Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Conner Chen and Version 1 by Monica Verdoia.
Cardiovascular disease is the leading cause of death worldwide and ischemic heart disease is responsible for approximately half of these deaths. Angina is the main symptom of ischemic heart disease; mirroring a mismatch between oxygen supply and demand.
- stable angina
- coronary artery disease
- nitrates
1. Introduction
Cardiovascular disease is the leading cause of death worldwide and ischemic heart disease is responsible for approximately half of these deaths [1,2][1][2]. Among its clinical manifestations, acute coronary syndromes typically need urgency or emergency management [3], while chronic coronary syndromes can be usually managed with an outpatient approach for both diagnosis and treatment, in absence of identified high-risk features [4]. Patients with stable angina pectoris have a 3 to 4% annual incidence of myocardial infarction and death and the principal therapies available (lifestyle modifications, medications, percutaneous coronary intervention, and coronary artery bypass grafting) have the primary aim of reducing the risk of death, myocardial infarction and stroke and improve quality of life by reducing symptoms [5].
2. Pathophysiology of Angina Pectoris
Angina pectoris is caused by episodes of myocardial ischemia provoked by an imbalance in myocardial oxygen supply-demand, which may be due either to an increase in oxygen demand (depending on wall tension, i.e., intraventricular pressure, left ventricular radius—and volume—and wall thickness; heart rate and myocardial contractility) or a decrease in oxygen supply (reduction in coronary blood flow, anaemia and other causes reducing oxygen-carrying capacity of the blood).
Angina typically occurs when oxygen demand increases in presence of a reduced coronary low reserve, often due to an atherosclerotic coronary plaque obstructing the vessel lumen. Some patients may report symptoms and have instrumental signs of ischemia—without angiographically-significant coronary lesions—due to anomalies in the coronary circulation (vasospastic angina, coronary microvascular dysfunction) [6,7][6][7]. Abrupt reduction or interruption of blood flow, typically caused by plaque rupture/erosion and coronary thrombosis is the typical mechanism involved in acute coronary syndromes [8].
Therefore, drugs that reduce the progression of atherosclerosis and prevent plaque remodelling are recommended in coronary artery disease (CAD) patients to reduce the risk of myocardial infarction (MI) and antianginal drugs may have prognostic benefits in certain populations [4].
The management of chronic stable angina encompasses a broad spectrum of measures, from lifestyle modifications, various medications (which may affect prognosis and symptoms) and revascularization [4]. A stepwise approach for the management of chronic angina is proposed in Figure 1.
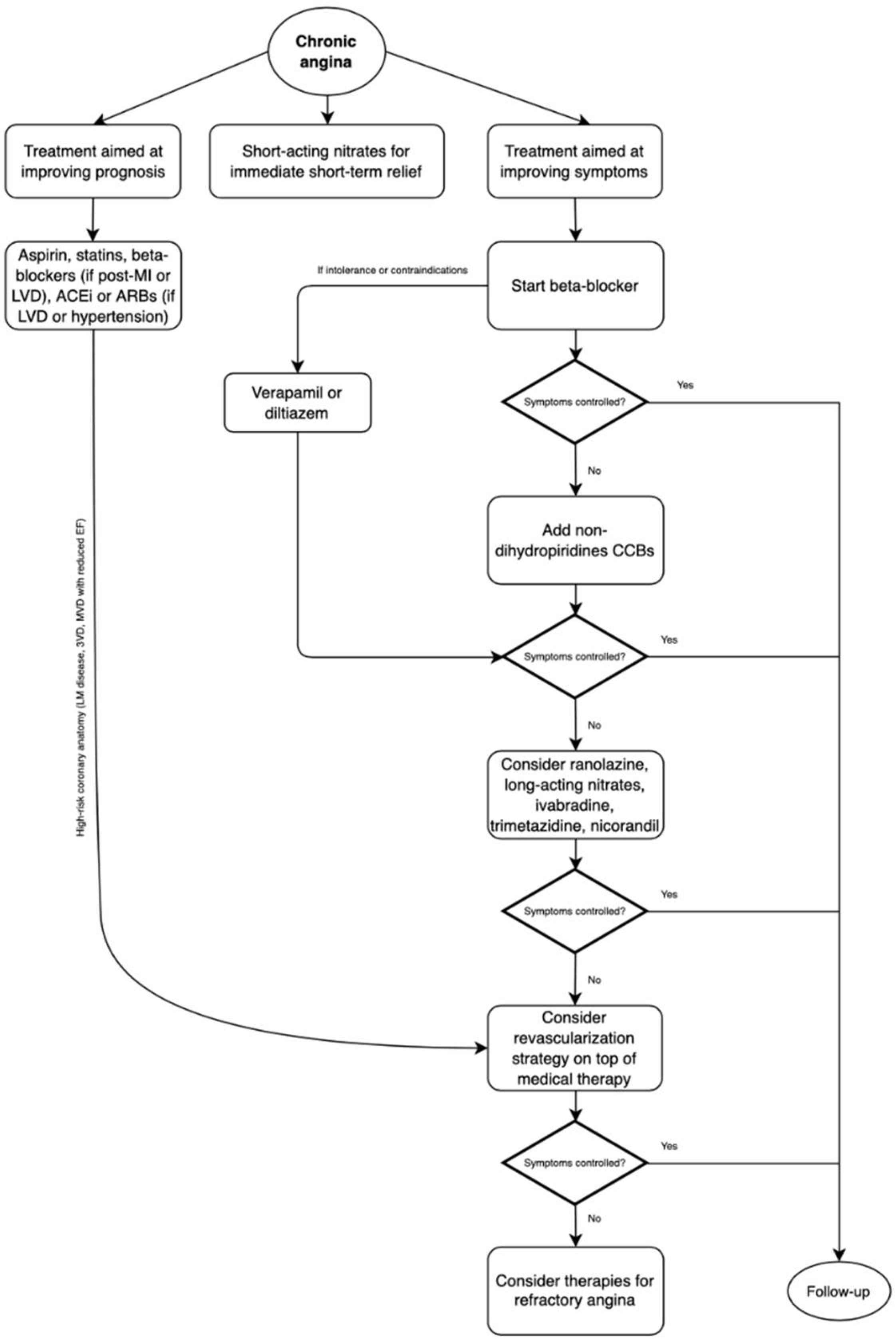
Figure 1. Management of chronic angina due to obstructive coronary artery disease. MI: myocardial infarction, LVD: left ventricular dysfunction, ACEi: angiotensin-converting enzyme inhibitors, ARBs: angiotensin-receptor blockers, CCBs: calcium channel blockers.
3. Lifestyle Modifications
Healthy lifestyle behaviour should be encouraged in all patients, in particular regular exercise, healthy (i.e., Mediterranean) diet pattern, intentional weight loss and smoking cessation.
Cigarette smoking is responsible for 50% of deaths in smokers and a lifetime smoker has on average a 10-year life loss [9]. Smoking cessation improves the prognosis in patients with chronic coronary syndromes with a 36% reduction in mortality risk in quitters [10].
A Mediterranean diet, rich in fruit, vegetables, fibre, polyunsaturated fats, and legumes, with limited intake of red meat, processed foods, saturated fats and dairy is recommended in the European guidelines [11].
The lifetime risk of incident cardiovascular disease is higher in overweight and obese, and a healthy weight (BMI < 25) should be obtained and maintained, since intentional weight loss was associated with a lower risk of clinical outcomes in patients with CAD [12]. A physically active lifestyle (30–60 min moderate physical activity) should be encouraged, since it has been associated with lower mortality, with the lowest risk among those achieving high levels of activity [13].
4. Percutaneous Coronary Intervention and Coronary Artery Bypass Graft
Revascularization in stable ischemic heart disease has two main indications: to improve symptoms and to improve survival. In fact, in patients undergoing CABG for stable ischemic heart disease, survival benefits were observed only in the case of left main disease, triple vessel disease or ischemic cardiomyopathy [14,15,16,17][14][15][16][17]. However, most of these trials were conducted before the introduction of the most recent pharmacological agents for optimal medical therapy (OMT), such as intensive lipid-lowering therapy, dual antiplatelet therapy, heart failure medications and novel antidiabetic agents.
Inversely, the STICH trial randomized over 1200 patients with ischemic left ventricular dysfunction (LVEF < 35%) to receive medical therapy alone or CABG and medical therapy and showed the superiority of revascularization and medical therapy at 10-year follow-up for all-cause mortality, while the primary endpoint was not met at 5-year follow-up [14,15][14][15].
Similarly, no trial has shown a survival benefit of percutaneous coronary intervention over OMT in patients with stable angina [19,20,21][18][19][20]—neither if physiology guided [22][21]—even if a larger benefit could have been expected in subjects with high surgical risk, as those currently being treated for ischemic heart disease (IHD) [23,24][22][23].
The ISCHEMIA trial randomly assigned >5000 patients with moderate-to-severe ischemia (approximately 90% with a history of angina pectoris) to an initial invasive strategy with angiography and revascularization when feasible or to medical therapy alone and angiography if medical therapy failed. There was no significant difference between the two groups for the occurrence of ischemic cardiovascular events or death over a median of 3.2 years [21][20]; participants in the invasive strategy arm had larger improvements in angina-related health status than those randomized to conservative strategy arm, with minimal benefit in asymptomatic patients and larger benefits in those who had angina at baseline [25][24]. It is noteworthy that the trial excluded patients with recent acute coronary syndrome, unprotected left main disease > 50%, ejection fraction < 35%, NYHA class III or IV heart failure and unacceptable angina despite maximal medical therapy, therefore these results cannot be generalized to all chronic angina patients [21][20].
Furthermore, subgroup analysis of ISCHEMIA showed that neither moderate nor severe ischemia was associated with increased mortality relative to mild/no ischemia, while increasing CAD severity was associated with death and myocardial infarction for the most versus the least severe CAD subgroup. The most severe CAD subgroup had inferior rate of CV death or MI in the invasive strategy arm, with similar 4-year all-cause mortality [26][25].
The recent REVIVED-BCIS2 randomized 700 patients with ejection fraction ≤35%, extensive coronary artery disease suitable for PCI and demonstrable myocardial viability to receive PCI + OMT or OMT alone. Over a median of 41 months, there was no difference between the two groups for the primary outcome (death or heart failure hospitalization) and left ventricular ejection fraction, while quality-of-life scores appeared to favor the PCI group. However, since most patients had little or no angina at enrollment, these findings cannot be extrapolated to patients with moderate-to-severe angina and acute coronary syndromes [27][26].
Similarly, the management of the left main disease is still a matter of debate [28,29,30,31][27][28][29][30]. The NOBLE trial showed inferior clinical outcomes at 5-years follow-up with PCI when compared to CABG, mortality rates among groups were similar but higher rates of non-procedural MI and repeat revascularization were noticed in the PCI arm [29][28]. The EXCEL trial showed noninferiority of unprotected left main PCI in low-to-intermediate anatomic complexity (SYNTAX score <33) for the composite endpoint of death, stroke and myocardial infarction [31][30]. However, data with newer generations of drug eluting stents, which could provide more favorable results for PCI, are still lacking.
5. “Event-Reducing” Drugs
Medical therapies for cardiovascular risk reduction have the primary purpose of reducing the likelihood of a coronary event and its downstream complications (such heart failure, arrhythmias). This purpose may be achieved by stabilizing the atherosclerotic disease progression and reducing the response to thrombosis pathway [8].
Lipid-lowering therapies are a cornerstone in CAD managment and statins are usually the most prescribed drugs since their wide availability and solid evidence on cardiovascular adverse events reduction [32,33,34,35,36][31][32][33][34][35]. Adding ezetimibe to statin therapy resulted in further LDL cholesterol lowering and in a risk reduction of occurrence of the primary composite endpoint (CV death, nonfatal MI, unstable angina requiring rehospitalization, coronary revascularization or nonfatal stroke) on a median follow-up of 6 years [37][36]. Proprotein convertase subtilisin-kexin type 9 (PCSK-9) inhibitors alirocumab and evolocumab were both effective in reducing the risk of cardiovascular events in high-risk populations in the FOURIER and ODISSEY without significant safety concerns [38,39][37][38].
In patients with elevated triglyceride levels (135 to 499 mg/dl) despite the use of statins, icosapent ethyl significantly reduced the risk of ischemic events, including CV death, when compared to placebo [40][39]. Inclisiran and bempedoic acid have been proven effective in reducing LDL cholesterol of approximately 50% and 16%, respectively, when compared to placebo [41,42][40][41].
Antithrombotic therapy with aspirin is effective in reducing all-cause mortality in secondary prevention after ACS, while only combination of ASA + ticagrelor and ASA + clopidogrel + very low dose rivaroxaban were associated with better outcomes than aspirin alone (OR 0.8 for CV mortality in ASA + ticagrelor group, OR 0.64 for ASA + clopidogrel + rivaroxaban for all-cause mortality), with major bleeding increasing by 45–95% with dual antiplatelet therapy and 2–6 fold with triple antithrombotic therapy [43][42]. Some studies suggest use of clopidogrel over ASA for single antiplatelet therapy [44,45][43][44]. Dual antiplatelet therapy is recommended after PCI to reduce ischemic events in both chronic and acute coronary syndromes, even if its length may vary according to setting (ACS vs. CCS) and patient-specific ischemic (clinical and procedure-related factors) and bleeding risks [4,23,46,47][4][22][45][46]. In stable cardiovascular disease setting, low-dose rivaroxaban was associated with lower occurrence of the primary composite outcome (CV death, stroke, MI) and death from any cause than aspirin alone, at a cost of higher risk of major bleeding [48][47].
ACE-inhibitors (or angiotensin-receptor blockers in case of ACE-i intolerance) are recommended in patients with chronic coronary syndrome and coexisting hypertension, left ventricular dysfunction (LVEF ≤ 40%), chronic kidney disease or diabetes, while their benefit tend to disappear in the absence of heart failure or high cardiovascular risk [4,49,50,51][4][48][49][50].
Mineralocorticoid receptor antagonists such spironolactone are recommended in post-MI patients already on ACEi and beta-blocker with significant left ventricular dysfunction (LVEF ≤ 35%) and diabetes or heart failure [52][51]. Antidiabetic medications such GLP-1 receptor agonists and SGLT-2 inhibitors are increasingly used in cardiovascular disease since they reduce all-cause mortality, CV mortality, nonfatal myocardial infarction and kidney failure [4,5,23,53][4][5][22][52].
Colchicine has a broad anti-inflammatory effect, it may accumulate in neutrophils and macrophages and prevent the assembly of inflammasome and the expression of IL-1β, and proinflammatory cytokines [54][53]. Various RCTs showed its efficacy in reducing cardiovascular events in both chronic and acute coronary syndrome cohorts, but it is also noteworthy that some studies found a possible increase in non-CV deaths and in COPS trials (which did not meet the primary composite outcome in ACS patients) in the colchicine arm had higher all-cause mortality [55,56,57][54][55][56].
Further evidence on the negative effects of inflammation in the progression of cardiovascular disease is provided by the CANTOS trial, in which canakinumab showed efficacy in reducing the occurrence of the composite endpoint (nonfatal MI, nonfatal stroke, CV death) in patients with previous myocardial infarction and elevated CRP [58][57].
6. Angina with Non-Obstructed Coronary Arteries
Angina with non-obstructed coronary arteries may encompass a wide spectrum of clinical presentations [118,119][58][59]. The two main mechanisms are coronary microvascular dysfunction and vasospastic angina. Microvascular angina is due to myocardial ischemia caused by coronary microvascular dysfunction, which may result from structural alteration or vasomotor disorders or both [120,121][60][61]. Vasospastic angina is due to dynamic epicardial coronary obstruction in a context of a vasomotor disorder [6,122][6][62]. Despite the absence of obstructive CAD, the prognosis, and quality of life of patients are affected negatively, especially if ischemia is documented by non-invasive functional tests [6]. In the diagnostic framework, invasive coronary angiography, measurement of left ventricular end-diastolic pressure, functional tests with adenosine and vasoreactivity test with acetylcholine are performed in an attempt to classify the patient in one of four categories: non-cardiac pain, epicardial vasospastic angina, microvascular angina, or a mixed form [120,123][60][63]. Information provided by left heart catheterization is of invaluable importance since management differs deeply. According to EAPCI consensus, patients with microvascular angina may be treated accordingly with β-blocker (e.g., nebivolol), dihydropyridines CCBs, ranolazine, trimetazidine, and ACEi/ARBs. VSA should be treated with CCBs (either DHP or non-DHP), nitrates or nicorandil. In mixed forms in which microvascular and vasospastic angina coexist, therapeutic options rely on CCBs, nicorandil, trimetazidine, ACEi/ARBs, and statins [6]. The recent EDIT-CMD trial randomized patients with angina with non-obstructive coronary arteries (ANOCA) to receive diltiazem or placebo. After 6 weeks, diltiazem therapy did not improve coronary vasomotor dysfunction, symptoms or quality of life, but did reduce the prevalence of epicardial spasm [92][64]. Equally important should be the prescription of drugs for risk factor management (hypertension, diabetes, dyslipidemia) and the advice to follow a healthy lifestyle [6,124][6][65]. Nowadays, ischemia with non-obstructed coronary arteries remains still an under-recognized and undertreated condition since the lack of uniform management and the lack of wide availability of invasive diagnostic tools [125][66].7. Refractory Angina
The prevalence of refractory angina may vary between 5 and 10% of stable CAD patients. Refractory angina was defined as “a chronic condition characterized by the presence of angina caused by coronary insufficiency in the presence of coronary artery disease which cannot be controlled by a combination of medical therapy, angioplasty and coronary artery bypass surgery” [126][67]. Reasons to withhold further revascularization procedures are various and may range from intrinsic anatomic complexity of CAD with unsuitable anatomy to patient-related conditions with lack of graft material, poor LV function, severe extracardiac diseases, and advanced age. Patients with refractory angina have a very poor quality of life with frequent hospitalizations, and therapeutic options, despite increasing, have often a low level of evidence in their favour [4]. Therapeutic nonpharmacological technologies in refractory angina address neural processing or myocardial perfusion (promoting collateral growth or transmurally redistributing blood flow). The ESC guidelines address with a low class of recommendation (IIb) enhanced external counterpulsation, coronary sinus reducer therapy and spinal cord stimulation to ameliorate symptoms and quality of life [4]. CD34+/CD133+ cell therapy is showing promising results, even in patients with coronary microvascular dysfunction, but it is still limited to research purposes [127][68]. Other therapies such as viral-transfer-based angiogenesis, transmyocardial laser revascularization, extracorporeal shockwave myocardial revascularization, and transcutaneous and subcutaneous electrical nerve stimulation lack solid placebo-controlled evidence [128][69].References
- Vos, T.; Lim, S.S.; Abbafati, C.; Abbas, K.M.; Abbasi, M.; Abbasifard, M.; Abbasi-Kangevari, M.; Abbastabar, H.; Abd-Allah, F.; Abdelalim, A.; et al. Global Burden of 369 Diseases and Injuries in 204 Countries and Territories, 1990–2019: A Systematic Analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222.
- Saglietto, A.; Manfredi, R.; Elia, E.; D’Ascenzo, F.; De Ferrari, G.M.; Biondi-Zoccai, G.; Munzel, T. Cardiovascular Disease Burden: Italian and Global Perspectives. Minerva Cardiol. Angiol. 2021, 69, 231–240.
- Bergmark, B.A.; Mathenge, N.; Merlini, P.A.; Lawrence-Wright, M.B.; Giugliano, R.P. Acute Coronary Syndromes. Lancet 2022, 399, 1347–1358.
- Neumann, F.J.; Sechtem, U.; Banning, A.P.; Bonaros, N.; Bueno, H.; Bugiardini, R.; Chieffo, A.; Crea, F.; Czerny, M.; Delgado, V.; et al. 2019 ESC Guidelines for the Diagnosis and Management of Chronic Coronary Syndromes. Eur. Heart J. 2020, 41, 407–477.
- Joshi, P.H.; de Lemos, J.A. Diagnosis and Management of Stable Angina: A Review. J. Am. Med. Assoc. 2021, 325, 1765–1778.
- Kunadian, V.; Chieffo, A.; Camici, P.G.; Berry, C.; Escaned, J.; Maas, A.H.E.M.; Prescott, E.; Karam, N.; Appelman, Y.; Fraccaro, C.; et al. An EAPCI Expert Consensus Document on Ischaemia with Non-Obstructive Coronary Arteries in Collaboration with European Society of Cardiology Working Group on Coronary Pathophysiology & Microcirculation Endorsed by Coronary Vasomotor Disorders International Study Group. Eur. Heart J. 2020, 41, 3504–3520.
- Perera, D.; Berry, C.; Hoole, S.P.; Sinha, A.; Rahman, H.; Morris, P.D.; Kharbanda, R.K.; Petraco, R.; Channon, K. Invasive Coronary Physiology in Patients with Angina and Non-Obstructive Coronary Artery Disease: A Consensus Document from the Coronary Microvascular Dysfunction Workstream of the British Heart Foundation/National Institute for Health Research Partnership. Heart 2022.
- Arbab-Zadeh, A.; Nakano, M.; Virmani, R.; Fuster, V. Acute Coronary Events. Circulation 2012, 125, 1147–1156.
- Doll, R.; Peto, R.; Boreham, J.; Sutherland, I. Mortality in Relation to Smoking: 50 Years’ Observations on Male British Doctors. BMJ 2004, 328, 1519.
- Critchley, J.A.; Capewell, S. Mortality Risk Reduction Associated With Smoking Cessation in Patients With Coronary Heart Disease. JAMA 2003, 290, 86.
- Sofi, F.; Abbate, R.; Gensini, G.F.; Casini, A. Accruing Evidence on Benefits of Adherence to the Mediterranean Diet on Health: An Updated Systematic Review and Meta-Analysis. Am. J. Clin. Nutr. 2010, 92, 1189–1196.
- Khan, S.S.; Ning, H.; Wilkins, J.T.; Allen, N.; Carnethon, M.; Berry, J.D.; Sweis, R.N.; Lloyd-Jones, D.M. Association of Body Mass Index With Lifetime Risk of Cardiovascular Disease and Compression of Morbidity. JAMA Cardiol. 2018, 3, 280.
- Moholdt, T.; Lavie, C.J.; Nauman, J. Sustained Physical Activity, Not Weight Loss, Associated With Improved Survival in Coronary Heart Disease. J. Am. Coll. Cardiol. 2018, 71, 1094–1101.
- Velazquez, E.J.; Lee, K.L.; Deja, M.A.; Jain, A.; Sopko, G.; Marchenko, A.; Ali, I.S.; Pohost, G.; Gradinac, S.; Abraham, W.T.; et al. Coronary-Artery Bypass Surgery in Patients with Left Ventricular Dysfunction. N. Engl. J. Med. 2011, 364, 1607–1616.
- Velazquez, E.J.; Lee, K.L.; Jones, R.H.; Al-Khalidi, H.R.; Hill, J.A.; Panza, J.A.; Michler, R.E.; Bonow, R.O.; Doenst, T.; Petrie, M.C.; et al. Coronary-Artery Bypass Surgery in Patients with Ischemic Cardiomyopathy. N. Engl. J. Med. 2016, 374, 1511–1520.
- The Veterans Administration Coronary Artery Bypass Surgery Cooperative Study Group Eleven-Year Survival in the Veterans Administration Randomized Trial of Coronary Bypass Surgery for Stable Angina. N. Engl. J. Med. 1984, 311, 1333–1339.
- Alderman, E.L.; Fisher, L.D.; Litwin, P.; Kaiser, G.C.; Myers, W.O.; Maynard, C.; Levine, F.; Schloss, M. Results of Coronary Artery Surgery in Patients with Poor Left Ventricular Function (CASS). Circulation 1983, 68, 785–795.
- Boden, W.E.; O’Rourke, R.A.; Teo, K.K.; Hartigan, P.M.; Maron, D.J.; Kostuk, W.J.; Knudtson, M.; Dada, M.; Casperson, P.; Harris, C.L.; et al. Optimal Medical Therapy with or without PCI for Stable Coronary Disease. N. Engl. J. Med. 2007, 356, 1503–1516.
- The BARI 2D Study Group. A Randomized Trial of Therapies for Type 2 Diabetes and Coronary Artery Disease. N. Engl. J. Med. 2009, 360, 2503–2515.
- Maron, D.J.; Hochman, J.S.; Reynolds, H.R.; Bangalore, S.; O’Brien, S.M.; Boden, W.E.; Chaitman, B.R.; Senior, R.; López-Sendón, J.; Alexander, K.P.; et al. Initial Invasive or Conservative Strategy for Stable Coronary Disease. N. Engl. J. Med. 2020, 382, 1395–1407.
- de Bruyne, B.; Pijls, N.H.J.; Kalesan, B.; Barbato, E.; Tonino, P.A.L.; Piroth, Z.; Jagic, N.; Möbius-Winkler, S.; Rioufol, G.; Witt, N.; et al. Fractional Flow Reserve–Guided PCI versus Medical Therapy in Stable Coronary Disease. N. Engl. J. Med. 2012, 367, 991–1001.
- Lawton, J.S.; Tamis-Holland, J.E.; Bangalore, S.; Bates, E.R.; Beckie, T.M.; Bischoff, J.M.; Bittl, J.A.; Cohen, M.G.; DiMaio, J.M.; Don, C.W.; et al. 2021 ACC/AHA/SCAI Guideline for Coronary Artery Revascularization. J. Am. Coll. Cardiol. 2022, 79, e21–e129.
- Hueb, W.; Lopes, N.; Gersh, B.J.; Soares, P.R.; Ribeiro, E.E.; Pereira, A.C.; Favarato, D.; Rocha, A.S.C.; Hueb, A.C.; Ramires, J.A.F. Ten-Year Follow-Up Survival of the Medicine, Angioplasty, or Surgery Study (MASS II). Circulation 2010, 122, 949–957.
- Spertus, J.A.; Jones, P.G.; Maron, D.J.; O’Brien, S.M.; Reynolds, H.R.; Rosenberg, Y.; Stone, G.W.; Harrell, F.E.; Boden, W.E.; Weintraub, W.S.; et al. Health-Status Outcomes with Invasive or Conservative Care in Coronary Disease. N. Engl. J. Med. 2020, 382, 1408–1419.
- Reynolds, H.R.; Shaw, L.J.; Min, J.K.; Page, C.B.; Berman, D.S.; Chaitman, B.R.; Picard, M.H.; Kwong, R.Y.; O’Brien, S.M.; Huang, Z.; et al. Outcomes in the ISCHEMIA Trial Based on Coronary Artery Disease and Ischemia Severity. Circulation 2021, 144, 1024–1038.
- Perera, D.; Clayton, T.; O’Kane, P.D.; Greenwood, J.P.; Weerackody, R.; Ryan, M.; Morgan, H.P.; Dodd, M.; Evans, R.; Canter, R.; et al. Percutaneous Revascularization for Ischemic Left Ventricular Dysfunction. N. Engl. J. Med. 2022, 387, 1351–1360.
- Mäkikallio, T.; Holm, N.R.; Lindsay, M.; Spence, M.S.; Erglis, A.; Menown, I.B.A.; Trovik, T.; Eskola, M.; Romppanen, H.; Kellerth, T.; et al. Percutaneous Coronary Angioplasty versus Coronary Artery Bypass Grafting in Treatment of Unprotected Left Main Stenosis (NOBLE): A Prospective, Randomised, Open-Label, Non-Inferiority Trial. Lancet 2016, 388, 2743–2752.
- Holm, N.R.; Mäkikallio, T.; Lindsay, M.M.; Spence, M.S.; Erglis, A.; Menown, I.B.A.; Trovik, T.; Kellerth, T.; Kalinauskas, G.; Mogensen, L.J.H.; et al. Percutaneous Coronary Angioplasty versus Coronary Artery Bypass Grafting in the Treatment of Unprotected Left Main Stenosis: Updated 5-Year Outcomes from the Randomised, Non-Inferiority NOBLE Trial. Lancet 2020, 395, 191–199.
- Park, D.-W.; Ahn, J.-M.; Park, H.; Yun, S.-C.; Kang, D.-Y.; Lee, P.H.; Kim, Y.-H.; Lim, D.-S.; Rha, S.-W.; Park, G.-M.; et al. Ten-Year Outcomes After Drug-Eluting Stents Versus Coronary Artery Bypass Grafting for Left Main Coronary Disease. Circulation 2020, 141, 1437–1446.
- Stone, G.W.; Kappetein, A.P.; Sabik, J.F.; Pocock, S.J.; Morice, M.-C.; Puskas, J.; Kandzari, D.E.; Karmpaliotis, D.; Brown, W.M.; Lembo, N.J.; et al. Five-Year Outcomes after PCI or CABG for Left Main Coronary Disease. N. Engl. J. Med. 2019, 381, 1820–1830.
- Scandinavian Simvastatin Survival Study Group. Randomised Trial of Cholesterol Lowering in 4444 Patients with Coronary Heart Disease: The Scandinavian Simvastatin Survival Study (4S). Lancet 1994, 344, 1383–1389.
- Schwartz, G.G. Effects of Atorvastatin on Early Recurrent Ischemic Events in Acute Coronary Syndromes; The MIRACL Study: A Randomized Controlled Trial. JAMA 2001, 285, 1711.
- Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. Prevention of Cardiovascular Events and Death with Pravastatin in Patients with Coronary Heart Disease and a Broad Range of Initial Cholesterol Levels. N. Engl. J. Med. 1998, 339, 1349–1357.
- Sacks, F.M.; Pfeffer, M.A.; Moye, L.A.; Rouleau, J.L.; Rutherford, J.D.; Cole, T.G.; Brown, L.; Warnica, J.W.; Arnold, J.M.O.; Wun, C.-C.; et al. The Effect of Pravastatin on Coronary Events after Myocardial Infarction in Patients with Average Cholesterol Levels. N. Engl. J. Med. 1996, 335, 1001–1009.
- Lloyd-Jones, D.M.; Morris, P.B.; Ballantyne, C.M.; Birtcher, K.K.; Covington, A.M.; DePalma, S.M.; Minissian, M.B.; Orringer, C.E.; Smith, S.C.; Waring, A.A.; et al. 2022 ACC Expert Consensus Decision Pathway on the Role of Nonstatin Therapies for LDL-Cholesterol Lowering in the Management of Atherosclerotic Cardiovascular Disease Risk. J. Am. Coll. Cardiol. 2022, 80, 1366–1418.
- Cannon, C.P.; Blazing, M.A.; Giugliano, R.P.; McCagg, A.; White, J.A.; Theroux, P.; Darius, H.; Lewis, B.S.; Ophuis, T.O.; Jukema, J.W.; et al. Ezetimibe Added to Statin Therapy after Acute Coronary Syndromes. N. Engl. J. Med. 2015, 372, 2387–2397.
- Sabatine, M.S.; Giugliano, R.P.; Keech, A.C.; Honarpour, N.; Wiviott, S.D.; Murphy, S.A.; Kuder, J.F.; Wang, H.; Liu, T.; Wasserman, S.M.; et al. Evolocumab and Clinical Outcomes in Patients with Cardiovascular Disease. N. Engl. J. Med. 2017, 376, 1713–1722.
- Schwartz, G.G.; Steg, P.G.; Szarek, M.; Bhatt, D.L.; Bittner, V.A.; Diaz, R.; Edelberg, J.M.; Goodman, S.G.; Hanotin, C.; Harrington, R.A.; et al. Alirocumab and Cardiovascular Outcomes after Acute Coronary Syndrome. N. Engl. J. Med. 2018, 379, 2097–2107.
- Bhatt, D.L.; Steg, P.G.; Miller, M.; Brinton, E.A.; Jacobson, T.A.; Ketchum, S.B.; Doyle, R.T.; Juliano, R.A.; Jiao, L.; Granowitz, C.; et al. Cardiovascular Risk Reduction with Icosapent Ethyl for Hypertriglyceridemia. N. Engl. J. Med. 2019, 380, 11–22.
- Ray, K.K.; Wright, R.S.; Kallend, D.; Koenig, W.; Leiter, L.A.; Raal, F.J.; Bisch, J.A.; Richardson, T.; Jaros, M.; Wijngaard, P.L.J.; et al. Two Phase 3 Trials of Inclisiran in Patients with Elevated LDL Cholesterol. N. Engl. J. Med. 2020, 382, 1507–1519.
- Ray, K.K.; Bays, H.E.; Catapano, A.L.; Lalwani, N.D.; Bloedon, L.T.; Sterling, L.R.; Robinson, P.L.; Ballantyne, C.M. Safety and Efficacy of Bempedoic Acid to Reduce LDL Cholesterol. N. Engl. J. Med. 2019, 380, 1022–1032.
- Fanaroff, A.C.; Hasselblad, V.; Roe, M.T.; Bhatt, D.L.; James, S.K.; Steg, P.G.; Gibson, C.M.; Ohman, E.M. Antithrombotic Agents for Secondary Prevention after Acute Coronary Syndromes: A Systematic Review and Network Meta-Analysis. Int. J. Cardiol. 2017, 241, 87–96.
- CAPRIE Steering Committee. A Randomised, Blinded, Trial of Clopidogrel versus Aspirin in Patients at Risk of Ischaemic Events (CAPRIE). Lancet 1996, 348, 1329–1339.
- Kang, J.; Park, K.W.; Lee, H.; Hwang, D.; Yang, H.-M.; Rha, S.-W.; Bae, J.-W.; Lee, N.H.; Hur, S.H.; Han, J.-K.; et al. Aspirin vs. Clopidogrel for Chronic Maintenance Monotherapy after Percutaneous Coronary Intervention: The HOST-EXAM Extended Study. Circulation 2022, 397, 2487–2496.
- Udell, J.A.; Bonaca, M.P.; Collet, J.-P.; Lincoff, A.M.; Kereiakes, D.J.; Costa, F.; Lee, C.W.; Mauri, L.; Valgimigli, M.; Park, S.-J.; et al. Long-Term Dual Antiplatelet Therapy for Secondary Prevention of Cardiovascular Events in the Subgroup of Patients with Previous Myocardial Infarction: A Collaborative Meta-Analysis of Randomized Trials. Eur. Heart J. 2016, 37, 390–399.
- Bonaca, M.P.; Bhatt, D.L.; Cohen, M.; Steg, P.G.; Storey, R.F.; Jensen, E.C.; Magnani, G.; Bansilal, S.; Fish, M.P.; Im, K.; et al. Long-Term Use of Ticagrelor in Patients with Prior Myocardial Infarction. N. Engl. J. Med. 2015, 372, 1791–1800.
- Eikelboom, J.W.; Connolly, S.J.; Bosch, J.; Dagenais, G.R.; Hart, R.G.; Shestakovska, O.; Diaz, R.; Alings, M.; Lonn, E.M.; Anand, S.S.; et al. Rivaroxaban with or without Aspirin in Stable Cardiovascular Disease. N. Engl. J. Med. 2017, 377, 1319–1330.
- Heart Outcomes Prevention Evaluation Study Investigators; Yusuf, S.; Sleight, P.; Pogue, J.; Bosch, J.; Davies, R.; Dagenais, G. Effects of an Angiotensin-Converting–Enzyme Inhibitor, Ramipril, on Cardiovascular Events in High-Risk Patients. N. Engl. J. Med. 2000, 342, 145–153.
- The PEACE Trial Investigators. Angiotensin-Converting–Enzyme Inhibition in Stable Coronary Artery Disease. N. Engl. J. Med. 2004, 351, 2058–2068.
- Telmisartan Randomised AssessmeNt Study in ACE iNtolerant subjects with cardiovascular Disease (TRANSCEND) Investigators; Yusuf, S.; Teo, K.; Anderson, C.; Pogue, J.; Dyal, L.; Copland, I.; Schumacher, H.; Dagenais, G.; Sleight, P. Effects of the Angiotensin-Receptor Blocker Telmisartan on Cardiovascular Events in High-Risk Patients Intolerant to Angiotensin-Converting Enzyme Inhibitors: A Randomised Controlled Trial. Lancet 2008, 372, 1174–1183.
- Pitt, B.; Zannad, F.; Remme, W.J.; Cody, R.; Castaigne, A.; Perez, A.; Palensky, J.; Wittes, J. The Effect of Spironolactone on Morbidity and Mortality in Patients with Severe Heart Failure. N. Engl. J. Med. 1999, 341, 709–717.
- Palmer, S.C.; Tendal, B.; Mustafa, R.A.; Vandvik, P.O.; Li, S.; Hao, Q.; Tunnicliffe, D.; Ruospo, M.; Natale, P.; Saglimbene, V.; et al. Sodium-Glucose Cotransporter Protein-2 (SGLT-2) Inhibitors and Glucagon-like Peptide-1 (GLP-1) Receptor Agonists for Type 2 Diabetes: Systematic Review and Network Meta-Analysis of Randomised Controlled Trials. BMJ 2021, 372, m4573.
- Imazio, M.; Nidorf, M. Colchicine and the Heart. Eur. Heart J. 2021, 42, 2745–2760.
- Nidorf, S.M.; Eikelboom, J.W.; Budgeon, C.A.; Thompson, P.L. Low-Dose Colchicine for Secondary Prevention of Cardiovascular Disease. J. Am. Coll. Cardiol. 2013, 61, 404–410.
- Nidorf, S.M.; Fiolet, A.T.L.; Mosterd, A.; Eikelboom, J.W.; Schut, A.; Opstal, T.S.J.; The, S.H.K.; Xu, X.-F.; Ireland, M.A.; Lenderink, T.; et al. Colchicine in Patients with Chronic Coronary Disease. N. Engl. J. Med. 2020, 383, 1838–1847.
- Tardif, J.-C.; Kouz, S.; Waters, D.D.; Bertrand, O.F.; Diaz, R.; Maggioni, A.P.; Pinto, F.J.; Ibrahim, R.; Gamra, H.; Kiwan, G.S.; et al. Efficacy and Safety of Low-Dose Colchicine after Myocardial Infarction. N. Engl. J. Med. 2019, 381, 2497–2505.
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131.
- Ciliberti, G.; Guerra, F.; Coiro, S.; Capucci, A. Is There an ‘Atherosclerotic Continuum’ from Angina with Unobstructed Coronary Arteries to MINOCA? Eur. Heart J. 2019, 40, 1987.
- Ciliberti, G.; Coiro, S.; Tritto, I.; Benedetti, M.; Guerra, F.; del Pinto, M.; Finocchiaro, G.; Cavallini, C.; Capucci, A.; Kaski, J.C.; et al. Predictors of Poor Clinical Outcomes in Patients with Acute Myocardial Infarction and Non-Obstructed Coronary Arteries (MINOCA). Int. J. Cardiol. 2018, 267, 41–45.
- Ong, P.; Camici, P.G.; Beltrame, J.F.; Crea, F.; Shimokawa, H.; Sechtem, U.; Kaski, J.C.; Bairey Merz, C.N. International Standardization of Diagnostic Criteria for Microvascular Angina. Int. J. Cardiol. 2018, 250, 16–20.
- Camici, P.G.; Crea, F. Coronary Microvascular Dysfunction. N. Engl. J. Med. 2007, 356, 830–840.
- Ciliberti, G.; Compagnucci, P.; Urbinati, A.; Bianco, F.; Stronati, G.; Lattanzi, S.; dello Russo, A.; Guerra, F. Myocardial Infarction without Obstructive Coronary Artery Disease (MINOCA): A Practical Guide for Clinicians. Curr. Probl. Cardiol. 2021, 46, 100761.
- Ciliberti, G.; Seshasai, S.R.K.; Ambrosio, G.; Kaski, J.C. Safety of Intracoronary Provocative Testing for the Diagnosis of Coronary Artery Spasm. Int. J. Cardiol. 2017, 244, 77–83.
- Jansen, T.P.J.; Konst, R.E.; de Vos, A.; Paradies, V.; Teerenstra, S.; van den Oord, S.C.H.; Dimitriu-Leen, A.; Maas, A.H.E.M.; Smits, P.C.; Damman, P.; et al. Efficacy of Diltiazem to Improve Coronary Vasomotor Dysfunction in ANOCA. JACC Cardiovasc. Imaging 2022, 15, 1473–1484.
- Ciliberti, G.; Mancone, M.; Guerra, F.; Capucci, A. Is Invasive Coronary Provocation Testing Cost-Effective among MINOCA Patients? Eur. Heart J. 2018, 39, 3334.
- Ciliberti, G.; Verdoia, M.; Merlo, M.; Zilio, F.; Vatrano, M.; Bianco, F.; Mancone, M.; Zaffalon, D.; Bonci, A.; Boscutti, A.; et al. Pharmacological Therapy for the Prevention of Cardiovascular Events in Patients with Myocardial Infarction with Non-Obstructed Coronary Arteries (MINOCA): Insights from a Multicentre National Registry. Int. J. Cardiol. 2021, 327, 9–14.
- Mannheimer, C. The Problem of Chronic Refractory Angina. Report from the ESC Joint Study Group on the Treatment of Refractory Angina. Eur. Heart J. 2002, 23, 355–370.
- Henry, T.D.; Bairey Merz, C.N.; Wei, J.; Corban, M.T.; Quesada, O.; Joung, S.; Kotynski, C.L.; Wang, J.; Lewis, M.; Schumacher, A.M.; et al. Autologous CD34+ Stem Cell Therapy Increases Coronary Flow Reserve and Reduces Angina in Patients with Coronary Microvascular Dysfunction. Circ. Cardiovasc. Interv. 2022, 15, e010802.
- Gallone, G.; Baldetti, L.; Tzanis, G.; Gramegna, M.; Latib, A.; Colombo, A.; Henry, T.D.; Giannini, F. Refractory Angina. JACC Cardiovasc. Interv. 2020, 13, 1–19.
More
