Canine inflammatory bowel diseases (IBD) are of increasing interest in veterinary medicine. They refer to complex and debilitating conditions of dogs’ gastrointestinal tract. Although little evidence for causal inferences is currently available, it is believed that IBD pathophysiology entails intricate interactions between environmental factors, the intestinal immune system, and the microbial communities that colonize the gut. To better understand the mechanisms underlying these disorders, leveraging factors associated with the development of these diseases is imperative. Of these factors, emerging evidence supports the role of dietary patterns as key players influencing the composition and function of gut microbes, with subsequent effects on health and disease.
- canine inflammatory bowel disease (IBD)
- diet
- gut microbiota
- holobiont
1. Introduction
2. Gut Microbiota in Canine IBD
Growing evidence suggests that bacteria present in a dog’s gut may play an essential role in its health and disease [26][28]. The gut microbiota of healthy dogs is known to comprise three main phyla: Fusobacterium, Bacteroidetes, and Firmicutes [27][29]. Within this core bacterial community, several taxa are members of the phylum Firmicutes, including bacilli and clostridia, most of which are short-chain fatty acid (SCFA) producers, such as Faecalibacterium spp. [28][29][30,31]. Bacteroidetes is another prominent phylum and includes the genera Bacteroides and Prevotella [30][32]. Similarly, the phylum Fusobacterium has been commonly associated with health in dogs [30][32]. Key roles of the gut microbiota include protecting against pathogens, shaping the immune system, and providing beneficial metabolites to host epithelial cells through fermentative reactions [26][28]. Microbial metabolites may influence host health, gut microbes, and multiple interacting communities, thereby maintaining the holobiont symbiosis [31][33]. They provide other beneficial effects, notably, immunomodulatory, anti-diarrheal and regulatory effects of GI motility [32][34]. Gut microbiota is also involved in the metabolism of bile acids (BA) as potential mediators linking gut bacteria to metabolic and inflammatory disorders [26][28]. Links between gut microbiota composition/function and a myriad of diseases have been widely reported. In fact, it was demonstrated in mice that gut microbiota causes several pathologies, including obesity and dyslipidemia [33][34][35,36]. Evidence suggests that microbial ecosystem imbalance or dysbiosis has been correlated with several inflammatory diseases in dogs, such as IBD [35][37]. Intestinal dysbiosis in dogs with IBD is often characterized by a decrease in bacterial richness and diversity [28][30]. Metagenomic analyses have highlighted a lower abundance of Firmicutes, while Proteobacteria increases in dogs with IBD compared to dogs with a healthy status [36][38]. The abundance of Faecalibacterium spp. and Fusobacterium spp. were also significantly decreased in dogs with IBD relative to healthy controls [37][39]. In addition, higher abundances of adherent and invasive Escherichia coli (AIEC) were noted in colonic biopsies from dogs with granulomatous colitis, thus highlighting a potential link with gut inflammation [38][40]. Metabolic alterations have also been reported, including impaired short chain fatty acids (SCFAs) and tryptophan metabolites production, which may influence intestinal homeostasis and immunological tolerance [39][40][41,42]. SCFAs (i.e., acetate, propionate and butyrate) are the main end products of intestinal bacterial fermentation of non-digestible food components, such as dietary fiber. Lower levels of acetate and propionate were detected in fecal samples from dogs with IBD compared to healthy subjects [41][43]. These SCFAs are known to hold therapeutic promises in IBD as they improve epithelial barrier integrity and alleviate gut inflammation in vivo. In addition to SCFAs deficiency, an altered BA metabolism has been demonstrated in canine IBD [42][44]. The conversion of primary BA to secondary BA is largely known to be achieved by gut microbes. BA play key roles in the emulsification and absorption of dietary lipids and serve as potent signaling molecules that act through the farnesoid X receptor (FXR) and Takeda G-protein-coupled receptor 5 (TGR5). By activating FXR and TGR5, BA can influence a variety of processes, including inflammation and lipid, glucose and energy metabolism. Accordingly, changes in gut bacterial populations have been suggested to influence inflammatory parameters and pathways through changes in BA metabolism [26][43][28,45]. In dogs with IBD, the decrease in the abundance of Clostridium hiranonis, a potent BA converter, is correlated with the alteration of the BA metabolism. Conversely, treatment of intestinal inflammation is accompanied by an increase in the abundance of C. hiranonis and a normalization of the BA metabolism [37][39]. The links between dietary interventions, SCFAs, BA metabolism and canine inflammation are yet to be explored. Similarly, the relevance of BA as potential therapeutic targets in dogs would need to be thoroughly addressed as investigations related to this field are still in their infancy.3. Diet-Microbiota Interactions in Canine IBD
3.1. Dietary Proteins
Diets with high protein levels were associated with a modification of the gut microbiota composition in healthy beagles, mainly characterized by an increase in the genus Lactobacillus abundance. This change was linked to high concentrations of butyrate in dogs that were fed a high-protein diet [44][46]. Furthermore, a high protein diet has been shown to promote the growth of Clostridium perfringens and to reduce the abundance of Clostridium cluster XIVa (also known as the Clostridium coccoides group) in a similar population of healthy dogs [45][47]. Although the findings from these studies suggest that high-protein diets exert significant effects on the canine gut community, as they elicit the growth of select Clostridium species, a major limitation of such trials is the relatively small size of the studied cohort, limited to only twelve and nine Beagles, respectively [44][45][46,47]. Other significant differences were observed in the microbiota composition, with a higher Firmicutes:Bacteroidetes ratio in response to a high protein-low carbohydrate (HPLC) diet when compared with a low protein-high carbohydrate (LPHC) diet. Several taxa became detectable in response to diet, such as Lactobacillus ruminis, which was detected in 59% of LPHC-fed dogs [46][48]. In another study, the fecal microbiota of dogs fed a HPLC diet showed an increased abundance of Bacteroidetes in addition to an enrichment in the phylum Firmicutes [47][49].3.2. Dietary Tryptophan and Indole Derivatives
In humans with IBD, reduced availability of tryptophan or tryptophan metabolites has been suggested to contribute to the disease [48][49][51,52]. Tryptophan represents a precursor of several microbial and host metabolites, including serotonin and vitamin B3 [50][53]. Tryptophan metabolites are known as one of the most important endogenous ligands of the aryl hydrocarbon receptor (AhR), a nuclear protein involved in the regulation of gene expression and in maintaining intestinal homeostasis [51][54]. Microbial metabolites or dietary factors may influence this pathway. Dogs with IBD and dogs with protein losing enteropathy have also been shown to exhibit lower plasma tryptophan levels than healthy dogs [52][53][55,56]. While these studies highlight a potential role of tryptophan in dogs with IBD and protein losing enteropathy, the small cohort size (10 dogs) in the IBD study and the retrospective study design for the protein losing enteropathy pathology represent major limitations. Further prospective studies with larger cohorts are needed. Further analysis of dog’s gut microbiota would be beneficial to such studies as the link between the decrease in the plasma concentration of tryptophan and dysbiosis is not yet established. In addition to the functional abnormality of the intestinal microbiota, an absorption defect linked to intestinal inflammation could also be involved.3.3. Dietary Fibers and SCFAs
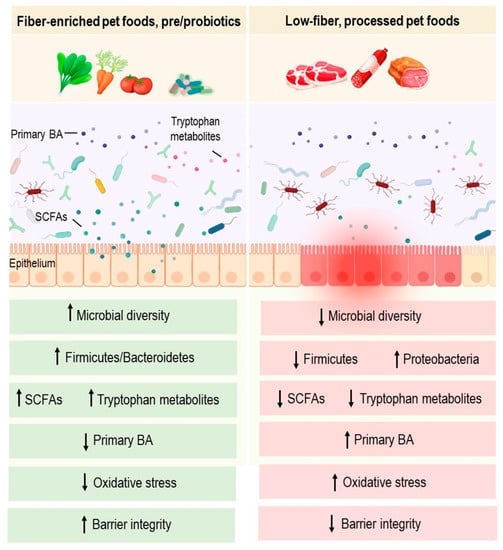
Fibers can be defined as non-digestible carbohydrates that come from plants. They can be classified according to their solubility or fermentability. Soluble or fermentable fibers, such as pectin, gum Arabic, and fructooligosaccharides, support normal GI microflora growth and provide fuel for colonocytes. Several human studies showed that they also delay gastric emptying and inhibit absorption in the small intestine [31][33]. Insoluble fibers, such as cellulose and oat fiber, were shown to increase the volume and water content of stools, to absorb toxins and to normalize colonic motility [31][33]. SCFAs, including butyrate, acetate and propionate, are well-studied microbial metabolites primarily produced by the bacterial fermentation of non-digestible dietary fibers. Thus far, most human clinical trials investigating the anti-inflammatory effects of dietary fibers have been linked with a higher luminal production of SCFAs following the intake of high-fiber foods [54][55][57,58]. It is well demonstrated that SCFAs not only contribute to the regulation of the mucosal barrier function but also provide immune regulatory functions [31][33]. In addition, their production provides an acidic luminal environment that inhibits the proliferation of pH-sensitive pathogenic bacteria such as Enterobacteriaceae [56][59]. Furthermore, in human studies, SCFAs are likely to modulate inflammation by increasing the production of anti-inflammatory cytokines, decreasing pro-inflammatory cytokines, and activating the transcription factor Foxp3 [57][60]. Studies applied to dogs in this regard are still in their infancy and few reports have explored the role of fiber-enriched diets in canine IBD. Interestingly, the intake of high-fiber diets has recently been shown to alleviate acute large-bowel diarrhea and to exhibit significant clinical benefits in dogs [58][61] (Figure 1). However, the use of antibiotic and antiparasitic treatments and the absence of microbiota analysis are important limitations in this enstrudy. More controlled studies are therefore required to confirm these effects. Further metagenomic analysis of dogs’ gut microbiomes would shed light on the functional potential of this community, and provide mechanistic knowledge linking dietary fiber, gut microbiota, and the treatment of canine IBD.