Given that females may be more likely to be affected by some ailments such as osteoarthritis, heart disease, cancer, and anxiety, it is imperative to study the effect of the gut microbiome and its role in female health. It is evident that the presence/ratio of microbial species is altered in polycystic ovarian syndrome, cancer, pregnancy, and menopause. Thus, potential probiotics should be developed and the administration of certain bacterial species should be considered, as novel independent or adjunct therapies for various female-related pathologies.
- gut microbiota
- estrobolome
- estrogen
- probiotic
- gut dysbiosis
1. Introduction
2. Gut Microbiome in Healthy Females
Gender-dependent differences in the gut microbiome have been reported [9]. Women’s gut microbiome composition markedly differs from men’s [13,14][10][11]. As depicted in Figure 1, women are reported to have a lower Bacteroides abundance but higher α diversity, a measure of diversity within an individual sample [13,14,15][10][11][12]. Female sex hormone levels affect the microbiota composition [16][13]. However, this influence is bidirectional. This is because the microbiome regulates steroid hormone levels including estrogens [17][14]. In a clinical trial employing 25 men, 7 postmenopausal women, and 19 premenopausal women, results imply that the intestinal microbiome indeed affects systemic estrogen levels [18][15]. Another study reports that gut microbial diversity is positively correlated with estrogen metabolites to parent estrogen ratio in a group of postmenopausal women [17][14]. However, further studies with a larger and more inclusive population should be carried out to investigate this linkage in women before menopause. Certain enteric bacteria, whose genome is called the estrobolome, metabolize estrogens [17,19][14][16]. To be excreted, estrogens are hepatically conjugated through glucuronidation or sulfonation [19][16]. These estrobolome bacterial species with their ß-glucuronidase activity can deconjugate excreted estrogens in the bile and prevent their excretion [17,19][14][16]. This may explain why fecal glucuronidase levels were reported to be inversely associated with gut estrogen levels [18][15]. The human gut also carries out various local and distant functions through hormonal metabolites and intermediates [19][16]. Remarkably, gut microbes also carry out the synthesis of estrogen-like compounds from nutrition [17][14]. In healthy females, probiotic administration has shown promise. While probiotic administration fails to engender persistent gut microbiota changes, it has been reported to improve vaginal lactobacilli concentration, female health system bowel movement, and immune system responses in healthy adults [20][17]. Moreover, probiotics help enhance local vaginal immunity and maintain female reproductive tract health [21][18]. Notably, certain bacterial strains, namely, Lactobacillus strains have been shown to prevent the recurrence of urinary tract infections and bacterial vaginosis [22,23][19][20].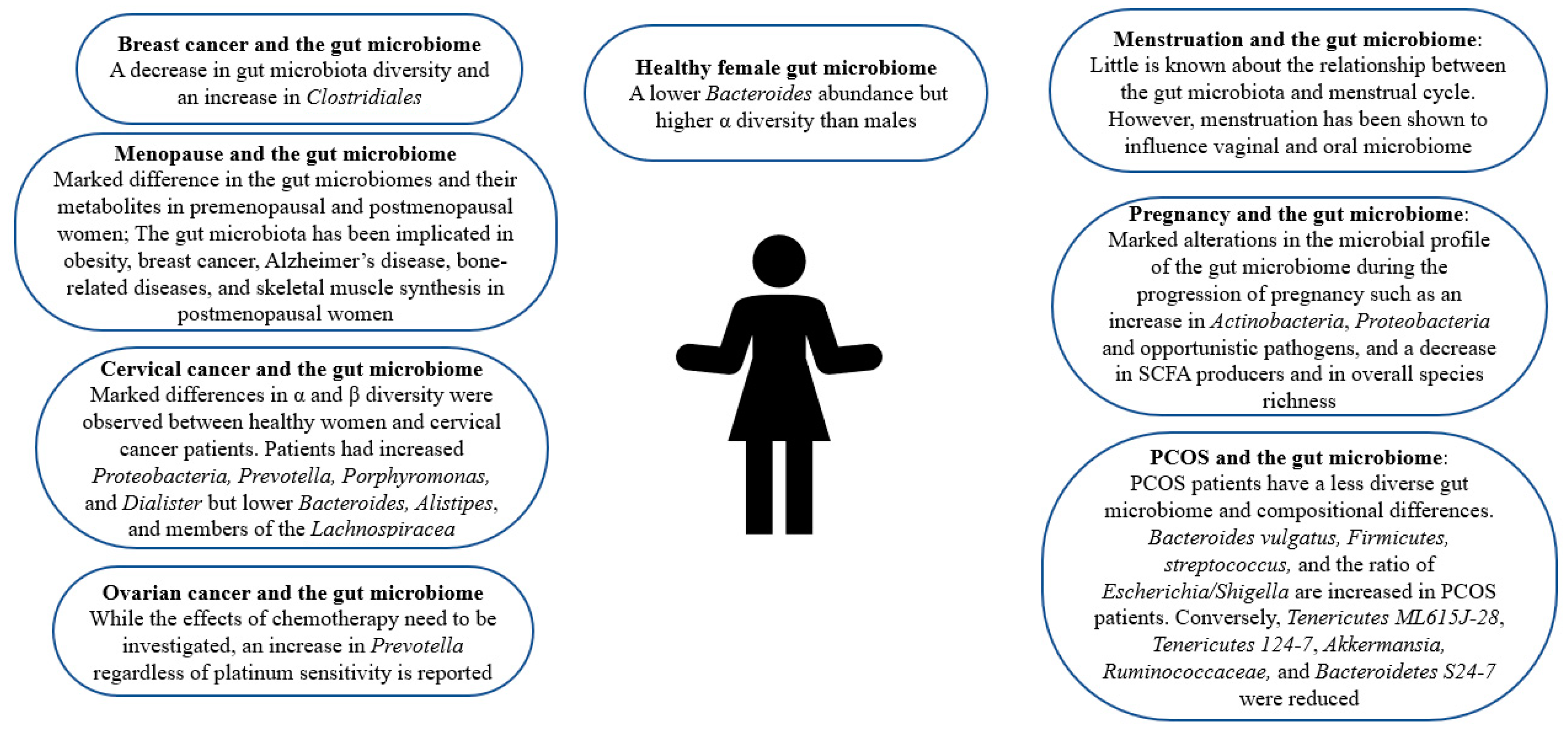
3. Gut Microbiome and PCOS
PCOS affects 8–13% of women worldwide in their reproductive age [24][21]. While the clinical phenotype may vary, insulin resistance and hyperandrogenism are the hallmarks of PCOS [25][22]. The gut microbiome of PCOS sufferers differs from that of controls [26][23]. As can be seen in Table 1 and Figure 1, PCOS patients have a less diverse gut microbiome, which is correlated with hyperandrogenism [25,27,28][22][24][25]. In a clinical trial, 43 healthy females and 50 PCOS patients were employed, taking into consideration the influence of body weight [26][23]. While Alpha (α) diversity was comparable in both groups, Beta (β) diversity, a measure of diversity among different samples, was markedly decreased in the gut microbiota of PCOS patients [15,26][12][23]. Conversely, a study reports that α diversity is altered in PCOS patients’ guts [29][26]. Another study confirms the alteration by reporting a decrease in α diversity in the guts of women with PCOS [30][27]. Notably, the abundance of a Bacteroides species responsible for the deconjugation of conjugated bile acids synthesized in the liver, Bacteroides vulgatus (B. vulgatus), was significantly higher in women with PCOS than in the controls [26][23]. Firmicutes, a phylum correlated with obesity, is also more abundant in PCOS patients while Tenericutes ML615J-28, Tenericutes 124-7, and Bacteroidetes S24-7 levels are reduced [25,27,28][22][24][25]. An increase in streptococcus and the ratio of Escherichia/Shigella was also reported in the gut of women with PCOS [25][22]. Opposingly, it was found that Akkermansia, a species reported to modulate energy metabolism and glucose tolerance in humans, and Ruminococcaceae, were less abundant in PCOS patients [25,31][22][28].|
Condition |
Dysbiosis |
Strains Increased |
Strains Decreased |
References |
|---|---|---|---|---|
|
Menstruation |
Little is known about the relationship between menstruation and the gut microbiome. |
|||
|
Pregnancy |
Gut microbial changes mediated by gestational hormonal changes |
Actinobacteria, Proteobacteria, and opportunistic pathogens |
SCFA producers and in overall species richness |
|
|
positive immune antibody-associated miscarriage |
Unreported |
Blautia and Bacteroides |
None reported |
|
|
Perimenopause |
Present |
Enterobacter |
Lactobacillus and Bifidobacteria |
|
|
Postmenopause |
Grows in resemblance to male gut microbiome |
Conflicting results |
Conflicting results |
|
|
Postmenopause-associated bone diseases |
decreased bacterial richness and diversity |
unclassified Clostridia and methanogenic archaea |
PrevotellaStudies report conflicting results on Bacteroides |
|
|
PCOS |
Present with decreased diversity |
Bacteroides vulgatus, Firmicutes, Streptococcus, and the ratio of Escherichia/Shigella |
Tenericutes ML615J-28, Tenericutes 124-7, Akkermansia, Ruminococcaceae, and Bacteroidetes S24-7 |
|
|
Breast cancer |
Present with a decrease in diversity |
Clostridiales |
None reported |
|
|
Ovarian cancer |
Present |
Prevotella, yet the effects of chemotherapy have not been accounted for. |
None reported |
|
|
Cervical cancer |
Present with changes in diversity |
Proteobacteria, Prevotella, Porphyromonas, and Dialister |
Bacteroides, Alistipes, and members of the Lachnospiracea |
4. Gut Microbiome and Cancer
Disturbances in the gut microbiome have been associated with and observed in various cancers including gastric, colorectal, hepatic, pancreatic, and prostate cancer [51,52,53][45][46][47]. Interestingly, in pancreatic cancer and melanoma murine models, a significant decrease in subcutaneous tumor burden was observed after depleting the gut microbiome through antibiotic administration [51][45]. Dysbiosis of the gut microbiota is also observed in various cancers affecting the female population including breast, cervical, and ovarian cancer as depicted in Table 1 and Figure 1 [45,46,47][42][43][44]. Furthermore, some studies imply a decrease in the diversity of the gut microbiota and an increase in the abundance of Clostridiales in breast cancer patients [45][42]. An analysis of the gut microbiota of postmenopausal women with breast cancer reveals that while the differences in relative species abundance in gut microbiota between premenopausal breast cancer patients and premenopausal controls was negligible, 45 species differed significantly in their relative abundance between postmenopausal patients and postmenopausal controls [54][48]. Moreover, in postmenopausal cancer patients, 38 species were overrepresented such as Escherichia coli, Actinomyces sp. HPA0247, Klebsiella sp_1_1_55, Prevotella amnii, and Shewanella. A study revealed that α and β diversity differed significantly between employed cervical cancer patients and cancer-free women [47][44]. Additionally, the study disclosed that cervical cancer patients had higher levels of Prevotella, Porphyromonas, and Dialister while cancer-free individuals had greater levels of Bacteroides, Alistipes, and members of the Lachnospiracea than controls [47][44]. In ovarian cancer patients, a shared increase in Prevotella regardless of platinum sensitivity was observed, but the study was unable to count for the potential effects of chemotherapy [46][43]. Aside from regulating the host’s immune system, the gut microbiome is involved in both oncogenesis and the suppression of malignant transformation [51,55][45][49]. Furthermore, bacterial metabolites produced by the gut microbiome also regulate cancer cell metabolism [45,56][42][50]. These secreted bacterial metabolites act like hormones since they can enter the circulation, reach far targets, and carry out important functions such as impacting mitochondrial metabolism and modulating the behavior of breast cancer cells, lithocholic acid (LCA), SCFAs, cadaverine, and deconjugated estrogens [45,56][42][50]. As mentioned in the previous section, the gut microbiome is an important player in estrogen metabolism. Since 80% of breast cancer cases are estrogen receptor-positive, the deconjugation of estrogens by the gut microbiome is of relevance [19,57,58,59,60,61][16][51][52][53][54][55]. Aside from being more abundant in the gut of breast cancer patients, Clostridiales reactivates estrogens and increases their serum levels [45][42]. Estrogen receptors play a direct role in the expression of nuclear-coded mitochondrial proteins [45][42]. An increase in oxidative phosphorylation promotes metastasis [45][42]. The gut microbiome also synthesizes estrogen-like compounds from dietary sources [17][14].5. Gut Microbiome and Pregnancy
Aside from infant health, evidence indicates that maternal microbiome niches influence maternal well-being and post-partum recovery [64][56]. Furthermore, gut microbiome disturbances have been linked with the clinical characteristics of preeclampsia, a pregnancy complication characterized by high blood pressure, and some even hypothesize that there is a link between the maternal gut microbiome and postpartum depression [64,65][56][57]. In fact, a Chinese herbal medicine has been shown to ameliorate postpartum depression through modulating the gut microbiota [66][58]. A study also indicates that the maternal gut microbiome may play a part in the immunological adaptations accompanying pregnancy, as shown in Table 1 and Figure 1 [67][59]. Interestingly, an investigation of gut microbiota changes in patients with positive immune antibody-associated recurrent miscarriage reveals that some highly abundant genera, such as Blautia and Bacteroides, may be incriminated in recurrent miscarriage [37][34]. Bidirectional interactions between the gut microbiome and pregnancy have been reported. For instance, bacterial growth can be influenced by hormonal changes [68,69][60][61]. Moreover, gut microbial changes during pregnancy are mediated by hormonal changes accompanying gestation [33,34][30][31]. Namely, it has been reported that fecal progesterone levels were negatively correlated with diversity during pregnancy [34][31]. Notably, profound alterations in the microbial profile of the gut microbiome have been observed during the progression of pregnancy such as an increase in Actinobacteria, Proteobacteria, and opportunistic pathogens, and a decrease in SCFA producers and in overall species richness [35,36][32][33]. In humans, an analysis of the gut microbiome of thirty-five women in their first and third trimesters of pregnancy reveals that Bifidobacterium, Blautia, unclassified Ruminococcaceae, Bacteroides, unclassified Lachnospiraceae, unclassified Clostridiales, Akkermansia, Faecalibacterium, Ruminococcus, and Prevotella were the generally dominant bacterial species. Interestingly, Bifidobacterium is crucial for human milk oligosaccharide degradation and Prevotella metabolizes estradiol and progesterone [36][33]. Differences were also observed between the two semesters. Furthermore, Bifidobacterium, Neisseria, Blautia, and Collinsella increased most significantly in the third semester while Dehalobacterium, Clostridium, and Bacteroidales were markedly higher in the first [36][33]. Another report disclosed that maternal microbiome biodiversity changes with the progression of pregnancy and is associated with gestational weight gain [70][62]. While studies imply that gut microbiota changes dramatically such as an increase in lactic acid-producing bacteria coupled with a decrease in butyrate-producing bacteria, a recent analysis conducted on Japanese women during early and late pregnancy negates differences between late and early pregnancy microbial composition and reveals that the recruited women did not show notable differences in gut microbiota related to pregnancy, except for the phylum TM7, which decreased in late pregnancy [71,72][63][64]. Similarly, another study confirms a lack of difference and mentions that the study carried out by [35][32], which reported significant changes associated with pregnancy, recruited women who were consuming probiotic supplementation [73][65]. Interestingly, studies imply that pregnancy-induced changes in the female gut microbiome occurring at the onset of pregnancy may be vulnerable to modulation by diet while being independent of maternal weight gain and even the number of successive pregnancies [74,75][66][67]. Thus, in late pregnancy, the microbiota readjusts carbohydrate-related functions expression in consistency with the high glucose availability [76][68]. Notably, the microbiome of pregnant women can also bring about metabolic alterations in germ-free hosts. Furthermore, a study disclosed that fecal transplantation from pregnant women to germ-free mice induced greater adiposity and insulin insensitivity [35][32]. Given the plethora of studies indicative of the effect of the gut microbiome in pregnancy, further work is warranted to comprehend a more detailed mechanistic understanding as well as work to develop pre/pro/postbiotics for pregnant women.6. Changes in Gut Microbiome during the Menstrual Cycle
The menstrual cycle extends 28 ± 4 days and is comprised of the follicular, luteal, and menstrual phases [80,81][69][70]. The menstrual cycle is accompanied by significant hormonal fluctuations. Moreover, the level of the steroid hormone estrogen soars in the middle of the follicular phase, drops after ovulation, and rises back again in the early luteal phase [80][69]. The early luteal phase is also characterized by an increase in the progesterone level [81][70]. The sudden drop in these two hormones in the late luteal phase brings about menses [81][70]. Many healthy females report variations in gastrointestinal (GI) symptoms during their menstrual cycle, which may be deduced to the presence of sex hormone receptors along the GI tract [81][70]. For instance, a study investigating the relationship between menstrual cycle phase, daily stool number, and consistency reveals looser stool consistency in the early menstrual period in comparison to midcycle in six out of the seven employed participants [82][71]. However, studies investigating GI transit throughout the menstrual cycle produce contradicting results. Furthermore, some disclose an increase in transit time during the luteal phase, accompanying the increased progesterone levels while others negate any change [13,83,84][10][72][73]. Hormonal fluctuations may impose pressures on the function and composition of the human microbiome [85][74]. Namely, the gut microbiome is reported to be influenced by estrogen [86][75]. Furthermore, estrogen levels are associated with gut microbiome alpha diversity and fecal Clostrdia taxa [13][10]. Given the influence estrogen has on the gut microbiome, the potential link between the GI disturbances and menstruation may potentially be mediated by the gut microbiome. The gut microbiota is also reported to be affected by the steroid hormone progesterone. A study reports the amelioration of depression and anxiety-like behaviors accompanying the premenstrual, post-partum, and premenopausal periods by progesterone in mice [84][73]. The influence between sex hormones and the gut microbiome is bidirectional. Moreover, bacteria can metabolize sex hormones through various enzymes such as hydroxysteroid dehydrogenase, regulating the balance between active and inactive steroids [80][69]. Namely, fecal bacteria carry out hydrolytic reductive and oxidative reactions of androgens and estrogen [80][69]. Furthermore, the gut microbiome markedly influences estrogen levels [86][75]. This is through the gut microbiome’s secretion of β-glucuronidase, which is the enzyme responsible for estrogen deconjugation [86][75]. A decrease in the gut microbiome diversity affects β-glucuronidase activity adversely, lowering estrogen levels [13][10]. Since estrogen is only biologically active if deconjugated, this deconjugation enables estrogen to bind to its receptors: estrogen receptor alpha (ERα) and estrogen receptor beta (ERβ) [86,87][75][76]. Estrogen is crucial for homeostasis in healthy premenopausal women and its decrease accompanying menopause drives metabolic rate reduction and weight gain, yet it also stimulates epithelial proliferation within the female reproductive tract, driving various proliferative diseases such as uterine fibroids and endometriosis [86,87][75][76].7. Gut Microbiota Composition Alterations Accompanying Menopause
Female sex hormones such as estrogen impact microbiota in various body sites, especially the gut [16][13]. When women possess sufficient estrogen, their gut microbiota displays species diversity where beneficial bacteria are dominant and harmful bacteria growth is inhibited [38][35]. The gut microbiome has been correlated with menopause: the cessation of menstruation accompanied by estrogen down-regulation, ovary function loss, and hormone receptors dysfunction [16,38][13][35]. Menopause is associated with a lower gut microbial species diversity [92][77]. Thus, there are marked differences in the gut microbiomes and their metabolites in premenopausal and postmenopausal women, as shown in Table 1 and Figure 1 [93][78]. Changes in the gut microbiome have been reported in the perimenopausal period, the period before menopause occurs. Namely, during the perimenopausal period, the relative abundance of beneficial bacteria such as Lactobacillus and Bifidobacteria is markedly reduced while that of harmful bacteria such as Enterobacter is increased in women [38][35]. In a study, bilateral ovariectomizing was employed to investigate gut microbiota changes accompanying perimenopause and it revealed that ovariectomized mice displayed the lowest abundances, which was regulated by estrogen supplementation, implying a bidirectional relationship between the microbiota and estrogen [38][35]. Moreover, the study discloses that obesity in peri- and post-menopausal women is associated with possessing a gut microbiota unable to metabolize the soy isoflavone daidzein to O-desmethylangolensin [94][79]. Of note, the gut microbiota of post-menopausal women was observed to be closer in resemblance to men than that of pre-menopausal women [39][36]. Namely, postmenopausal women, similar to age-matched men, have a lower abundance of SCFA-producing bacteria [39][36]. Furthermore, the number of species from genera that differentiate men from women decreased after menopause implying a masculinization of the gut microbiota composition postmenopause [39][36]. Studies report that premenopausal women have higher abundances of several Alistipes, Bifidobacterium, and Ruminococcus species and lower abundances of Bacteroides, Prevotella, and Haemophilus species, while postmenopausal women have fewer Firmicutes and Roseburia spp., and more Bacteroidetes and Tolumonasare in their fecal samples [39,93][36][78].8. The Role of the Gut Microbiome in Postmenopausal Female Health
The gut microbiome has been correlated with various diseases accompanying menopause. Obesity affects 65% of postmenopausal women and interestingly, the relationship between the gut microbiota and estrogen is speculated to mediate this weight gain [16][13]. Moreover, the gut microbiome has been related to obesity, and menopause is associated with a heightened risk of obesity [92,93][77][78]. Notably, other than the differences in Akkermansia muciniphila, Bifidobacterium animalis, Dorea, and Desulfovibrio, the gut microbial characteristics of diet-induced and bilaterally ovariectomized obese mice are reportedly similar [38][35]. However, a study reports that while menopausal obesity and dietary obesity led to similar gut microbiome structures, menopausal obesity engenders a different intestinal microbiota, namely, Bifidobacterium animalis, which was solely observed in the ovariectomized mice [96][80]. Notably, the gut microbiome was reported to impact skeletal muscle mass through its synthesis of SCFA butyrate in healthy menopausal women [97][81]. Moreover, increased capacity for gut microbial synthesis was markedly associated with serum butyrate levels and skeletal muscle index, and two main butyrate-producing bacterial species, Faecalibacterium prausnitzii, and Butyricimonas virosa, were positively associated with this increased capacity for gut microbial synthesis of butyrate and the skeletal muscle index [97][81]. Gut ecology was also reported to contribute to the mediation of the protective effects increased aerobic capacity may have against menopause-associated cardiometabolic risk, especially the production of signaling molecules such as short-chain fatty acids produced by the gut [98][82]. Moreover, another study reports that the response to physical exercise, which is reported to modify the intestinal microbiota composition, is actually contingent upon the initial microbiota profile [92][77]. Postmenopausal women with breast cancer have been reported to possess an altered composition and estrogen-independent low diversity of their microbiota [16,54][13][48]. An analysis reports that postmenopausal women recently diagnosed with breast cancer had a less diverse fecal microbiota with a composition that differs from that of postmenopausal women without breast cancer and higher urinary estrogens [99][83]. This was confirmed by another study reporting the potentially decreased postmenopausal breast cancer risk for women who possess high intestinal microbial diversity [100][84]. Additionally, the abundance of SCFA-producing bacteria was reduced in healthy premenopausal women while Pediococcus and Desulfovibrio were relatively characteristic of premenopausal breast cancer patients [101][85]. Thirty-eight species were increased in postmenopausal breast cancer patients, namely, Shewanella putrefaciens, Enterococcus gallinarum, Escherichia coli, Klebsiella sp_1_1_55, Prevotella amnii, Actinomyces sp. HPA0247, and Erwinia amylovora, while seven species were underrepresented such as Eubacterium eligens and Lactobacillus vaginalis [54][48].9. The Role of the Gut Microbiome and Postmenopausal Bone Health
Among postmenopausal women, osteoporosis and its precursor osteopenia are prevalent metabolic bone diseases [41][38]. The gut microbiome has also been implicated in bone-related diseases among postmenopausal women and their manifestations, as shown in Table 1 and Figure 1. Moreover, an increase in gut permeability, which is associated with lower bone mineral density, has been reported during perimenopause [105][86]. A study reports decreased bacterial richness and diversity, and significant differences in abundance levels among phyla and genera in the gut microbial community in postmenopausal osteoporosis [42][39]. However, the study negates any significant correlation between bacterial diversity and estrogen [42][39]. An analysis of fecal samples from postmenopausal women with osteoporosis and with normal bone mass reveals a marked discrepancy between the gut microbiota of both groups [43][40]. Namely, the proportion of the genus Prevotella was notably higher in postmenopausal women with normal bone mass, implying a potential bone-protective effect of Prevotella [43][40]. Moreover, fracture incidence was markedly higher in postmenopausal women with low Bacteroides abundance than in controls [44][41]. Conversely, a recent study reports that Bacteroides were more prevalent in osteoporosis and osteopenia groups [41][38]. That study also reveals significant taxonomic compositional differences in osteoporotic and osteopenic, and healthy postmenopausal women, such as a higher abundance of unclassified Clostridia and methanogenic archaea, than in healthy postmenopausal women [41][38]. Another study recognized taxa-specific variations in the intestinal microbiota associated with bone turnover markers, especially C-terminal cross-linking telopeptide of type I collagen (CTX) [106][87]. This could be elucidated by the hormonal changes characterizing menopause. Furthermore, the lack of female hormones brings about bone loss and osteoporosis [96][80]. Interestingly, probiotic administration was reported to ameliorate osteopenia in postmenopausal women. Moreover, the administration of probiotic treatment and bioavailable isoflavone attenuated bone mineral density loss brought about by estrogen deficiency, promoted a favorable estrogen metabolite profile, and improved bone turnover [107][88].References
- Kautzky-Willer, A.; Harreiter, J.; Pacini, G. Sex and gender differences in risk, pathophysiology and complications of type 2 diabetes mellitus. Endocr. Rev. 2016, 37, 278–316.
- What Health Issues or Conditions Affect Women Differently Than Men? Available online: https://www.nichd.nih.gov/health/topics/womenshealth/conditioninfo/howconditionsaffect (accessed on 1 February 2022).
- Friedson-Ridenour, S.; Dutcher, T.V.; Calderon, C.; Brown, L.D.; Olsen, C.W. Gender analysis for one health: Theoretical perspectives and recommendations for practice. Ecohealth 2019, 16, 306–316.
- Vitale, C.; Fini, M.; Spoletini, I.; Lainscak, M.; Seferovic, P.; Rosano, G.M. Under-representation of elderly and women in clinical trials. Int. J. Cardiol. 2017, 232, 216–221.
- Mirin, A.A. Gender disparity in the funding of diseases by the US National Institutes of Health. J. Women’s Health 2021, 30, 956–963.
- Thackray, V.G. Sex, Microbes, and Polycystic Ovary Syndrome. Trends Endocrinol. Metab. 2019, 30, 54–65.
- Org, E.; Mehrabian, M.; Parks, B.W.; Shipkova, P.; Liu, X.; Drake, T.A.; Lusis, A.J. Sex differences and hormonal effects on gut microbiota composition in mice. Gut Microbes. 2016, 7, 313–322.
- Rizzetto, L.; Fava, F.; Tuohy, K.M.; Selmi, C. Connecting the immune system, systemic chronic inflammation and the gut microbiome: The role of sex. J. Autoimmun. 2018, 92, 12–34.
- Siddiqui, R.; Mungroo, M.R.; Alharbi, A.M.; Alfahemi, H.; Khan, N.A. The Use of Gut Microbial Modulation Strategies as Interventional Strategies for Ageing. Microorganisms 2022, 10, 1869.
- Pugh, J.N.; Lydon, K.; O’Donovan, C.M.; O’Sullivan, O.; Madigan, S.M. More than a gut feeling: What is the role of the gastrointestinal tract in female athlete health? Eur. J. Sport Sci. 2022, 22, 755–764.
- Dominianni, C.; Sinha, R.; Goedert, J.J.; Pei, Z.; Yang, L.; Hayes, R.B.; Ahn, J. Sex, body mass index, and dietary fiber intake influence the human gut microbiome. PLoS ONE 2015, 10, e0124599.
- Nikolova, V.L.; Hall, M.R.; Hall, L.J.; Cleare, A.J.; Stone, J.M.; Young, A.H. Perturbations in gut microbiota composition in psychiatric disorders: A review and meta-analysis. JAMA Psychiatry 2021, 78, 1343–1354.
- Vieira, A.T.; Castelo, P.M.; Ribeiro, D.A.; Ferreira, C.M. Influence of Oral and Gut Microbiota in the Health of Menopausal Women. Front. Microbiol. 2017, 8, 1884.
- Parida, S.; Sharma, D. The Microbiome-Estrogen Connection and Breast Cancer Risk. Cells 2019, 8, 1642.
- Flores, R.; Shi, J.; Fuhrman, B.; Xu, X.; Veenstra, T.D.; Gail, M.H.; Gajer, P.; Ravel, J.; Goedert, J.J. Fecal microbial determinants of fecal and systemic estrogens and estrogen metabolites: A cross-sectional study. J. Transl. Med. 2012, 10, 253.
- Kwa, M.; Plottel, C.S.; Blaser, M.J.; Adams, S. The Intestinal Microbiome and Estrogen Receptor-Positive Female Breast Cancer. J. Natl. Cancer Inst. 2016, 108, djw029.
- Khalesi, S.; Bellissimo, N.; Vandelanotte, C.; Williams, S.; Stanley, D.; Irwin, C. A review of probiotic supplementation in healthy adults: Helpful or hype? Eur. J. Clin. Nutr. 2019, 73, 24–37.
- Mei, Z.; Li, D. The role of probiotics in vaginal health. Front. Cell. Infect. Microbiol. 2022, 12, 963868.
- Falagas, M.E.; Betsi, G.I.; Tokas, T.; Athanasiou, S. Probiotics for prevention of recurrent urinary tract infections in women. Drugs 2006, 66, 1253–1261.
- Reid, G. The development of probiotics for women’s health. Can. J. Microbiol. 2017, 63, 269–277.
- He, F.F.; Li, Y.M. Role of gut microbiota in the development of insulin resistance and the mechanism underlying polycystic ovary syndrome: A review. J. Ovarian Res. 2020, 13, 73.
- Zhou, L.; Ni, Z.; Cheng, W.; Yu, J.; Sun, S.; Zhai, D.; Yu, C.; Cai, Z. Characteristic gut microbiota and predicted metabolic functions in women with PCOS. Endocr. Connect. 2020, 9, 63–73.
- Qi, X.; Yun, C.; Sun, L.; Xia, J.; Wu, Q.; Wang, Y.; Wang, L.; Zhang, Y.; Liang, X.; Wang, L.; et al. Gut microbiota-bile acid-interleukin-22 axis orchestrates polycystic ovary syndrome. Nat. Med. 2019, 25, 1459.
- Lindheim, L.; Bashir, M.; Münzker, J.; Trummer, C.; Zachhuber, V.; Leber, B.; Horvath, A.; Pieber, T.R.; Gorkiewicz, G.; Stadlbauer, V.; et al. Alterations in Gut Microbiome Composition and Barrier Function Are Associated with Reproductive and Metabolic Defects in Women with Polycystic Ovary Syndrome (PCOS): A Pilot Study. PLoS ONE 2017, 12, e0168390.
- Zhao, X.; Jiang, Y.; Xi, H.; Chen, L.; Feng, X. Exploration of the Relationship Between Gut Microbiota and Polycystic Ovary Syndrome (PCOS): A Review. Geburtshilfe Frauenheilkd. 2020, 80, 161–171.
- Rizk, M.G.; Thackray, V.G. Intersection of Polycystic Ovary Syndrome and the Gut Microbiome. J. Endocr. Soc. 2020, 5, bvaa177.
- Torres, P.J.; Siakowska, M.; Banaszewska, B.; Pawelczyk, L.; Duleba, A.J.; Kelley, S.T.; Thackray, V.G. Gut Microbial Diversity in Women with Polycystic Ovary Syndrome Correlates with Hyperandrogenism. J. Clin. Endocrinol. Metab. 2018, 103, 1502–1511.
- Yoon, H.S.; Cho, C.H.; Yun, M.S.; Jang, S.J.; You, H.J.; Kim, J.H.; Han, D.; Cha, K.H.; Moon, S.H.; Lee, K.; et al. Akkermansia muciniphila secretes a glucagon-like peptide-1-inducing protein that improves glucose homeostasis and ameliorates metabolic disease in mice. Nat. Microbiol. 2021, 6, 563–573.
- NIH. The Relationship of the Intestinal Microbiome and the Menstrual Cycle. 2018. Available online: https://clinicaltrials.gov/ct2/show/NCT03581201 (accessed on 1 February 2022).
- Ji, Y.J.; Li, H.; Xie, P.F.; Li, Z.H.; Li, H.W.; Yin, Y.L.; Blachier, F.; Kong, X.F. Stages of pregnancy and weaning influence the gut microbiota diversity and function in sows. J. Appl. Microbiol. 2019, 127, 867–879.
- Mallott, E.K.; Borries, C.; Koenig, A.; Amato, K.R.; Lu, A. Reproductive hormones mediate changes in the gut microbiome during pregnancy and lactation in Phayre’s leaf monkeys. Sci. Rep. 2020, 10, 9961.
- Koren, O.; Goodrich, J.K.; Cullender, T.C.; Spor, A.; Laitinen, K.; Bäckhed, H.K.; Gonzalez, A.; Werner, J.J.; Angenent, L.T.; Knight, R.; et al. Host remodeling of the gut microbiome and metabolic changes during pregnancy. Cell 2012, 150, 470–480.
- Nuriel-Ohayon, M.; Neuman, H.; Ziv, O.; Belogolovski, A.; Barsheshet, Y.; Bloch, N.; Uzan, A.; Lahav, R.; Peretz, A.; Frishman, S.; et al. Progesterone increases Bifidobacterium relative abundance during late pregnancy. Cell Rep. 2019, 27, 730–736.e3.
- Jin, M.; Li, D.; Ji, R.; Liu, W.; Xu, X.; Feng, X. Changes in Gut Microorganism in Patients with Positive Immune Antibody-Associated Recurrent Abortion. BioMed Res. Int. 2020, 2020, 4673250.
- Meng, Q.; Ma, M.; Zhang, W.; Bi, Y.; Cheng, P.; Yu, X.; Fu, Y.; Chao, Y.; Ji, T.; Li, J.; et al. The gut microbiota during the progression of atherosclerosis in the perimenopausal period shows specific compositional changes and significant correlations with circulating lipid metabolites. Gut Microbes 2021, 13, 1–27.
- Mayneris-Perxachs, J.; Arnoriaga-Rodríguez, M.; Luque-Córdoba, D.; Priego-Capote, F.; Pérez-Brocal, V.; Moya, A.; Burokas, A.; Maldonado, R.; Fernández-Real, J.M. Gut microbiota steroid sexual dimorphism and its impact on gonadal steroids: Influences of obesity and menopausal status. Microbiome 2020, 8, 136.
- Santos-Marcos, J.A.; Rangel-Zuñiga, O.A.; Jimenez-Lucena, R.; Quintana-Navarro, G.M.; Garcia-Carpintero, S.; Malagon, M.M.; Landa, B.B.; Tena-Sempere, M.; Perez-Martinez, P.; Lopez-Miranda, J.; et al. Influence of gender and menopausal status on gut microbiota. Maturitas 2018, 116, 43–53.
- Rettedal, E.A.; Ilesanmi-Oyelere, B.L.; Roy, N.C.; Coad, J.; Kruger, M.C. The gut microbiome is altered in postmenopausal women with osteoporosis and osteopenia. JBMR Plus 2021, 5, e10452.
- He, J.; Xu, S.; Zhang, B.; Xiao, C.; Chen, Z.; Si, F.; Fu, J.; Lin, X.; Zheng, G.; Yu, G.; et al. Gut microbiota and metabolite alterations associated with reduced bone mineral density or bone metabolic indexes in postmenopausal osteoporosis. Aging 2020, 12, 8583–8604.
- Wang, Z.; Chen, K.; Wu, C.; Chen, J.; Pan, H.; Liu, Y.; Wu, P.; Yuan, J.; Huang, F.; Lang, J.; et al. An emerging role of Prevotella histicola on estrogen deficiency–induced bone loss through the gut microbiota–bone axis in postmenopausal women and in ovariectomized mice. Am. J. Clin. Nutr. 2021, 114, 1304–1313.
- Ozaki, D.; Kubota, R.; Maeno, T.; Abdelhakim, M.; Hitosugi, N. Association between gut microbiota, bone metabolism, and fracture risk in postmenopausal Japanese women. Osteoporos. Int. 2021, 32, 145–156.
- Mikó, E.; Kovács, T.; Sebő, É.; Tóth, J.; Csonka, T.; Ujlaki, G.; Sipos, A.; Szabó, J.; Méhes, G.; Bai, P. Microbiome-Microbial Metabolome-Cancer Cell Interactions in Breast Cancer-Familiar, but Unexplored. Cells 2019, 8, 293.
- Jacobson, D.; Moore, K.; Gunderson, C.; Rowland, M.; Austin, R.; Honap, T.P.; Xu, J.; Warinner, C.; Sankaranarayanan, K.; Lewis, C.M., Jr. Shifts in gut and vaginal microbiomes are associated with cancer recurrence time in women with ovarian cancer. PeerJ 2021, 9, e11574.
- Sims, T.T.; Colbert, L.E.; Zheng, J.; Delgado Medrano, A.Y.; Hoffman, K.L.; Ramondetta, L.; Jazaeri, A.; Jhingran, A.; Schmeler, K.M.; Daniel, C.R.; et al. Gut microbial diversity and genus-level differences identified in cervical cancer patients versus healthy controls. Gynecol. Oncol. 2019, 155, 237–244.
- Sethi, V.; Kurtom, S.; Tarique, M.; Lavania, S.; Malchiodi, Z.; Hellmund, L.; Zhang, L.; Sharma, U.; Giri, B.; Garg, B.; et al. Gut Microbiota Promotes Tumor Growth in Mice by Modulating Immune Response. Gastroenterol 2018, 155, 33–37.e6.
- Wong, S.H.; Kwong, T.; Wu, C.Y.; Yu, J. Clinical applications of gut microbiota in cancer biology. Semin. Cancer Biol. 2019, 55, 28–36.
- Akbar, N.; Khan, N.A.; Muhammad, J.S.; Siddiqui, R. The role of gut microbiome in cancer genesis and cancer prevention. Health Sci. Rev. 2022, 2, 100010.
- Zhu, J.; Liao, M.; Yao, Z.; Liang, W.; Li, Q.; Liu, J.; Yang, H.; Ji, Y.; Wei, W.; Tan, A.; et al. Breast cancer in postmenopausal women is associated with an altered gut metagenome. Microbiome 2018, 6, 1–13.
- Yi, M.; Yu, S.; Qin, S.; Liu, Q.; Xu, H.; Zhao, W.; Chu, Q.; Wu, K. Gut microbiome modulates efficacy of immune checkpoint inhibitors. J. Hematol. Oncol. 2018, 11, 47.
- Alpuim Costa, D.; Nobre, J.G.; Batista, M.V.; Ribeiro, C.; Calle, C.; Cortes, A.; Marhold, M.; Negreiros, I.; Borralho, P.; Brito, M.; et al. Human Microbiota and Breast Cancer-Is There Any Relevant Link?—A Literature Review and New Horizons Toward Personalised Medicine. Front. Microbiol. 2021, 12, 584332.
- Muccee, F.; Ghazanfar, S.; Ajmal, W.; Al-Zahrani, M. In-Silico Characterization of Estrogen Reactivating β-Glucuronidase Enzyme in GIT Associated Microbiota of Normal Human and Breast Cancer Patients. Genes 2022, 13, 1545.
- Lumachi, F.; Santeufemia, D.A.; Basso, S.M. Current medical treatment of estrogen receptor-positive breast cancer. World J. Biol. Chem. 2015, 6, 231–239.
- Suraya, R.; Nagano, T.; Kobayashi, K.; Nishimura, Y. Microbiome as a Target for Cancer Therapy. Integr. Cancer Ther. 2020, 19, 1534735420920721.
- Hanker, A.B.; Sudhan, D.R.; Arteaga, C.L. Overcoming endocrine resistance in breast cancer. Cancer Cell 2020, 37, 496–513.
- Scheidemann, E.R.; Shajahan-Haq, A.N. Resistance to CDK4/6 Inhibitors in Estrogen Receptor-Positive Breast Cancer. Int. J. Mol. Sci. 2021, 22, 12292.
- Mutic, A.D.; Jordan, S.; Edwards, S.M.; Ferranti, E.P.; Thul, T.A.; Yang, I. The postpartum maternal and newborn microbiomes. MCN. Am. J. Matern. Child Nurs. 2017, 42, 326–331.
- Huang, L.; Cai, M.; Li, L.; Zhang, X.; Xu, Y.; Xiao, J.; Huang, Q.; Luo, G.; Zeng, Z.; Jin, C.; et al. Gut microbiota changes in preeclampsia, abnormal placental growth and healthy pregnant women. BMC Microbiol. 2021, 21, 265.
- Tian, X.Y.; Xing, J.W.; Zheng, Q.Q.; Gao, P.F. 919 Syrup Alleviates Postpartum Depression by Modulating the Structure and Metabolism of Gut Microbes and Affecting the Function of the Hippocampal GABA/Glutamate System. Front. Cell. Infect. Microbiol. 2021, 11, 694443.
- Faas, M.M.; Liu, Y.; Borghuis, T.; van Loo-Bouwman, C.A.; Harmsen, H.; De Vos, P. Microbiota induced changes in the immune response in pregnant mice. Front. Immunol. 2020, 10, 2976.
- Pelzer, E.S.; Allan, J.A.; Theodoropoulos, C.; Ross, T.; Beagley, K.W.; Knox, C.L. Hormone-dependent bacterial growth, persistence and biofilm formation–a pilot study investigating human follicular fluid collected during IVF cycles. PLoS ONE 2012, 7, e49965.
- Huang, X.; Gao, J.; Zhao, Y.; He, M.; Ke, S.; Wu, J.; Zhou, Y.; Fu, H.; Yang, H.; Chen, C.; et al. Dramatic remodeling of the gut microbiome around parturition and its relationship with host serum metabolic changes in sows. Front. Microbiol. 2019, 10, 2123.
- Smid, M.C.; Ricks, N.M.; Panzer, A.; Mccoy, A.N.; Azcarate-Peril, M.A.; Keku, T.O.; Boggess, K.A. Maternal gut microbiome biodiversity in pregnancy. Am. J. Perinatol. 2018, 35, 24–30.
- Di Simone, N.; Santamaria Ortiz, A.; Specchia, M.; Tersigni, C.; Villa, P.; Gasbarrini, A.; Scambia, G.; D’Ippolito, S. Recent insights on the maternal microbiota: Impact on pregnancy outcomes. Front. Immunol. 2020, 11, 528202.
- Sakurai, K.; Kato, T.; Tanabe, H.; Taguchi-Atarashi, N.; Sato, Y.; Eguchi, A.; Watanabe, M.; Ohno, H.; Mori, C. Association between gut microbiota composition and glycoalbumin level during pregnancy in Japanese women: Pilot study from Chiba Study of Mother and Child Health. J. Diabetes Investig. 2020, 11, 699–706.
- DiGiulio, D.B.; Callahan, B.J.; McMurdie, P.J.; Costello, E.K.; Lyell, D.J.; Robaczewska, A.; Sun, C.L.; Goltsman, D.S.; Wong, R.J.; Shaw, G.; et al. Temporal and spatial variation of the human microbiota during pregnancy. Proc. Natl. Acad. Sci. USA 2015, 112, 11060–11065.
- Gohir, W.; Whelan, F.J.; Surette, M.G.; Moore, C.; Schertzer, J.D.; Sloboda, D.M. Pregnancy-related changes in the maternal gut microbiota are dependent upon the mother’s periconceptional diet. Gut Microbes 2015, 6, 310–320.
- Berry, A.S.; Pierdon, M.K.; Misic, A.M.; Sullivan, M.C.; O’Brien, K.; Chen, Y.; Murray, S.J.; Ramharack, L.A.; Baldassano, R.N.; Parsons, T.D.; et al. Remodeling of the maternal gut microbiome during pregnancy is shaped by parity. Microbiome 2021, 9, 1–15.
- Gosalbes, M.J.; Compte, J.; Moriano-Gutierrez, S.; Vallès, Y.; Jiménez-Hernández, N.; Pons, X.; Artacho, A.; Francino, M.P. Metabolic adaptation in the human gut microbiota during pregnancy and the first year of life. EbioMedicine 2019, 39, 497–509.
- Mulak, A.; Taché, Y.; Larauche, M. Sex hormones in the modulation of irritable bowel syndrome. WJG 2014, 20, 2433–2448.
- Bharadwaj, S.; Barber, M.D.; Graff, L.A.; Shen, B. Symptomatology of irritable bowel syndrome and inflammatory bowel disease during the menstrual cycle. Gastroenterol. Rep. 2015, 3, 185–193.
- Simmons, L.; Heitkemper, M.; Shaver, J. Gastrointestinal function during the menstrual cycle. Health Care Women Int. 1988, 9, 201–209.
- Wald, A.; Van Thiel, D.H.; Hoechstetter, L.; Gavaler, J.S.; Egler, K.M.; Verm, R.; Scott, L.; Lester, R. Gastrointestinal transit: The effect of the menstrual cycle. Gastroenterology 1981, 80, 1497–1500.
- Sovijit, W.N.; Sovijit, W.E.; Pu, S.; Usuda, K.; Inoue, R.; Watanabe, G.; Yamaguchi, H.; Nagaoka, K. Ovarian progesterone suppresses depression and anxiety-like behaviors by increasing the Lactobacillus population of gut microbiota in ovariectomized mice. Neurosci. Res. 2021, 168, 76–82.
- Bostanci, N.; Krog, M.C.; Hugerth, L.W.; Bashir, Z.; Fransson, E.; Boulund, F.; Belibasakis, G.N.; Wannerberger, K.; Engstrand, L.; Nielsen, H.S.; et al. Dysbiosis of the human oral microbiome during the menstrual cycle and vulnerability to the external exposures of smoking and dietary sugar. Front. Cell. Infect. Microbiol. 2021, 11, 625229.
- Baker, J.M.; Al-Nakkash, L.; Herbst-Kralovetz, M.M. Estrogen–gut microbiome axis: Physiological and clinical implications. Maturitas 2017, 103, 45–53.
- Chen, K.L.; Madak-Erdogan, Z. Estrogen and microbiota crosstalk: Should we pay attention? Trends Endocrinol. Metab. 2016, 27, 752–755.
- Dupuit, M.; Rance, M.; Morel, C.; Bouillon, P.; Boscaro, A.; Boisseau, N.; Vincent, M.; Vazeille, E.; Barnich, N.; Chassaing, B. Impact of concurrent training on body composition and gut microbiota in postmenopausal women with overweight or obesity. Med. Sci. Sport. Exerc. 2021, 54, 517–529.
- Zhao, H.; Chen, J.; Li, X.; Sun, Q.; Qin, P.; Wang, Q. Compositional and functional features of the female premenopausal and postmenopausal gut microbiota. FEBS Lett. 2019, 593, 2655–2664.
- Miller, L.M.; Lampe, J.W.; Newton, K.M.; Gundersen, G.; Fuller, S.; Reed, S.D.; Frankenfeld, C.L. Being overweight or obese is associated with harboring a gut microbial community not capable of metabolizing the soy isoflavone daidzein to O-desmethylangolensin in peri-and post-menopausal women. Maturitas 2017, 99, 37–42.
- Choi, S.; Hwang, Y.J.; Shin, M.J.; Yi, H. Difference in the gut microbiome between ovariectomy-induced obesity and diet-induced obesity. J. Microbiol. Biotechnol. 2017, 27, 2228–2236.
- Lv, W.Q.; Lin, X.; Shen, H.; Liu, H.M.; Qiu, X.; Li, B.Y.; Shen, W.D.; Ge, C.L.; Lv, F.Y.; Shen, J.; et al. Human gut microbiome impacts skeletal muscle mass via gut microbial synthesis of the short-chain fatty acid butyrate among healthy menopausal women. J. Cachexia Sarcopenia Muscle 2021, 12, 1860–1870.
- Cox-York, K.A.; Sheflin, A.M.; Foster, M.T.; Gentile, C.L.; Kahl, A.; Koch, L.G.; Britton, S.L.; Weir, T.L. Ovariectomy results in differential shifts in gut microbiota in low versus high aerobic capacity rats. Physiol. Rep. 2015, 3, e12488.
- Goedert, J.J.; Jones, G.; Hua, X.; Xu, X.; Yu, G.; Flores, R.; Falk, R.T.; Gail, M.H.; Shi, J.; Ravel, J.; et al. Investigation of the association between the fecal microbiota and breast cancer in postmenopausal women: A population-based case-control pilot study. JNCI J. Natl. Cancer Inst. 2015, 107, djv147.
- Fuhrman, B.J.; Feigelson, H.S.; Flores, R.; Gail, M.H.; Xu, X.; Ravel, J.; Goedert, J.J. Associations of the fecal microbiome with urinary estrogens and estrogen metabolites in postmenopausal women. J. Clin. Endocrinol. Metab. 2014, 99, 4632–4640.
- He, C.; Liu, Y.; Ye, S.; Yin, S.; Gu, J. Changes of intestinal microflora of breast cancer in premenopausal women. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 503–513.
- Shieh, A.; Epeldegui, M.; Karlamangla, A.S.; Greendale, G.A. Gut permeability, inflammation, and bone density across the menopause transition. JCI Insight 2020, 5, e134092.
- Chen, L.; Yan, S.; Yang, M.; Yu, F.; Wang, J.; Wang, X.; Xu, H.; Shi, J.; Pan, L.; Zeng, Y.; et al. The gut microbiome is associated with bone turnover markers in postmenopausal women. Am. J. Transl. Res. 2021, 13, 12601–12613.
- Lambert, M.N.T.; Thybo, C.B.; Lykkeboe, S.; Rasmussen, L.M.; Frette, X.; Christensen, L.P.; Jeppesen, P.B. Combined bioavailable isoflavones and probiotics improve bone status and estrogen metabolism in postmenopausal osteopenic women: A randomized controlled trial. Am. J. Clin. Nutr. 2017, 106, 909–920.
