Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Rita Xu and Version 1 by Cristina Maria Marginean.
COVID-19 pneumonia represents a maximum medical challenge due to the virus’s high contagiousness, morbidity, and mortality and the still limited possibilities of the health systems. The differential diagnosis of COVID-19 pneumonia is challenging because there may be standard radiologic features such as ground-glass opacities, crazy paving patterns, and consolidations. A multidisciplinary approach is crucial to define a correct final diagnosis, as an overlap of COVID-19 pneumonia with pre-existing lung diseases is often possible and suggests possible differential diagnoses.
- COVID-19
- pneumonia
- differential diagnosis
1. Introduction
The new coronavirus (COVID-19) has caused a severe pandemic involving several nations. It is a significant concern and an enormous challenge, not only for doctors and researchers but also for the whole population.
It is caused by SARS-CoV-2, an RNA virus of the Sarbecovirus family, similar to the SARS virus [1]. This virus binds to the human angiotensin-converting enzyme receptor (hACE2), triggering general and respiratory symptoms [2]. The primary transmission mode is human-to-human, and the average incubation period is about four days [3]. Symptoms most observed in different cohorts of COVID-19 patients are fever (83–98%), followed by fatigue (70%) and dry cough (59%) [4]; gastrointestinal symptoms were relatively rare.
The disease is usually mild (80%), and the recovery period is, on average, about two weeks [5]. COVID-19 frequently occurs in elderly and middle-aged men, with the highest incidence of death (8–15%) amongst people aged >80 years. The onset of illness begins with fever, dry cough, fatigue, and myalgia, progressing to dyspnea and ARDS within 6 and 8 days of exposure, respectively [2,6][2][6]. The underlying comorbidities also increase the mortality rates of COVID-19. The poor prognosis includes age, comorbidities, severe lymphopenia, high CRP, and dimer D > 1 μg/L [7]. The overall mortality rate varies between 1.5 and 3.6% [8].
2. Pathophysiology
The infection progresses through a replication stage in the first few days, followed by an adaptive immunity phase in the next few days [9]. In the replication stage, the virus replicates, leading to a flu-like illness characterized by mild symptoms due to the direct cytopathic effect of the virus on type II alveolar cells. In the adaptive immune stage, viral levels decrease as the immune system becomes activated. However, the cytokine storm leads to tissue destruction and clinical damage—which explains why patients remain relatively well in the early stages before deteriorating suddenly. The implications of this are early initiation of antiviral therapies for better outcomes and the use of immunosuppressive therapies in targeting adaptive immunity [4].3. Incubation Period (IP)
The incubation period for COVID-19 has been defined as the potential interval between the first contact with the source of transmission (wildlife or suspected or confirmed case) and the first possible appearance of symptoms (cough, fever, fatigue, or myalgia). Generally, this is less than 14 days after exposure, with an average incubation period of four days (2–7 days)—50% of cases are reported within this period [10].4. Demographic Features and General Symptoms of COVID-19
The spectrum of disease ranges from mild disease (mild pneumonia) in 81% of cases, with a typical recovery period of about two weeks, to severe illness (dyspnea, hypoxia) with >50% pulmonary affection on imaging in 14% of patients, with a recovery period of approximately 3–6 weeks, until critical illness (ARDS, sepsis, septic shock, or MODS) in 5% of cases; this was observed in data from 44,500 confirmed cases of COVID-19 [5]. COVID-19 mainly affects men (58.1%), and the predominantly affected age groups are middle-aged and elderly, with fewer cases reported in children (0.9–2%). The elderly are more seriously affected and have a higher mortality rate (8–15%) [11]. Most studies on hospitalized patients reveal that the common symptoms of COVID-19 are fever (83–98%), fatigue (70%), dry cough (59%), anorexia (40%), myalgia (35%), dyspnea (31%) or a productive cough (27%) [4,11,12,13][4][11][12][13]. Fever in COVID-19 has been described in different cohorts, with variations between low fever (37–38 °C) and persistent fever lasting up to 14 days. In a study of 1099 patients, only 43.8% had a fever on admission and 88.7% during hospitalization [13]. Gastrointestinal symptoms such as nausea, vomiting (5%), and diarrhea (3.8%) are less common, differentiating COVID-19 from SARS and MERS. Asymptomatic infection was also observed, but the frequency is unknown.5. Clinical Evolution
Symptoms of COVID-19 (Figure 1) initially begin with fatigue, intermittent or prolonged fever, myalgia, dry cough, and shortness of breath, symptoms improved with early administration of conservative therapy or worsen and progress to dyspnea and productive cough [4]. The average time to onset of dyspnea in various studies was 6 days after exposure.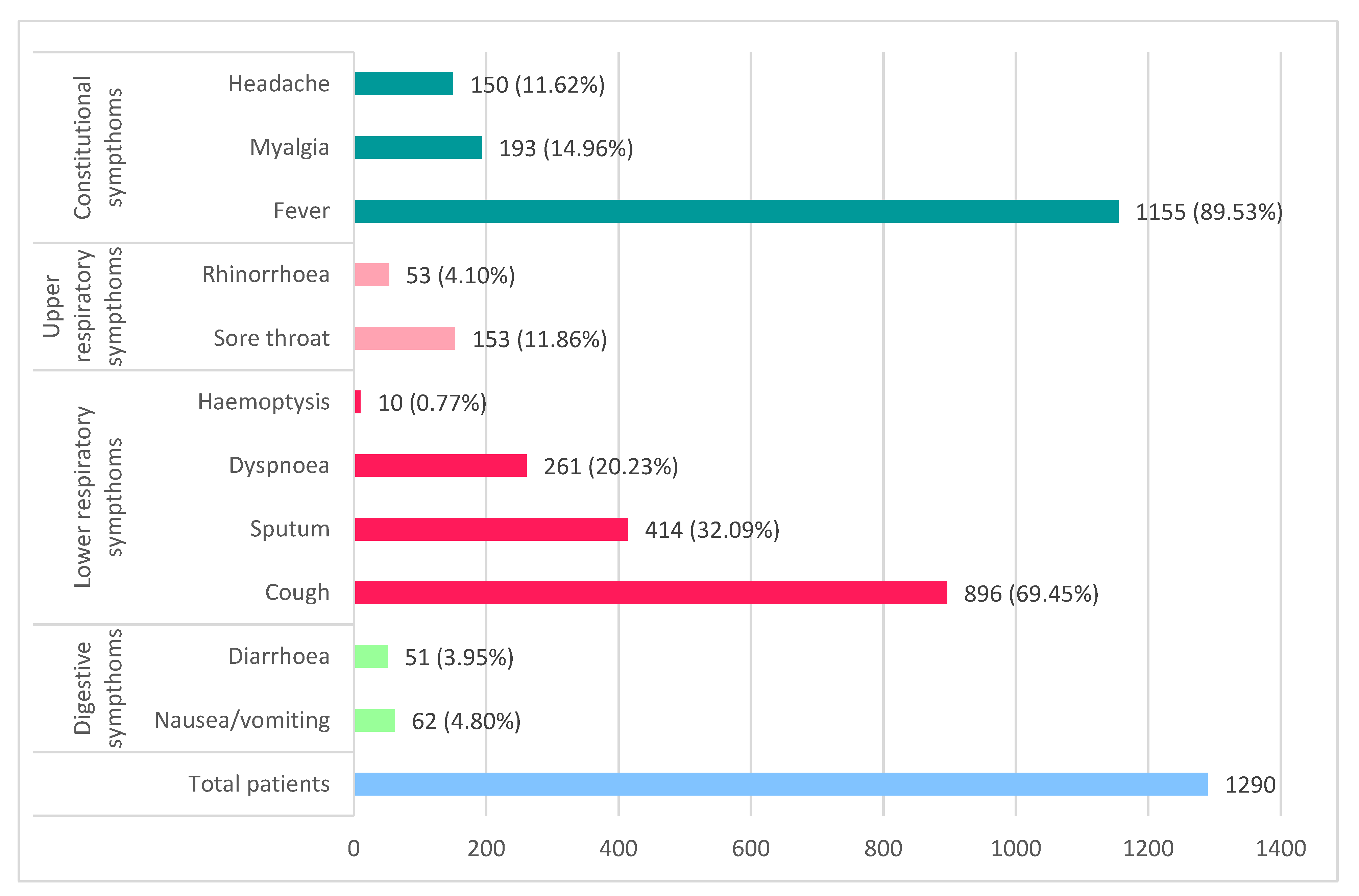
6. Underlying Comorbidities Related to the Evolution of COVID-19
The main comorbidities that are worsening the evolution of COVID-19, with increased disease severity, use of mechanical ventilation, and increased mortality, include uncontrolled hypertension, diabetes, coronary heart disease, cerebrovascular disease, chronic obstructive pulmonary disease, chronic liver disease, and other such as cancer, chronic kidney disease, and immunodeficiency. Guan et al. (2020) [13] revealed in a study that hypertension (15%) was predominant, also diabetes (7.4%), probably due to the hACE-2 receptor polymorphism in the Asian population (Figure 2).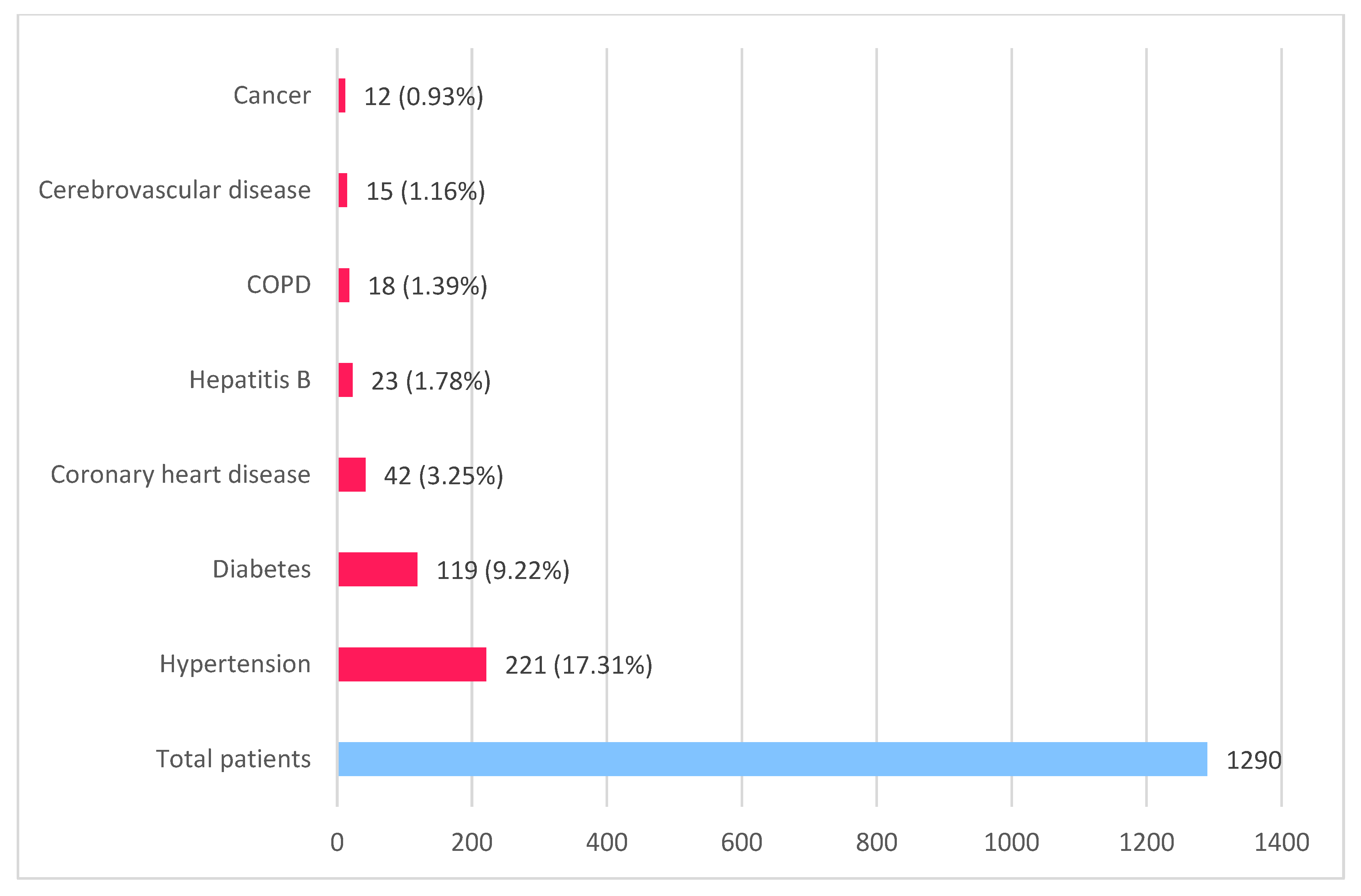
Figure 2. Main comorbidities in COVID-19 patients (based on data published by Guan et al. [13] and Zhou et al. [11]).
7. Prognostic Factors in COVID-19
The strong independent predictors of elevated mortality are age (over 70 years), comorbidities such as uncontrolled hypertension, diabetes, coronary heart disease, chronic obstructive pulmonary disease, neoplastic diseases, severe lymphopenia, and elevated D-dimer [7]. Additional negative prognostic factors are elevated C-reactive protein, LDH, ALT, serum ferritin, IL-6, and high-sensitivity cardiac troponin [14,15][14][15]. Serum sodium, glomerular filtration rate, and creatinine are useful in predicting the clinical outcome of patients with moderate forms of the disease [16].
8. Diagnostic Imaging
Chest radiographs can be used to diagnose COVID-19 due to their availability and low cost. It is helpful in the emergency room and in cases where the patient cannot be mobilized for computed tomography. At the onset, chest X-ray has low sensitivity and may even be normal [17]. Ground-glass opacities may not be thick enough to be evident by radiography, and, if they have a basal and retro-cardiac location, they may be hidden by the diaphragm or mediastinal structures [18].
Computed tomography is a method with higher sensitivity and specificity compared to chest X-ray, and it can also discover abnormalities during the early stages of COVID-19. It is routinely used in patients with clinical suspicion of COVID-19 in the screening and differential diagnosis of pneumonia. Still, a normal chest CT scan does not exclude a positive diagnosis of COVID-19.
High-resolution computed tomography (HRCT) reveals single or multiple GGOs with a predominantly subpleural distribution, crazy pavement, or segmental pulmonary consolidations [19].
Ultrasound (US) can be used in the triage of suspected patients to assess the severity and evolution of the disease, being a method without the risks of radiation, especially in children and pregnant women, but having disadvantages for the examiner (increased exposure time). US is useful in evaluating consolidations and interstitial lung involvement, with increased sensitivity and specificity than chest radiographs, as well as in the differential diagnosis of cardiogenic versus noncardiogenic acute pulmonary edema [20].
Although a susceptible method in detecting COVID-19 pneumonia, the US features are not pathognomonic, being similar in all interstitial and alveolar lung diseases, including viral pneumonia, idiopathic pulmonary fibrosis, hypersensitivity pneumonia, heart failure, and diffuse alveolar hemorrhages. Characteristics for COVID-19 US features are pleural thickening; vertical B lines determined by the decrease in lung aeration secondary to interstitial lung damage (focal, multifocal, or confluent); consolidations with aeric bronchograms, pleural effusions (their presence may lead to differential diagnoses such as bacterial pneumonia or congestive heart failure) [20].
Magnetic resonance imaging (MRI) is used to diagnose cardiac or central nervous system complications of COVID-19. On MRI, lung parenchymal changes appear as regions of increased signal intensity, corresponding to ground glass opacities, or consolidations also highlighted by chest X-ray or CT [21].
COVID-19 Pneumonia
COVID-19 pneumonia has been divided into four stages, which may have overlapping radiological elements [22]:
1. Early phase/Stage 1—days 0–4. Ground glass opacities represent the main radiological characteristic [12] (Figure 3A);
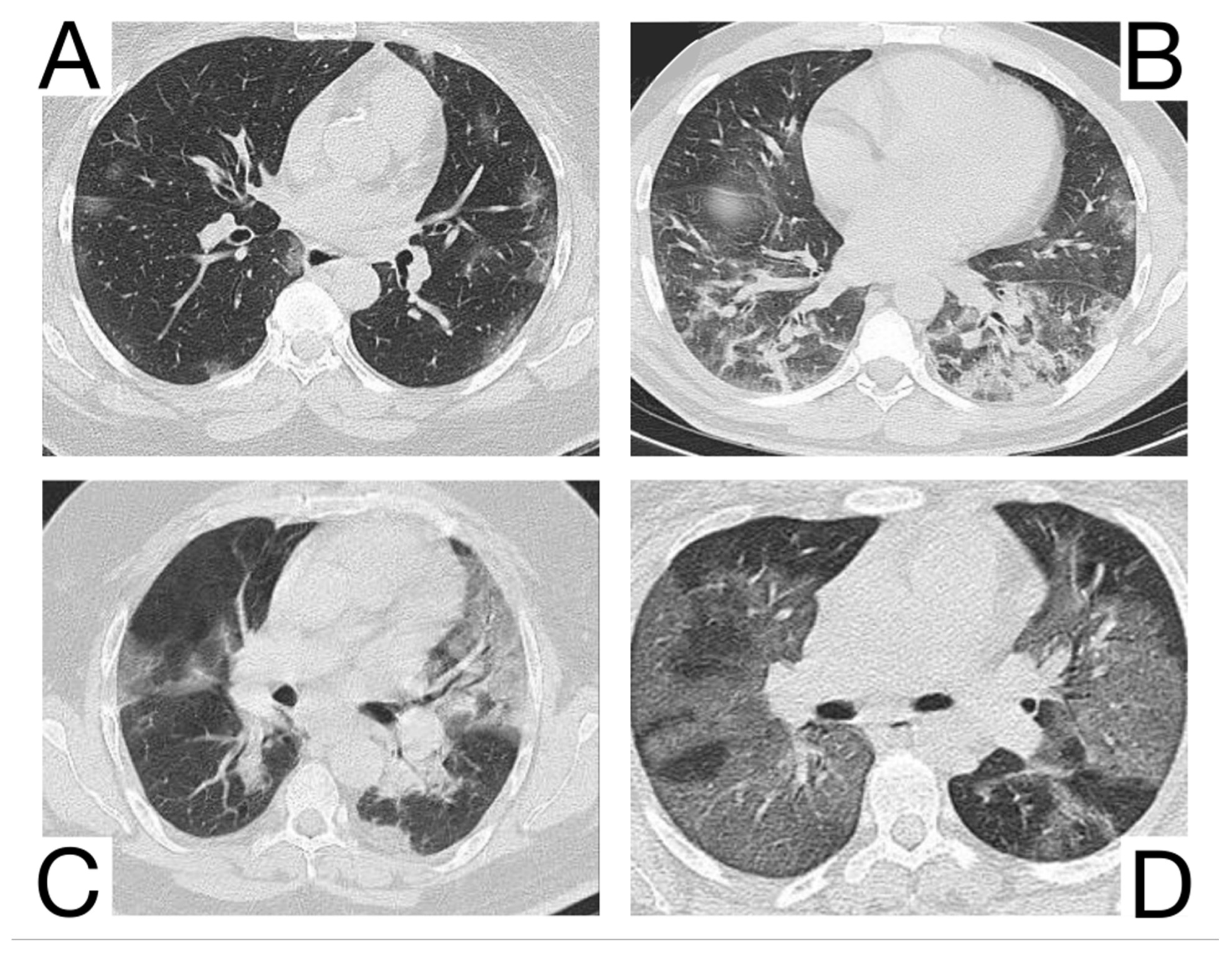

Figure 3. Imagistic findings in COVID-19 (A). Multiple areas of ground glass infiltration (patient on the third day of symptoms) (B). Bilateral patches of ground glass and subsegmental consolidation (C). Ground glass and consolidation with air bronchogram (8 days after onset) (D). Diffuse ground glass infiltration (white lung appearance). Note. Adapted from Hefeda et al. (2020) [22].
2. The progressive phase/Stage 2 refers to days 5–8, and the hallmark is a cobblestone appearance (Figure 3B) coexisting with extensive ground-glass opacities and condensation foci [23];
3. Peak phase/Stage 3 is typical for days 9–13, and CT shows pulmonary condensations (Figure 3C), sometimes surrounded by a halo of ground glass.
4. The absorption phase/Stage 4 begins around day 14; areas of ground glass together with linear condensations are appreciable (Figure 3D).
Opacities are usually bilateral and subpleural, having an apicobasal gradient of distribution. Additional radiological features are enlargement of the peripheral pulmonary vessels, while pleural effusions, pulmonary nodules, and mediastinal lymphadenopathy are rare [24].
Clinical conditions may worsen suddenly, with patients presenting wheezing, dyspnea, and tachypnea with low blood oxygen saturation. These features indicate the progression of COVID-19 pneumonia to ARDS (acute respiratory distress syndrome). ARDS is highlighted on HRCT as patchy ground-glass confluent areas and pulmonary condensations [19]. Clinical and radiological monitoring is key to the early identification and treatment of ARDS in COVID-19 pneumonia [23,24][23][24]. Opacities are usually bilateral and subpleural, with an apicobasal gradient of distribution. Additional radiological features are enlargement of the peripheral pulmonary vessels, while pleural effusions, pulmonary nodules, and mediastinal lymphadenopathy are rare [24,25][24][25].
References
- Cottin, V.; Cordier, J.-F. Eosinophilic pneumonias. Allergy 2005, 60, 841–857.
- Pollard, C.A.; Morran, M.P.; Nestor-Kalinoski, A.L. The COVID-19 pandemic: A global health crisis. Physiol. Genom. 2020, 52, 549–557.
- Eastin, C.; Eastin, T. Clinical Characteristics of Coronavirus Disease 2019 in China. J. Emerg. Med. 2020, 58, 711–712.
- Sharma, R.; Agarwal, M.; Gupta, M.; Somendra, S.; Saxena, S.K. Clinical Characteristics and Differential Clinical Diagnosis of Novel Coronavirus Disease 2019 (COVID-19). In Coronavirus Disease 2019 (COVID-19); Springer: Singapore, 2020; pp. 55–70.
- Wu, Z.; McGoogan, J.M. Characteristics of and Important Lessons from the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72,314 Cases from the Chinese Center for Disease Control and Prevention. JAMA 2020, 323, 1239–1242.
- Umakanthan, S.; Sahu, P.; Ranade, A.V.; Bukelo, M.M.; Rao, J.S.; Lf, A.-M.; Dahal, S.; Kumar, H.; Kv, D. Origin, transmission, diagnosis and management of coronavirus disease 2019 (COVID-19). Postgrad. Med. J. 2020, 96, 753–758.
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 2020, 395, 507–513.
- Baud, D.; Qi, X.; Nielsen-Saines, K.; Musso, D.; Pomar, L.; Favre, G. Real estimates of mortality following COVID-19 infection. Lancet Infect. Dis. 2020, 20, 773.
- Karakike, E.; Giamarellos-Bourboulis, E.J. Macrophage Activation-Like Syndrome: A Distinct Entity Leading to Early Death in Sepsis. Front. Immunol. 2019, 10, 55.
- McAloon, C.; Collins, Á.; Hunt, K.; Barber, A.; Byrne, A.W.; Butler, F.; Casey, M.; Griffin, J.; Lane, E.; McEvoy, D.; et al. Incubation period of COVID-19: A rapid systematic review and meta-analysis of observational research. BMJ Open 2020, 10, e039652.
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062.
- Shi, H.; Han, X.; Jiang, N.; Cao, Y.; Alwalid, O.; Gu, J.; Fan, Y.; Zheng, C. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: A descriptive study. Lancet Infect. Dis. 2020, 20, 425–434.
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Qu, C.Q.; He, J.X.; Liu, L.; Shan, H.; Lei, C.L.; Hui, D.S.C.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720.
- Henry, B.M.; de Oliveira, M.H.S.; Benoit, S.; Plebani, M.; Lippi, G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): A meta-analysis. Clin. Chem. Lab. Med. 2020, 58, 1021–1028.
- Grasselli, G.; Greco, M.; Zanella, A.; Albano, G.; Antonelli, M.; Bellani, G.; Bonanomi, E.; Cabrini, L.; Carlesso, E.; Castelli, G.; et al. Risk Factors Associated with Mortality Among Patients with COVID-19 in Intensive Care Units in Lombardy, Italy. JAMA Intern. Med. 2020, 180, 1345–1355.
- Venturini, S.; Orso, D.; Cugini, F.; Crapis, M.; Fossati, S.; Callegari, A.; Pellis, T.; Tonizzo, M.; Grembiale, A.; Rosso, A.; et al. Classification and analysis of outcome predictors in non-critically ill COVID-19 patients. Intern. Med. J. 2021, 51, 506–514.
- Fatima, S.; Ratnani, I.; Husain, M.; Surani, S. Radiological Findings in Patients with COVID-19. Cureus 2020, 12, e7651.
- Li, B.; Li, X.; Wang, Y.; Han, Y.; Wang, Y.; Wang, C.; Zhang, G.; Jin, J.; Jia, H.; Fan, F.; et al. Diagnostic value and key features of computed tomography in Coronavirus Disease 2019. Emerg. Microbes Infect. 2020, 9, 787–793.
- Hani, C.; Trieu, N.; Saab, I.; Dangeard, S.; Bennani, S.; Chassagnon, G.; Revel, M.-P. COVID-19 pneumonia: A review of typical CT findings and differential diagnosis. Diagn. Interv. Imaging 2020, 101, 263–268.
- Vetrugno, L.; Baciarello, M.; Bignami, E.; Bonetti, A.; Saturno, F.; Orso, D.; Girometti, R.; Cereser, L.; Bove, T. The “pandemic” increase in lung ultrasound use in response to COVID-19: Can we complement computed tomography findings? A narrative review. Ultrasound J. 2020, 12, 39.
- Filatov, A.; Sharma, P.; Hindi, F.; Espinosa, P.S. Neurological Complications of Coronavirus Disease (COVID-19): Encephalopathy. Cureus 2020, 12, e7352.
- Hefeda, M.M. CT chest findings in patients infected with COVID-19: Review of literature. Egypt. J. Radiol. Nucl. Med. 2020, 51, 239.
- Pan, F.; Ye, T.; Sun, P.; Gui, S.; Liang, B.; Li, L.; Zheng, D.; Wang, J.; Hesketh, R.L.; Yang, L.; et al. Time Course of Lung Changes at Chest CT during Recovery from Coronavirus Disease 2019 (COVID-19). Radiology 2020, 295, 715–721.
- Zhang, K.; Liu, X.; Shen, J.; Li, Z.; Sang, Y.; Wu, X.; Zha, Y.; Liang, W.; Wang, C.; Wang, K.; et al. Clinically Applicable AI System for Accurate Diagnosis, Quantitative Measurements, and Prognosis of COVID-19 Pneumonia Using Computed Tomography. Cell 2020, 181, 1423–1433.e11.
- Parekh, M.; Donuru, A.; Balasubramanya, R.; Kapur, S. Review of the Chest CT Differential Diagnosis of Ground-Glass Opacities in the COVID Era. Radiology 2020, 297, E289–E302.
More
