2. Gut Microbiome in Healthy Females
Gender-dependent differences in the gut microbiome have been reported
[9]. Women’s gut microbiome composition markedly differs from men’s
[10][11][13,14]. As depicted in
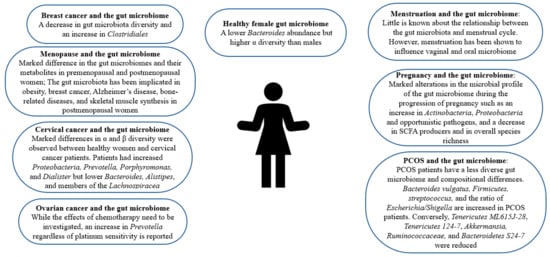
Figure 1, women are reported to have a lower
Bacteroides abundance but higher α diversity, a measure of diversity within an individual sample
[10][11][12][13,14,15]. Female sex hormone levels affect the microbiota composition
[13][16]. However, this influence is bidirectional. This is because the microbiome regulates steroid hormone levels including estrogens
[14][17]. In a clinical trial employing 25 men, 7 postmenopausal women, and 19 premenopausal women, results imply that the intestinal microbiome indeed affects systemic estrogen levels
[15][18]. Another study reports that gut microbial diversity is positively correlated with estrogen metabolites to parent estrogen ratio in a group of postmenopausal women
[14][17]. However, further studies with a larger and more inclusive population should be carried out to investigate this linkage in women before menopause. Certain enteric bacteria, whose genome is called the estrobolome, metabolize estrogens
[14][16][17,19]. To be excreted, estrogens are hepatically conjugated through glucuronidation or sulfonation
[16][19]. These estrobolome bacterial species with their ß-glucuronidase activity can deconjugate excreted estrogens in the bile and prevent their excretion
[14][16][17,19]. This may explain why fecal glucuronidase levels were reported to be inversely associated with gut estrogen levels
[15][18]. The human gut also carries out various local and distant functions through hormonal metabolites and intermediates
[16][19]. Remarkably, gut microbes also carry out the synthesis of estrogen-like compounds from nutrition
[14][17]. In healthy females, probiotic administration has shown promise. While probiotic administration fails to engender persistent gut microbiota changes, it has been reported to improve vaginal lactobacilli concentration, female health system bowel movement, and immune system responses in healthy adults
[17][20]. Moreover, probiotics help enhance local vaginal immunity and maintain female reproductive tract health
[18][21]. Notably, certain bacterial strains, namely,
Lactobacillus strains have been shown to prevent the recurrence of urinary tract infections and bacterial vaginosis
[19][20][22,23].
Figure 1.
The role of gut microbiome in female health.
3. Gut Microbiome and PCOS
PCOS affects 8–13% of women worldwide in their reproductive age
[21][24]. While the clinical phenotype may vary, insulin resistance and hyperandrogenism are the hallmarks of PCOS
[22][25]. The gut microbiome of PCOS sufferers differs from that of controls
[23][26]. As can be seen in
Table 1 and
Figure 1, PCOS patients have a less diverse gut microbiome, which is correlated with hyperandrogenism
[22][24][25][25,27,28]. In a clinical trial, 43 healthy females and 50 PCOS patients were employed, taking into consideration the influence of body weight
[23][26]. While Alpha (α) diversity was comparable in both groups, Beta (β) diversity, a measure of diversity among different samples, was markedly decreased in the gut microbiota of PCOS patients
[12][23][15,26]. Conversely, a study reports that α diversity is altered in PCOS patients’ guts
[26][29]. Another study confirms the alteration by reporting a decrease in α diversity in the guts of women with PCOS
[27][30]. Notably, the abundance of a Bacteroides species responsible for the deconjugation of conjugated bile acids synthesized in the liver,
Bacteroides vulgatus (
B. vulgatus), was significantly higher in women with PCOS than in the controls
[23][26]. Firmicutes, a phylum correlated with obesity, is also more abundant in PCOS patients while
Tenericutes ML615J-28,
Tenericutes 124-7, and
Bacteroidetes S24-7 levels are reduced
[22][24][25][25,27,28]. An increase in
streptococcus and the ratio of
Escherichia/Shigella was also reported in the gut of women with PCOS
[22][25]. Opposingly, it was found that
Akkermansia, a species reported to modulate energy metabolism and glucose tolerance in humans, and
Ruminococcaceae, were less abundant in PCOS patients
[22][28][25,31].
Table 1.
The impact of gut microbiota on female health.
4. Gut Microbiome and Cancer
Disturbances in the gut microbiome have been associated with and observed in various cancers including gastric, colorectal, hepatic, pancreatic, and prostate cancer
[45][46][47][51,52,53]. Interestingly, in pancreatic cancer and melanoma murine models, a significant decrease in subcutaneous tumor burden was observed after depleting the gut microbiome through antibiotic administration
[45][51]. Dysbiosis of the gut microbiota is also observed in various cancers affecting the female population including breast, cervical, and ovarian cancer as depicted in
Table 1 and
Figure 1 [42][43][44][45,46,47]. Furthermore, some studies imply a decrease in the diversity of the gut microbiota and an increase in the abundance of
Clostridiales in breast cancer patients
[42][45]. An analysis of the gut microbiota of postmenopausal women with breast cancer reveals that while the differences in relative species abundance in gut microbiota between premenopausal breast cancer patients and premenopausal controls was negligible, 45 species differed significantly in their relative abundance between postmenopausal patients and postmenopausal controls
[48][54]. Moreover, in postmenopausal cancer patients, 38 species were overrepresented such as
Escherichia coli, Actinomyces sp
. HPA0247, Klebsiella sp
_1_1_55, Prevotella amnii, and
Shewanella. A study revealed that α and β diversity differed significantly between employed cervical cancer patients and cancer-free women
[44][47]. Additionally, the study disclosed that cervical cancer patients had higher levels of
Prevotella, Porphyromonas, and
Dialister while cancer-free individuals had greater levels of
Bacteroides, Alistipes, and members of the
Lachnospiracea than controls
[44][47]. In ovarian cancer patients, a shared increase in
Prevotella regardless of platinum sensitivity was observed, but the study was unable to count for the potential effects of chemotherapy
[43][46].
Aside from regulating the host’s immune system, the gut microbiome is involved in both oncogenesis and the suppression of malignant transformation
[45][49][51,55]. Furthermore, bacterial metabolites produced by the gut microbiome also regulate cancer cell metabolism
[42][50][45,56]. These secreted bacterial metabolites act like hormones since they can enter the circulation, reach far targets, and carry out important functions such as impacting mitochondrial metabolism and modulating the behavior of breast cancer cells, lithocholic acid (LCA), SCFAs, cadaverine, and deconjugated estrogens
[42][50][45,56]. As mentioned in the previous section, the gut microbiome is an important player in estrogen metabolism. Since 80% of breast cancer cases are estrogen receptor-positive, the deconjugation of estrogens by the gut microbiome is of relevance
[16][51][52][53][54][55][19,57,58,59,60,61]. Aside from being more abundant in the gut of breast cancer patients,
Clostridiales reactivates estrogens and increases their serum levels
[42][45]. Estrogen receptors play a direct role in the expression of nuclear-coded mitochondrial proteins
[42][45]. An increase in oxidative phosphorylation promotes metastasis
[42][45]. The gut microbiome also synthesizes estrogen-like compounds from dietary sources
[14][17].
5. Gut Microbiome and Pregnancy
Aside from infant health, evidence indicates that maternal microbiome niches influence maternal well-being and post-partum recovery
[56][64]. Furthermore, gut microbiome disturbances have been linked with the clinical characteristics of preeclampsia, a pregnancy complication characterized by high blood pressure, and some even hypothesize that there is a link between the maternal gut microbiome and postpartum depression
[56][57][64,65]. In fact, a Chinese herbal medicine has been shown to ameliorate postpartum depression through modulating the gut microbiota
[58][66]. A study also indicates that the maternal gut microbiome may play a part in the immunological adaptations accompanying pregnancy, as shown in
Table 1 and
Figure 1 [59][67]. Interestingly, an investigation of gut microbiota changes in patients with positive immune antibody-associated recurrent miscarriage reveals that some highly abundant genera, such as
Blautia and
Bacteroides, may be incriminated in recurrent miscarriage
[34][37]. Bidirectional interactions between the gut microbiome and pregnancy have been reported. For instance, bacterial growth can be influenced by hormonal changes
[60][61][68,69]. Moreover, gut microbial changes during pregnancy are mediated by hormonal changes accompanying gestation
[30][31][33,34]. Namely, it has been reported that fecal progesterone levels were negatively correlated with diversity during pregnancy
[31][34]. Notably, profound alterations in the microbial profile of the gut microbiome have been observed during the progression of pregnancy such as an increase in
Actinobacteria, Proteobacteria, and opportunistic pathogens, and a decrease in SCFA producers and in overall species richness
[32][33][35,36].
In humans, an analysis of the gut microbiome of thirty-five women in their first and third trimesters of pregnancy reveals that
Bifidobacterium, Blautia, unclassified
Ruminococcaceae,
Bacteroides, unclassified
Lachnospiraceae, unclassified
Clostridiales, Akkermansia, Faecalibacterium, Ruminococcus, and
Prevotella were the generally dominant bacterial species. Interestingly,
Bifidobacterium is crucial for human milk oligosaccharide degradation and
Prevotella metabolizes estradiol and progesterone
[33][36]. Differences were also observed between the two semesters. Furthermore,
Bifidobacterium, Neisseria, Blautia, and
Collinsella increased most significantly in the third semester while
Dehalobacterium, Clostridium, and
Bacteroidales were markedly higher in the first
[33][36]. Another report disclosed that maternal microbiome biodiversity changes with the progression of pregnancy and is associated with gestational weight gain
[62][70]. While studies imply that gut microbiota changes dramatically such as an increase in lactic acid-producing bacteria coupled with a decrease in butyrate-producing bacteria, a recent analysis conducted on Japanese women during early and late pregnancy negates differences between late and early pregnancy microbial composition and reveals that the recruited women did not show notable differences in gut microbiota related to pregnancy, except for the phylum TM7, which decreased in late pregnancy
[63][64][71,72]. Similarly, another study confirms a lack of difference and mentions that the study carried out by
[32][35], which reported significant changes associated with pregnancy, recruited women who were consuming probiotic supplementation
[65][73]. Interestingly, studies imply that pregnancy-induced changes in the female gut microbiome occurring at the onset of pregnancy may be vulnerable to modulation by diet while being independent of maternal weight gain and even the number of successive pregnancies
[66][67][74,75]. Thus, in late pregnancy, the microbiota readjusts carbohydrate-related functions expression in consistency with the high glucose availability
[68][76]. Notably, the microbiome of pregnant women can also bring about metabolic alterations in germ-free hosts. Furthermore, a study disclosed that fecal transplantation from pregnant women to germ-free mice induced greater adiposity and insulin insensitivity
[32][35]. Given the plethora of studies indicative of the effect of the gut microbiome in pregnancy, further work is warranted to comprehend a more detailed mechanistic understanding as well as work to develop pre/pro/postbiotics for pregnant women.
6. Changes in Gut Microbiome during the Menstrual Cycle
The menstrual cycle extends 28 ± 4 days and is comprised of the follicular, luteal, and menstrual phases
[69][70][80,81]. The menstrual cycle is accompanied by significant hormonal fluctuations. Moreover, the level of the steroid hormone estrogen soars in the middle of the follicular phase, drops after ovulation, and rises back again in the early luteal phase
[69][80]. The early luteal phase is also characterized by an increase in the progesterone level
[70][81]. The sudden drop in these two hormones in the late luteal phase brings about menses
[70][81]. Many healthy females report variations in gastrointestinal (GI) symptoms during their menstrual cycle, which may be deduced to the presence of sex hormone receptors along the GI tract
[70][81]. For instance, a study investigating the relationship between menstrual cycle phase, daily stool number, and consistency reveals looser stool consistency in the early menstrual period in comparison to midcycle in six out of the seven employed participants
[71][82]. However, studies investigating GI transit throughout the menstrual cycle produce contradicting results. Furthermore, some disclose an increase in transit time during the luteal phase, accompanying the increased progesterone levels while others negate any change
[10][72][73][13,83,84].
Hormonal fluctuations may impose pressures on the function and composition of the human microbiome
[74][85]. Namely, the gut microbiome is reported to be influenced by estrogen
[75][86]. Furthermore, estrogen levels are associated with gut microbiome alpha diversity and fecal
Clostrdia taxa
[10][13]. Given the influence estrogen has on the gut microbiome, the potential link between the GI disturbances and menstruation may potentially be mediated by the gut microbiome. The gut microbiota is also reported to be affected by the steroid hormone progesterone. A study reports the amelioration of depression and anxiety-like behaviors accompanying the premenstrual, post-partum, and premenopausal periods by progesterone in mice
[73][84].
The influence between sex hormones and the gut microbiome is bidirectional. Moreover, bacteria can metabolize sex hormones through various enzymes such as hydroxysteroid dehydrogenase, regulating the balance between active and inactive steroids
[69][80]. Namely, fecal bacteria carry out hydrolytic reductive and oxidative reactions of androgens and estrogen
[69][80]. Furthermore, the gut microbiome markedly influences estrogen levels
[75][86]. This is through the gut microbiome’s secretion of β-glucuronidase, which is the enzyme responsible for estrogen deconjugation
[75][86]. A decrease in the gut microbiome diversity affects β-glucuronidase activity adversely, lowering estrogen levels
[10][13]. Since estrogen is only biologically active if deconjugated, this deconjugation enables estrogen to bind to its receptors: estrogen receptor alpha (ERα) and estrogen receptor beta (ERβ)
[75][76][86,87]. Estrogen is crucial for homeostasis in healthy premenopausal women and its decrease accompanying menopause drives metabolic rate reduction and weight gain, yet it also stimulates epithelial proliferation within the female reproductive tract, driving various proliferative diseases such as uterine fibroids and endometriosis
[75][76][86,87].
7. Gut Microbiota Composition Alterations Accompanying Menopause
Female sex hormones such as estrogen impact microbiota in various body sites, especially the gut
[13][16]. When women possess sufficient estrogen, their gut microbiota displays species diversity where beneficial bacteria are dominant and harmful bacteria growth is inhibited
[35][38]. The gut microbiome has been correlated with menopause: the cessation of menstruation accompanied by estrogen down-regulation, ovary function loss, and hormone receptors dysfunction
[13][35][16,38]. Menopause is associated with a lower gut microbial species diversity
[77][92]. Thus, there are marked differences in the gut microbiomes and their metabolites in premenopausal and postmenopausal women, as shown in
Table 1 and
Figure 1 [78][93]. Changes in the gut microbiome have been reported in the perimenopausal period, the period before menopause occurs. Namely, during the perimenopausal period, the relative abundance of beneficial bacteria such as
Lactobacillus and
Bifidobacteria is markedly reduced while that of harmful bacteria such as
Enterobacter is increased in women
[35][38]. In a study, bilateral ovariectomizing was employed to investigate gut microbiota changes accompanying perimenopause and it revealed that ovariectomized mice displayed the lowest abundances, which was regulated by estrogen supplementation, implying a bidirectional relationship between the microbiota and estrogen
[35][38]. Moreover, the study discloses that obesity in peri- and post-menopausal women is associated with possessing a gut microbiota unable to metabolize the soy isoflavone daidzein to
O-desmethylangolensin
[79][94].
Of note, the gut microbiota of post-menopausal women was observed to be closer in resemblance to men than that of pre-menopausal women
[36][39]. Namely, postmenopausal women, similar to age-matched men, have a lower abundance of SCFA-producing bacteria
[36][39]. Furthermore, the number of species from genera that differentiate men from women decreased after menopause implying a masculinization of the gut microbiota composition postmenopause
[36][39]. Studies report that premenopausal women have higher abundances of several
Alistipes,
Bifidobacterium, and
Ruminococcus species and lower abundances of
Bacteroides,
Prevotella, and
Haemophilus species, while postmenopausal women have fewer
Firmicutes and
Roseburia spp., and more
Bacteroidetes and
Tolumonasare in their fecal samples
[36][78][39,93].
8. The Role of the Gut Microbiome in Postmenopausal Female Health
The gut microbiome has been correlated with various diseases accompanying menopause. Obesity affects 65% of postmenopausal women and interestingly, the relationship between the gut microbiota and estrogen is speculated to mediate this weight gain
[13][16]. Moreover, the gut microbiome has been related to obesity, and menopause is associated with a heightened risk of obesity
[77][78][92,93]. Notably, other than the differences in
Akkermansia muciniphila,
Bifidobacterium animalis, Dorea, and
Desulfovibrio, the gut microbial characteristics of diet-induced and bilaterally ovariectomized obese mice are reportedly similar
[35][38]. However, a study reports that while menopausal obesity and dietary obesity led to similar gut microbiome structures, menopausal obesity engenders a different intestinal microbiota, namely,
Bifidobacterium animalis, which was solely observed in the ovariectomized mice
[80][96].
Notably, the gut microbiome was reported to impact skeletal muscle mass through its synthesis of SCFA butyrate in healthy menopausal women
[81][97]. Moreover, increased capacity for gut microbial synthesis was markedly associated with serum butyrate levels and skeletal muscle index, and two main butyrate-producing bacterial species,
Faecalibacterium prausnitzii, and
Butyricimonas virosa, were positively associated with this increased capacity for gut microbial synthesis of butyrate and the skeletal muscle index
[81][97]. Gut ecology was also reported to contribute to the mediation of the protective effects increased aerobic capacity may have against menopause-associated cardiometabolic risk, especially the production of signaling molecules such as short-chain fatty acids produced by the gut
[82][98]. Moreover, another study reports that the response to physical exercise, which is reported to modify the intestinal microbiota composition, is actually contingent upon the initial microbiota profile
[77][92].
Postmenopausal women with breast cancer have been reported to possess an altered composition and estrogen-independent low diversity of their microbiota
[13][48][16,54]. An analysis reports that postmenopausal women recently diagnosed with breast cancer had a less diverse fecal microbiota with a composition that differs from that of postmenopausal women without breast cancer and higher urinary estrogens
[83][99]. This was confirmed by another study reporting the potentially decreased postmenopausal breast cancer risk for women who possess high intestinal microbial diversity
[84][100]. Additionally, the abundance of SCFA-producing bacteria was reduced in healthy premenopausal women while
Pediococcus and
Desulfovibrio were relatively characteristic of premenopausal breast cancer patients
[85][101]. Thirty-eight species were increased in postmenopausal breast cancer patients, namely,
Shewanella putrefaciens, Enterococcus gallinarum, Escherichia coli, Klebsiella sp_1_1_55
, Prevotella amnii, Actinomyces sp. HPA0247
, and
Erwinia amylovora, while seven species were underrepresented such as
Eubacterium eligens and
Lactobacillus vaginalis [48][54].
9. The Role of the Gut Microbiome and Postmenopausal Bone Health
Among postmenopausal women, osteoporosis and its precursor osteopenia are prevalent metabolic bone diseases
[38][41]. The gut microbiome has also been implicated in bone-related diseases among postmenopausal women and their manifestations, as shown in
Table 1 and
Figure 1. Moreover, an increase in gut permeability, which is associated with lower bone mineral density, has been reported during perimenopause
[86][105]. A study reports decreased bacterial richness and diversity, and significant differences in abundance levels among phyla and genera in the gut microbial community in postmenopausal osteoporosis
[39][42]. However, the study negates any significant correlation between bacterial diversity and estrogen
[39][42]. An analysis of fecal samples from postmenopausal women with osteoporosis and with normal bone mass reveals a marked discrepancy between the gut microbiota of both groups
[40][43]. Namely, the proportion of the genus
Prevotella was notably higher in postmenopausal women with normal bone mass, implying a potential bone-protective effect of
Prevotella [40][43]. Moreover, fracture incidence was markedly higher in postmenopausal women with low
Bacteroides abundance than in controls
[41][44]. Conversely, a recent study reports that
Bacteroides were more prevalent in osteoporosis and osteopenia groups
[38][41]. That study also reveals significant taxonomic compositional differences in osteoporotic and osteopenic, and healthy postmenopausal women, such as a higher abundance of unclassified
Clostridia and methanogenic archaea, than in healthy postmenopausal women
[38][41]. Another study recognized taxa-specific variations in the intestinal microbiota associated with bone turnover markers, especially C-terminal cross-linking telopeptide of type I collagen (CTX)
[87][106]. This could be elucidated by the hormonal changes characterizing menopause. Furthermore, the lack of female hormones brings about bone loss and osteoporosis
[80][96]. Interestingly, probiotic administration was reported to ameliorate osteopenia in postmenopausal women. Moreover, the administration of probiotic treatment and bioavailable isoflavone attenuated bone mineral density loss brought about by estrogen deficiency, promoted a favorable estrogen metabolite profile, and improved bone turnover
[88][107].