from the complexity of the processing methods, significant energy demand or/and unsatisfactory quality of the obtained products. The best method for managing plastics is their depolymerization to monomers (input ingredients for the production of plastics). However, this method is applicable, with good efficiency, to a few, selected polymers (polyethylene terephthalate, polymethyl methacrylate, polystyrene) [19,20,21][13][14][15]. In addition, the raw material must be clean and free from any additives of other materials. Unfortunately, this method cannot be applied to polyolefins, which accounts for approximately 70–76% of the waste plastic stream [22][16]. In the case of polyolefins, the best method for their management is material (mechanical) recycling to regranulate. This method is considered the most environmentally friendly due to the low value of CO2 emissions compared to other methods [23][17], but it is not free from drawbacks. The feedstock for processing must be very well selected and clean, and unfortunately the material loses quality with each subsequent processing cycle. In addition, the use of different types of plastics on various parts of packaging (e.g., labels, caps, packaging) and the use of additives and fillers for plastics make it difficult to recycle and recover individual groups of materials and reduce the quality of the obtained final products [24,25][18][19]. Post-industrial plastics (such as cuttings and trimmings from production, runners from injection moulding or granulation residues) are best-suited for such recycling because they have a known and homogeneous composition and contain few impurities [26,27][20][21]. Regarding post-consumer PW, which is a complex mixture of different plastics and other materials, a sorting process should be carried out to obtain separate components should. Selected components, depending on their quality, can be mechanically recycled (for example, into outdoor furniture, flower pots, elements of terraces), directed to chemical recycling or sent to incineration [28][22]. However, due to the difficulty of completely separating the individual materials and contaminations of post-consumer waste, chemical recycling methods seem to be the most appropriate for their management [29][23]. Figure 1 shows a schematic diagram of the main directions of plastic waste management.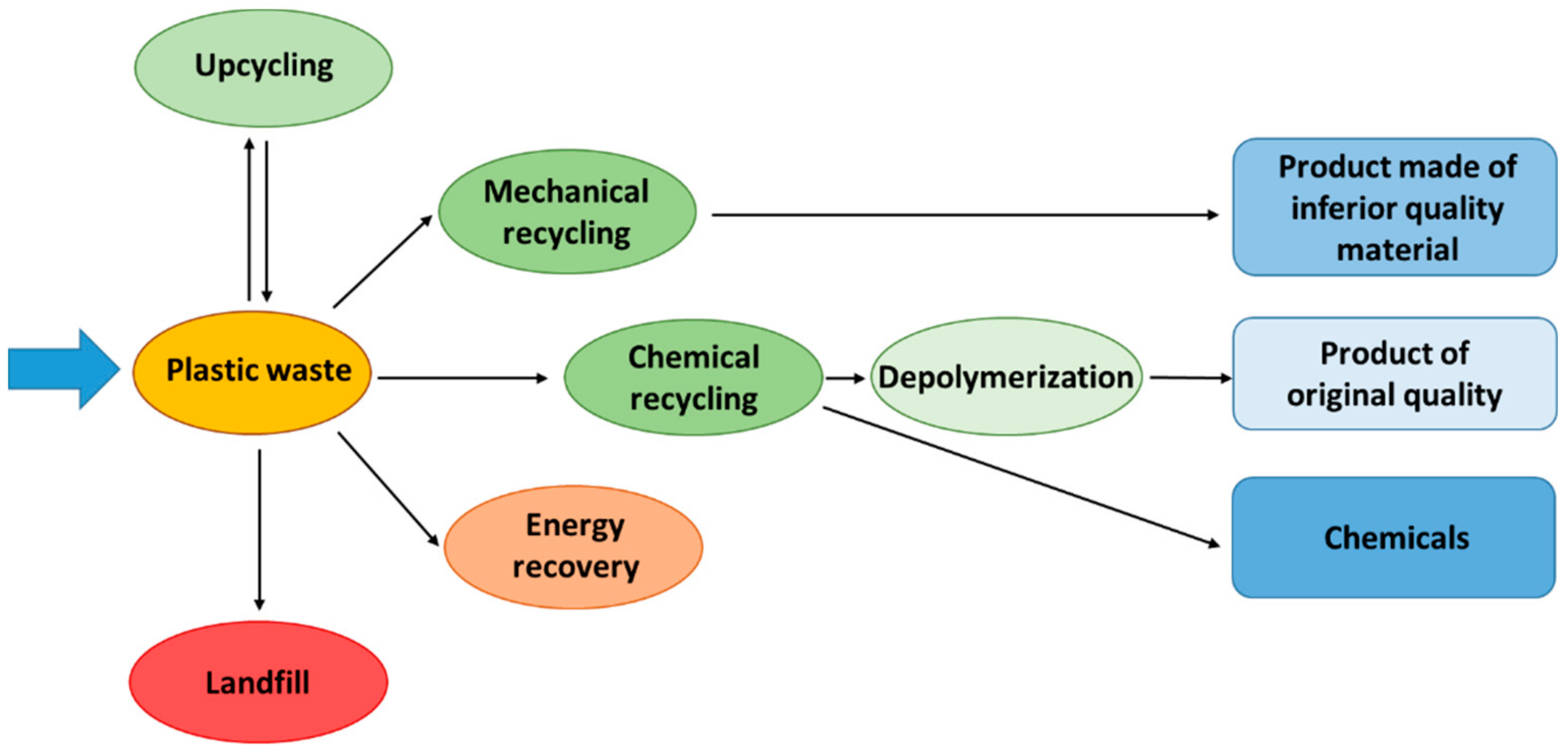
3. Pyrolysis
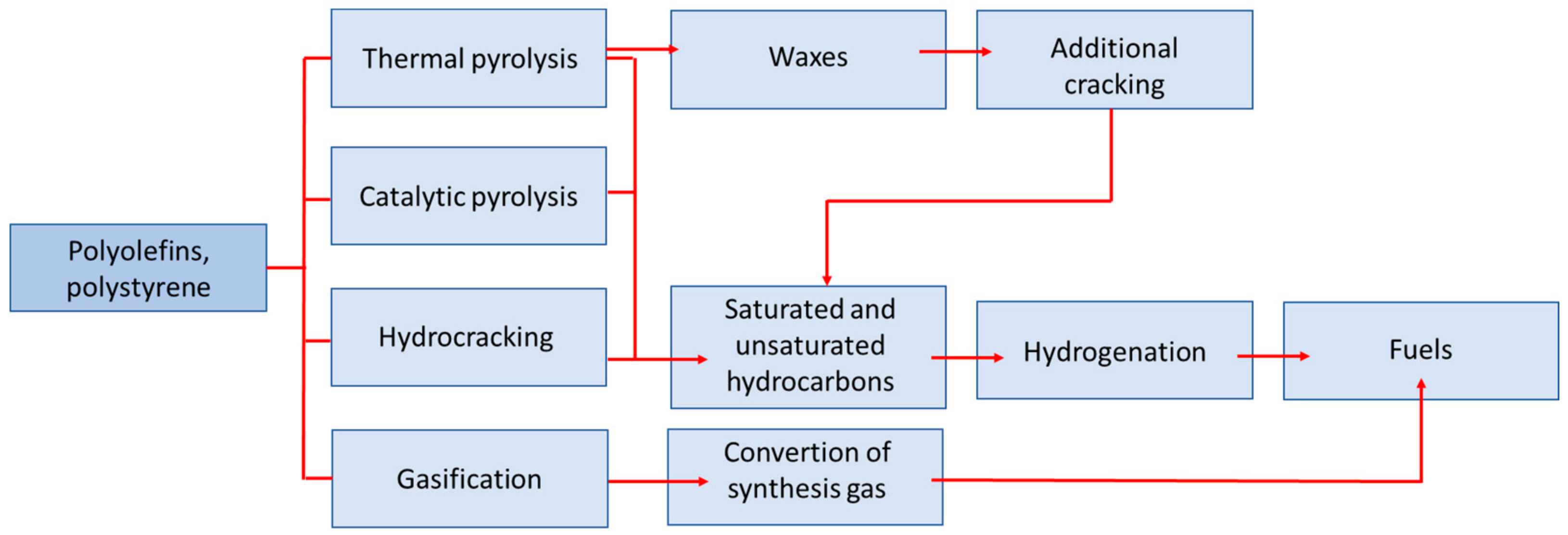
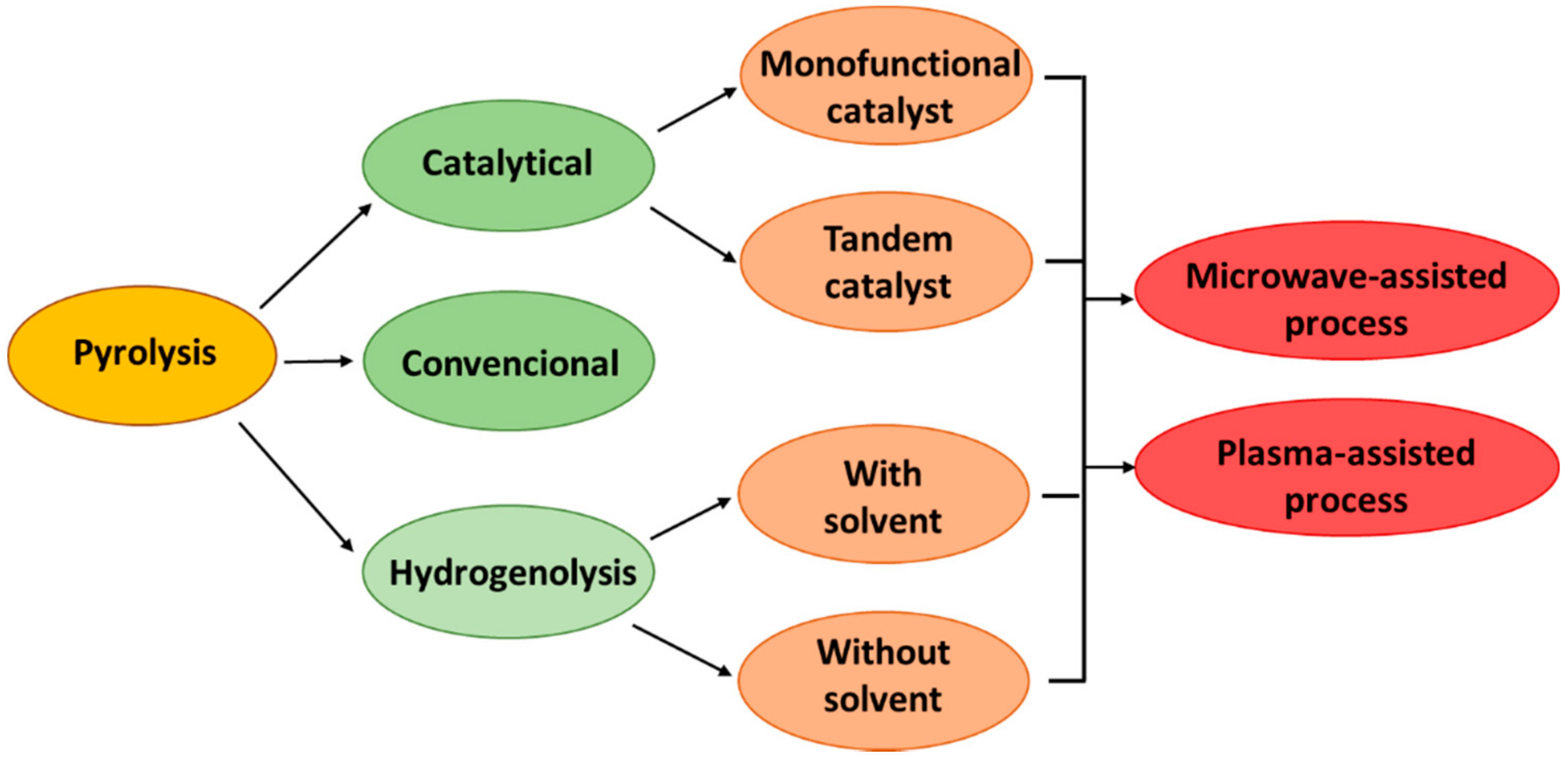
One of the methods of converting waste plastics to hydrocarbon fractions is pyrolysis. This enables the transformation of high-molecular compounds, such as waste plastics, into compounds with much shorter carbon chains. It is considered a promising method for the conversion of addition polymers such as polyethylene and polypropylene (polyolefins) [31,32][25][26]. It offers the opportunity to recycle this polyolefin stream, which cannot be economically managed in conventional mechanical recycling, thus avoiding their incineration and the generation of hazardous substances such as dioxins and furans [33,34][27][28]. The pyrolysis oil (a mixture of waxes and a liquid fraction) obtained as a result of pyrolysis can be used as a substitute for heavy fuel oil or undergo further processing, e.g., in the petrochemical industry [35,36][29][30]. Furthermore, as shown in Reference [37][31], large-scale pyrolysis is economically viable. For example, in the case of an investment in installation that processes 100,000 kg/h of waste plastics, income is generated within the first year and has a positive net present value. The advantage of pyrolysis is that pre-segregated, mixed and contaminated plastic waste with both organic and inorganic material can be subjected to this process [38,39][32][33]. The pyrolysis process is based on the thermochemical decomposition of various materials of organic and synthetic origin at elevated temperatures and in an oxygen-free atmosphere. Typically, the process is carried out at temperatures in the range of 500–800 °C and, in the case of plastics, it follows four types of mechanism: end-chain scission or depolymerization, random-chain scission, chain stripping and cross-linking [40][34]. The main products of the pyrolysis process are pyrolysis oil, gaseous products and the solid fraction (char). On average, depending on the technology used, 45–50% of the oil fraction, 35–40% of the gaseous fraction and 10–20% of char is obtained in the process [41][35]. It was generally found that with the increase of the process temperature, the yield of the liquid fraction increases, while the yield of the solid fraction decreases [42][36]. However, for selected plastics (e.g., for PE over catalyst), it is possible to obtain a liquid fraction in an amount exceeding even 90% [43,44][37][38]. The high propensity of plastics to generate significant amounts of oil fraction during pyrolysis is the result of high volatile matter and low ash content in relation to other materials (e.g., biomass). These two parameters are the key factors influencing the pyrolysis oil yield [45][39]. Due to the process conditions (vapor residence time in the reactor, temperature, raw material heating rate), pyrolysis is divided into three types [46,47][40][41]:-
Slow pyrolysis—process temperature from 350 °C to 550 °C, heating rate from 1 to 10 °C/min, extended steam residence time, where char is the main product’s first bullet;
-
Fast pyrolysis—the process temperature usually ranges from 500 °C to 700 °C, the raw material heating rate is above 100 °C/min, and the vapor residence time is usually within a few seconds; the main product is the liquid fraction, and in the case of polyolefin, pyrolysis also waxes;
-
Flash pyrolysis—the process temperature usually exceeds 700 °C, the heating rate of the raw material >200 °C/s, and the vapor residence time is in the millisecond range.
|
Type of Reactor |
Type of Feedstock Materials |
|||
|---|---|---|---|---|
|
Type of Reactor | References | |||
Advantages |
Disadvantages |
|||
|
Batch reactor |
||||
|
Batch reactor |
PS, PE, PP and PET |
| ||
|
HDPE |
[31] | ||
|
Semi-batch reactor | [25] |
|||
|
|
Plastic medical wastes |
[65 | |
|
Fixed-bed reactor | ][60] |
|||
|
|
Semi-batch reactor |
PS, HDPE, LDPE, PP |
|
|
Fluidized-bed reactor |
| |||
|
PE |
|||
|
Rotary kiln reactor | ||||
|
|
Polyolefin |
[ | |
|
Stirred tank reactor | ||||
|
|
Continuous reactor |
Plastic waste from landfill |
|
|
Conical spouted bed reactor |
| |||
|
HDPE |
|||
|
Fixed bed reactor |
PP, HDPE, LDPE |
|||
|
HDPE |
||||
|
Fluidized bed reactor |
LDPE |
|||
|
PP, PE |
||||
|
PE |
||||
|
CSBR |
HDPE, LDPE, PP |
|||
|
HDPE, LDPE, PP, PS, PET, PMMA |
||||
|
PE |
References
- World Energy Outlook 2021. Report of International Energy Agency. October 2021. Available online: https://iea.blob.core.windows.net/assets/4ed140c1-c3f3-4fd9-acae-789a4e14a23c/WorldEnergyOutlook2021.pdf (accessed on 10 January 2022).
- Short-Term Energy Outlook. Report of U.S. Energy Information Adsministration. December 2021. Available online: https://www.eia.gov/outlooks/steo/pdf/steo_full.pdf (accessed on 10 January 2022).
- Umar, M.; Farid, S.; Naeem, M.A. Time-frequency connectedness among clean-energy stocks and fossil fuel markets: Comparison between financial, oil and pandemic crisis. Energy 2022, 240, 122702.
- Tang, C.; Aruga, K. Relationships among the Fossil Fuel and Financial Markets during the COVID-19 Pandemic: Evidence from Bayesian DCC-MGARCH Models. Sustainability 2022, 14, 51.
- Strielkowski, W.; Civín, L.; Tarkhanova, E.; Tvaronavičienė, M.; Petrenko, Y. Renewable Energy in the Sustainable Development of Electrical Power Sector: A Review. Energies 2021, 14, 8240.
- Pisciotta, M.; Pilorgé, H.; Feldmann, J.; Jacobson, R.; Davids, J.; Swett, S.; Sasso, Z.; Wilcox, J. Current state of industrial heating and opportunities for decarbonization. Prog. Energy Combust. Sci. 2022, 91, 100982.
- Special Report. Global Warming of 1.5 °C. Available online: https://www.ipcc.ch/sr15/ (accessed on 10 January 2022).
- Shooshtarian, S.; Maqsood, T.; Caldera, S.; Ryley, T. Transformation towards a circular economy in the Australian construction and demolition waste management system. Sustain. Prod. Consum. 2022, 30, 89–106.
- Sikdar, S. Circular economy: Is there anything new in this concept? Clean Technol. Environ. Policy 2019, 21, 1173–1175.
- Plastics—The Facts 2021—Report Plastics Europe. The Association of Plastics Manufacturers in Europe. Available online: https://plasticseurope.org/pl/knowledge-hub/plastics-the-facts-2021/ (accessed on 13 January 2022).
- Menyuka, N.N.; Sibanda, M.; Bob, U. Perceptions of the Challenges and Opportunities of Utilising Organic Waste through Urban Agriculture in the Durban South Basin. Int. J. Environ. Res. Public Health 2020, 17, 1158.
- Jha, K.K.; Kannan, T.T.M. Recycling of plastic waste into fuel by pyrolysis—A review. Mater. Today Proc. 2021, 37, 3718–3720.
- Kang, M.J.; Yu, H.J.; Jegal, J.; Kim, H.S.; Cha, H.G. Depolymerization of PET into terephthalic acid in neutral media catalyzed by the ZSM-5 acidic catalyst. Chem. Eng. J. 2020, 398, 125655.
- Balema, V.P.; Hlova, I.Z.; Carnahan, S.L.; Seyedi, M.; Dolotko, O.; Rossini, A.J.; Luzinov, I. Depolymerization of polystyrene under ambient conditions. New J. Chem. 2021, 45, 2935–2938.
- Dos Santos, P.B.; da Silva Ribeiro, H.J.; Ferreira, A.C.; Ferreira, C.C.; Bernar, L.P.; da Costa Assunção, F.P.; de Castro, D.A.R.; Santos, M.C.; Duvoisin, S.; Borges, L.E.P.; et al. Process Analysis of PMMA-Based Dental Resins Residues Depolymerization: Optimization of Reaction Time and Temperature. Energies 2022, 15, 91.
- Lahtela, V.; Hyvärinen, M.; Kärki, T. Composition of Plastic Fractions in Waste Streams: Toward More Efficient Recycling and Utilization. Polymers 2019, 11, 69.
- Jubinville, D.; Esmizadeh, E.; Saikrishnan, S.; Tzoganakis, C.; Mekonnen, T. A comprehensive review of global production and recycling methods of polyolefin (PO) based products and their post-recycling applications. Sustain. Mater. Technol. 2020, 25, e00188.
- Shen, L.; Worrell, E. Chapter 13—Plastic Recycling. In Handbook of Recycling. State-of-the-Art for Practitioners, Analysts, and Scientists; Worrell, E., Reuter, M.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 179–190.
- Singh, N.; Hui, D.; Singh, R.; Ahuja, I.P.S.; Feo, L.; Fraternali, F. Recycling of plastic solid waste: A state of art review and future applications. Compos. Part B Eng. 2017, 115, 409–422.
- Ignatyev, I.A.; Thielemans, W.; Vander Beke, B. Recycling of Polymers: A Review. ChemSusChem 2014, 7, 1579–1593.
- Schwabl, D.; Bauer, M.; Lehner, M. Advancing Plastic Recycling by Wet-Mechanical Processing of Mixed Waste Fractions. Processes 2021, 9, 493.
- Ragaert, K.; Huysveld, S.; Vyncke, G.; Hubo, S.; Veelaert, L.; Dewulf, J.; Du Bois, E. Design from recycling: A complex mixed plastic waste case study. Resour. Conserv. Recycl. 2020, 155, 104646.
- Solis, M.; Silveira, S. Technologies for chemical recycling of household plastics—A technical review and TRL assessment. Waste Manag. 2020, 105, 128–138.
- Li, N.; Liu, H.X.; Cheng, Z.N.; Yan, B.B.; Chen, G.Y.; Wang, S.B. Conversion of plastic waste into fuels: A critical review. J. Hazard. Mater. 2022, 424, 127460.
- Palos, R.; Gutiérrez, A.; Vela, F.J.; Maña, J.A.; Hita, I.; Asueta, A.; Arnaiz, S.; Arandes, J.M.; Bilbao, J. Assessing the potential of the recycled plastic slow pyrolysis for the production of streams attractive for refineries. J. Anal. Appl. Pyrolysis 2019, 142, 104668.
- Prajapati, R.; Kohli, K.; Maity, S.K.; Sharma, B.K. Potential Chemicals from Plastic Wastes. Molecules 2021, 26, 3175.
- Gebre, S.H.; Sendeku, M.G.; Bahri, M. Recent Trends in the Pyrolysis of Non-Degradable Waste Plastics. ChemistryOpen 2021, 10, 1202–1226.
- Ma, H.; Cao, Y.; Lu, X.; Ding, Z.; Zhou, W. Review of Typical Municipal Solid Waste Disposal Status and Energy Technology. Energy Procedia 2016, 88, 589–594.
- Arandes, J.M.; Torre, I.; Castaño, P.; Olazar, M.; Bilbao, J. Catalytic Cracking of Waxes Produced by the Fast Pyrolysis of Polyolefins. Energy Fuels 2007, 21, 561–569.
- Rodríguez, E.; Gutiérrez, A.; Palos, R.; Vela, F.J.; Arandes, J.M.; Bilbao, J. Fuel production by cracking of polyolefins pyrolysis waxes under fluid catalytic cracking (FCC) operating conditions. Waste Manag. 2019, 93, 162–172.
- Fivga, A.; Dimitriou, I. Pyrolysis of plastic waste for production of heavy fuel substitute: A techno-economic assessment. Energy 2018, 149, 865–874.
- Fekhar, B.; Zsinka, V.; Miskolczi, N. Value added hydrocarbons obtained by pyrolysis of contaminated waste plastics in horizontal tubular reactor: In situ upgrading of the products by chlorine capture. J. Clean. Prod. 2019, 241, 118166.
- Kaminsky, W. Chemical recycling of plastics by fluidized bed pyrolysis. Fuel Commun. 2021, 8, 100023.
- Syamsiro, M.; Saptoadi, H.; Norsujianto, T.; Noviasri, P.; Cheng, S.; Alimuddin, Z.; Yoshikawa, K. Fuel Oil Production from Municipal Plastic Wastes in Sequential Pyrolysis and Catalytic Reforming Reactors. Energy Procedia 2014, 47, 180–188.
- Bezergianni, S.; Dimitriadis, A.; Faussone, G.C.; Karonis, D. Alternative Diesel from Waste Plastics. Energies 2017, 10, 1750.
- Nanda, S.; Berruti, F. Thermochemical conversion of plastic waste to fuels: A review. Environ. Chem. Lett. 2021, 19, 123–148.
- Marcilla, A.; Beltrán, M.I.; Navarro, R. Thermal and catalytic pyrolysis of polyethylene over HZSM5 and HUSY zeolites in a batch reactor under dynamic conditions. Appl. Catal. B Environ. 2009, 86, 78–86.
- Fahim, I.; Mohsen, O.; ElKayaly, D. Production of Fuel from Plastic Waste: A Feasible Business. Polymers 2021, 13, 915.
- Sharuddin, S.D.A.; Abnisa, F.; Daud, W.M.A.W.; Aroua, M.K. A review on pyrolysis of plastic wastes. Energy Convers. Manag. 2016, 115, 308–326.
- Papari, S.; Bamdad, H.; Berruti, F. Pyrolytic Conversion of Plastic Waste to Value-Added Products and Fuels: A Review. Materials 2021, 14, 2586.
- Papari, S.; Hawboldt, K. Development and Validation of a Process Model to DescribePyrolysis of Forestry Residues in an Auger Reactor. Energy Fuels 2017, 31, 10833–10841.
- Suresh, A.; Alagusundaram, A.; Kumar, P.S.; Vo, D.V.N.; Christopher, F.C.; Balaji, B.; Viswanathan, V.; Sankar, S. Microwave pyrolysis of coal, biomass and plastic waste: A review. Environ. Chem. Lett. 2021, 19, 3609–3629.
- Miandad, R.; Nizami, A.S.; Rehan, M.; Barakat, M.A.; Khan, M.I.; Mustafa, A.; Ismail, I.M.I.; Murphy, J.D. Influence of temperature and reaction time on the conversion of polystyrene waste to pyrolysis liquid oil. Waste Manag. 2016, 58, 250–259.
- Diaz-Silvarrey, L.S.; Zhang, K.; Phan, A.N. Monomer recovery through advanced pyrolysis of waste high density polyethylene (HDPE). Green Chem. 2018, 20, 1813–1823.
- Miandad, R.; Barakat, M.A.; Aburiazaiza, A.S.; Rehan, M.; Ismail, I.M.I.; Nizami, A.S. Effect of plastic waste types on pyrolysis liquid oil. Int. Biodeterior. Biodegrad. 2017, 119, 239–252.
- Grabda, M.; Oleszek, S.; Shibata, E.; Nakamura, T. Study on simultaneous recycling of EAF dust and plastic waste containing TBBPA. J. Hazard. Mater. 2014, 278, 97–106.
- Fekhar, B.; Gombor, L.; Miskolczi, N. Pyrolysis of chlorine contaminated municipal plastic waste: In-situ upgrading of pyrolysis oils by Ni/ZSM-5, Ni/SAPO-11, red mud and Ca(OH)2 containing catalysts. J. Energy Inst. 2019, 92, 1270–1283.
- Hahladakis, J.N.; Velis, C.A.; Weber, R.; Iacovidou, E.; Purnell, P. An overview of chemical additives present in plastics: Migration, release, fate and environmental impact during their use, disposal and recycling. J. Hazard. Mater. 2018, 344, 179–199.
- Oleszek, S.; Kumagai, S.; Grabda, M.; Shiota, K.; Yoshioka, T.; Takaoka, M. Mitigation of bromine-containing products during pyrolysis of polycarbonate-based tetrabromobisphenol A in the presence of copper(I) oxide. J. Hazard. Mater. 2021, 409, 124972.
- Ma, C.; Yu, J.; Wang, B.; Song, Z.; Xiang, J.; Hu, S.; Su, S.; Sun, L. Chemical recycling of brominated flame retarded plastics from e-waste for clean fuels production: A review. Renew. Sustain. Energy Rev. 2016, 61, 433–450.
- Terakado, O.; Ohhashi, R.; Hirasawa, M. Bromine fixation by metal oxide in pyrolysis of printed circuit board containing brominated flame retardant. J. Anal. Appl. Pyrolysis 2013, 103, 216–221.
- Jung, S.H.; Kim, S.J.; Kim, J.S. Thermal degradation of acrylonitrile–butadiene–styrene (ABS) containing flame retardants using a fluidized bed reactor: The effects of Ca-based additives on halogen removal. Fuel Process Technol. 2012, 96, 265–270.
- Brebu, M.; Bhaskar, T.; Murai, K.; Muto, A.; Sakata, Y.; Uddin, M.A. Removal of nitrogen, bromine, and chlorine from PP/PE/PS/PVC/ABS–Br pyrolysis liquid products using Fe- and Ca-based catalysts. Polym. Degrad. Stab. 2005, 87, 225–230.
- Manžuch, Z.; Akelytė, R.; Camboni, M.; Carlander, D. Chemical Recycling of Polymeric Materials from Waste in the Circular Economy. RPA Europe Final Report. August 2021. Available online: https://echa.europa.eu/documents/10162/1459379/chem_recycling_final_report_en.pdf/887c4182-8327-e197-0bc4-17a5d608de6e?t=1636708465520, (accessed on 14 January 2022).
- Charitopoulou, M.A.; Kalogiannis, K.G.; Lappas, A.A.; Achilias, D.S. Novel trends in the thermo-chemical recycling of plastics from WEEE containing brominated flame retardants. Environ. Sci. Pollut. Res. 2021, 28, 59190–59213.
- Al-Salem, S.M.; Antelava, A.; Constantinou, A.; Manos, G.; Dutta, A. A review on thermal and catalytic pyrolysis of plastic solid waste (PSW). J. Environ. Manag. 2017, 197, 177–198.
- Wong, S.L.; Ngadi, N.; Abdullah, T.A.T.; Inuwa, I.M. Current state and future prospects of plastic waste as source of fuel: A review. Renew. Sustain. Energy Rev. 2015, 50, 1167–1180.
- Lewandowski, W.M.; Januszewicz, K.; Kosakowski, W. Efficiency and proportions of waste tyre pyrolysis products depending on the reactor type—A review. J. Anal. Appl. Pyrolysis 2019, 140, 25–53.
- Uddin, M.N.; Techato, K.; Taweekun, J.; Rahman, M.M.; Rasul, M.G.; Mahlia, T.M.I.; Ashrafur, S.M. An Overview of Recent Developments in Biomass Pyrolysis Technologies. Energies 2018, 11, 3115.
- Rasul, S.B.; Som, U.; Hossain, S.; Rahman, W. Liquid fuel oil produced from plastic based medical wastes by thermal cracking. Sci. Rep. 2021, 11, 17048.
- Lee, K.H.; Shin, D.H. Characteristics of liquid product from the pyrolysis of waste plastic mixture at low and high temperatures: Influence of lapse time of reaction. Waste Manag. 2007, 27, 168–176.
- Pan, R.; Martins, M.F.; Debenest, G. Pyrolysis of waste polyethylene in a semi-batch reactor to produce liquid fuel: Optimization of operating conditions. Energy Convers. Manag. 2021, 237, 114114.
- Torres, D.; Jiang, Y.; Sanchez Monsalve, D.A.; Leeke, G.A. Chlorine removal from the pyrolysis of urban polyolefinic waste in a semi-batch reactor. J. Environ. Chem. Eng. 2021, 9, 104920.
- Al-Salem, S.M.; Yang, Y.; Wang, J.; Leeke, G.A. Pyro-Oil and Wax Recovery from Reclaimed Plastic Waste in a Continuous Auger Pyrolysis Reactor. Energies 2020, 13, 2040.
- Zhou, N.; Dai, L.; Lv, Y.; Li, H.; Deng, W.; Guo, F.; Chen, P.; Lei, H.; Ruan, R. Catalytic pyrolysis of plastic wastes in a continuous microwave assisted pyrolysis system for fuel production. Chem. Eng. J. 2021, 418, 129412.
- Tulashie, S.K.; Boadu, E.K.; Dapaah, S. Plastic waste to fuel via pyrolysis: A key way to solving the severe plastic waste problem in Ghana. Therm. Sci. Eng. Prog. 2019, 11, 417–424.
- Papuga, S.V.; Gvero, P.M.; Vukić, L.M. Temperature and time influence on the waste plastics pyrolysis in the fixed bed reactor. Therm. Sci. 2016, 20, 731–741.
- Li, H.; Mašek, O.; Harper, A.; Ocone, R. Kinetic study of pyrolysis of high-density polyethylene (HDPE) waste at different bed thickness in a fixed bed reactor. Can. J. Chem. Eng. 2021, 99, 1733–1744.
- Milne, B.J.; Behie, L.A.; Berruti, F. Recycling of waste plastics by ultrapyrolysis using an internally circulating fluidized bed reactor. J. Anal. Appl. Pyrolysis 1999, 51, 157–166.
- Jung, S.H.; Cho, M.H.; Kang, B.S.; Kim, J.S. Pyrolysis of a fraction of waste polypropylene and polyethylene for the recovery of BTX aromatics using a fluidized bed reactor. Fuel Process. Technol. 2010, 91, 277–284.
- Mastral, J.F.; Berrueco, C.; Ceamanos, J. Modelling of the pyrolysis of high density polyethylene: Product distribution in a fluidized bed reactor. J. Anal. Appl. Pyrolysis 2007, 79, 313–322.
- Arabiourrutia, M.; Elordi, G.; Lopez, G.; Borsella, E.; Bilbao, J.; Olazar, M. Characterization of the waxes obtained by the pyrolysis of polyolefin plastics in a conical spouted bed reactor. J. Anal. Appl. Pyrolysis 2012, 94, 230–237.
- Orozco, S.; Alvarez, J.; Lopez, G.; Artetxe, M.; Bilbao, J.; Olazar, M. Pyrolysis of plastic wastes in a fountain confined conical spouted bed reactor: Determination of stable operating conditions. Energy Convers. Manag. 2021, 229, 113768.
- Artetxe, M.; Lopez, G.; Elordi, G.; Amutio, M.; Bilbao, J.; Olazar, M. Production of Light Olefins from Polyethylene in a Two-Step Process: Pyrolysis in a Conical Spouted Bed and Downstream High-Temperature Thermal Cracking. Ind. Eng. Chem. Res. 2012, 51, 13915–13923.
