Iron oxides are chemical compounds which havedifferent polymorphic forms, including γ-Fe2O3 (maghemite), Fe3O4(magnetite), and FeO (wustite). Among them, the most studiedare γ-Fe2O3 and Fe3O4,as they possess extraordinary properties at the nanoscale (such as superparamagnetism, high specific surface area, biocompatible etc.),because at this size scale, the quantum effectsaffect matter behavior and optical, electrical and magnetic properties.Therefore, in the nanoscale, these materials become ideal for surface functionalization and modification in various applications such as separation techniques, magnetic sorting (cells and other biomolecules etc.), drug delivery, cancer hyperthermia, sensing etc., and also for increased surface area-to-volume ratio, which allows for excellent dispersibility in the solution form.The current methods used are partially and passively mixed reactants, and,thus, every reaction has a different proportion of all factors which causes further difficulties in reproducibility.Direct active and complete mixing and automated approaches could be solutions to thissize- and shape-controlled synthesis, playing a key role in its exploitation for scientific or technological purposes. An ideal synthesis method should be able to allow reliable adjustment of parameters andcontrol over the following: fluctuation in temperature;pH, stirring rate;particle distribution; size control;concentration; and control over nanoparticle shape andcomposition i.e., crystallinity, purity, and rapid screening. Iron oxide nanoparticle (IONP)-based available clinical applications are RNA/DNAextraction and detection of infectious bacteria andviruses. Such technologies are important at POC (point of care) diagnosis.IONPs can play a key role in these perspectives.Although there are various methods for synthesis of IONPs,one of the most crucial goals is to control size and properties with high reproducibility to accomplish successful applications.Using multiple characterization techniques to identify and confirm the oxide phase of iron can provide better characterization capability. It is very important to understand the in-depth IONP formation mechanism, enabling better control over parameters and overall reaction and, by extension, properties of IONPs.This workprovides an in-depth overview ofdifferent properties, synthesis methods, and mechanisms of iron oxide nanoparticles (IONPs) formation, and the diverse range of theirapplications. Differentcharacterization factors and strategiesto confirm phase purity in the IONP synthesis field are reviewed.First, properties of IONPs and various synthesis routes with their merits and demerits are described. We also describe different synthesis strategies and formation mechanisms for IONPs such as for: wustite (FeO), hematite(α-Fe2O3), maghemite(ɤ-Fe2O3) and magnetite(Fe3O4). We also describe characterization of these nanoparticles and various applications in detail. In conclusion, we present a detailed overview on the properties, size-controlled synthesis, formation mechanisms and applications of IONPs.
- iron oxide nanoparticles(IONPs)
- formation mechanisms
- reproducible
- biomedical
1. Introduction
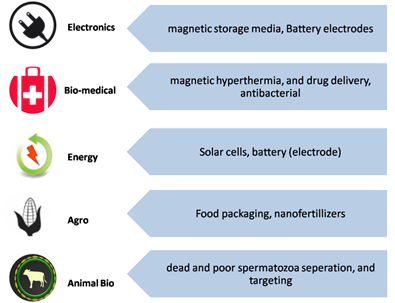
Iron oxide nanoparticle (IONP)-bas are chemical compounds which have different polymorphic fored technologies are catalyzing rapid developments in nanotechnology. Due to technological importance, extensive research has been carried out on the development of various synthetic routes to yield IONPs with desired properties[1]. Among IONPs, inclumainly Fe3O4 anding γ-Fe2O3 are extensively studied[2]. In general, iron oxides are classified into different phases (magnetite, hemite), Fatite, maghemite, wustite). In the nano form, a material possesses intere3O4 (sting optical, magnetite)c, and FeO (wustite). Among them,electrical properties which cannot be found in their bulk form. This phenomenon can be described as the "quantum size effect"[3][4][5]. In the nanomost studied are γ-Feter range of IONPs, the quantum effect dominates the behavior-affecting magnetic, electric, and optical properties of the2O3 mand Ftter. In the3O4, nas they possess extraordinarynoscale, there is an impact of specific individual atoms or molecules, while in the bulk form, properties at the nanoscale (suchy is attributed to the average of all the quantum forces that affect all of the atoms. For example, magnetic Fe3O4 nas super noparticles are superparamagnetisc below the size of 20 nm[6]. As the nanoparticle size decreases, thigh specific surface area, biocompatible etc.), because at this size scales property tends towards paramagnetic or superparamagnetic magnetization. Therefore, a decrease in nanoparticle size will enhance superparamagnetic behavior and decrease ferromagnetic behavior. As the size of nanoparticles decreases, the quantum effects affect matter behavrelative oxygen concentration decreases; therefore, a slight reduction in the iron valance state occurs. Because of this ferrous ion content increase, an increase in magnetization should also be observed[7]. Simiolar ly, γ-Fe2O3 nand optical, electrical and articles have gained technological importance due to their magnetic and catalytic properties. Therefore, in the nanoscale, these materials become ideal forHigh magnetization and hysteretic heating make them potential candidates in separation and biomedical areas, and the semiconducting property and chemically active surface functionalization and modificatiallow catalytic activities such as photocatalytic ability[8][9]. Iron oxin variousde nanoparticles (IONPs) have a broad range of significant applications in electronics[10][11], biomedicine[12][13][14], energy[15][16], agriculture[11][18], and animal biotechnology[19][20], as shown in Figureparation techniques, 1. In a small size of about 10–20 nm, the superparamagnetic sorting (cproperties of Fells3O4 and γ-Fe2O3 nanoparther biomolecules etc.), drug delivery, cancer hyperthermia, sensing etc., and also foricles become apparent, therefore, better performance can be achieved for the above-mentioned applications. Additionally, due to the increased surface areae-to-volume ratio, which allows forthey show excellent dispersinsability in the solutions[21].
Figure 1. Various applications of iron oxide nanoparticles (IONPs).
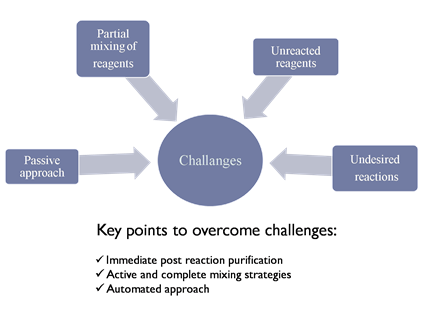
However, reproducible synthesis ofor IONPs with desired properties is still a problem[22]. This is be currentcause existing synthesis methods used are partially and passively mixedshow a passive approach towards synthesis reaction. The main challenges and key points to overcome them are explained in Figure 2. In existing methods, reactants, and, thus, every reaction has a different p are mixed partially and passively. Unreacted components therefore effect the final product when undesired reactions takes place, as the proportion of all these factors which causes further is different in every reaction, making it difficulties in repro to achieve reproducibility in the desired properties[23]. Immediate pucibilityrification of nanoparticles after reaction becomes necessary to minimize error. Direct active and complete mixing anof reactants and automated approaches could be solutions to this solve this issue. Researchers are mainly focused on size- and shape-controlled synthesis, playingas size determines the surface area, which plays a key role in its exploitation for scientific or technological purposes[24].
Figure 2. Challenges and key points in reproducible synthesis of nanoparticles.
2. Manipulation of reaction parameters
Manipulation Aof reaction ideal synthesis methodparameters is necessary to obtain controlled nanoparticles in terms of size, shape, purity, crystallinity, and morphology. A synthetic route should be able to allow reliable adjustment ofenable control over reaction parameters and control over the following:: temperature; concentration; fluctuation in temperature; pH,; stirring rate; particle distribution; size control; concentration; and controtrol over shape; nanoparticle shape and e composition i.e.,and structure, which includes crystallinity, purity, and rapid screening. Iron oxide nanoparticle (IONP)-based available, and reliable adjustment of parameters[22][25][26][27].
In clinicalour applications are RNA/DNA extraction and detection of infectious bacteria and viruses. Such technologies are important at POC (point of care) diagnosis. IONPs can play a key role in these perspectives. Although there are various opinion, the established synthetic routes of iron oxide nanoparticles have difficulty in controlling the particle size, shape, and properties. Many of the reported methods for synthesis of IONPs, one of the most crucial goals is to control size and properties with highhave their own pros and cons, as described in Table 1. It is necessary to develop a new synthetic route for IONPs that yields nanoparticles in a reproducibility to accomplish successful applications. Using multiple characterization techniques to identify and confirm the oxide phase of iron can provide better characterization capability. It is very important to understand the in-depth IONP formation mechanism, enabling better control over parameters and overall reaction and, by extension, properties of IONPsle manner with excellent size control. This review explains various dimensions associated with synthesis of IONPs and their applications, and different synthesis mechanisms are summarized. Figures S1–7 represented in supplementary materials corresponds to various IONPs synthesis methods graphically presented which also includes key points for each corresponding method.
1. Introduction
Iron oxide nanoparticle (IONP)-based technologies are catalyzing rapid developments in nanotechnology. Due to technological importance, extensive research has been carried out on the development of various synthetic routes to yield IONPs with desired properties [1]. Among IONPs, mainly Fe3O4 and γ-Fe2O3 are extensively studied [2]. In general, iron oxides are classified into different phases (magnetite, hematite, maghemite, wustite). In the nano form, a material possesses interesting optical, magnetic, and electrical properties which cannot be found in their bulk form. This phenomenon can be described as the “quantum size effect” [3–5]. In the nanometer range of IONPs, the quantum effect dominates the behavior-affecting magnetic, electric, and optical properties of the matter. In the nanoscale, there is an impact of specific individual atoms or molecules, while in the bulk form, property is attributed to the average of all the quantum forces that affect all of the atoms. For example, magnetic Fe3O4 nanoparticles are superparamagnetic below the size of 20 nm [6]. As the nanoparticle size decreases, this property tends towards paramagnetic or superparamagnetic magnetization. Therefore, a decrease in nanoparticle size will enhance superparamagnetic behavior and decrease ferromagnetic behavior. As the size of nanoparticles decreases, the relative oxygen concentration decreases; therefore, a slight reduction in the iron valance state occurs. Because of this ferrous ion content increase, an increase in magnetization should also be observed [7]. Similarly, γ-Fe2O3 nanoparticles have gained technological importance due to their magnetic and catalytic properties. High magnetization and hysteretic heating make them potential candidates in separation and biomedical areas, and the semiconducting property and chemically active surface allow catalytic activities such as photocatalytic ability [8,9]. Iron oxide nanoparticles (IONPs) have a broad range of significant applications in electronics [10,11], biomedicine [12–14], energy [15,16], agriculture [17,18], and animal biotechnology [19,20], as shown in Figure 1. In a small size of about 10–20 nm, the superparamagnetic properties of Fe3O4 and γ-Fe2O3 nanoparticles become apparent, therefore, better performance can be achieved for the above-mentioned applications. Additionally, due to the increased surface-to-volume ratio, they show excellent dispensability in solutions [21].
Figure 1. Various applications of iron oxide nanoparticles (IONPs).
However, reproducible synthesis of IONPs with desired properties is still a problem [22]. This is because existing synthesis methods show a passive approach towards synthesis reaction. The main challenges and key points to overcome them are explained in Figure 2. In existing methods, reactants are mixed partially and passively. Unreacted components therefore effect the final product when undesired reactions takes place, as the proportion of all these factors is different in every reaction, making it difficult to achieve reproducibility in the desired properties [23]. Immediate purification of nanoparticles after reaction becomes necessary to minimize error. Direct active and complete mixing of reactants and automated approaches could solve this issue. Researchers are mainly focused on size- and shape-controlled synthesis, as size determines the surface area, which plays a key role in its exploitation for scientific or technological purposes [24].
Figure 2. Challenges and key points in reproducible synthesis of nanoparticles.
Manipulation of reaction parameters is necessary to obtain controlled nanoparticles in terms of size, shape, purity, crystallinity, and morphology. A synthetic route should enable control over reaction parameters: temperature; concentration; fluctuation in temperature; pH; stirring rate; particle distribution; size control; control over shape; nanoparticle composition and structure, which includes crystallinity, purity, rapid screening, and reliable adjustment of parameters [22,25–27].
In our opinion, the established synthetic routes of iron oxide nanoparticles have difficulty in controlling the particle size, shape, and properties. Many of the reported methods have their own pros and cons, as described in Table 1. It is necessary to develop a new synthetic route for IONPs that yields nanoparticles in a reproducible manner with excellent size control. This review explains various dimensions associated with synthesis of IONPs and their applications, and different synthesis mechanisms are summarized. Figures S1–7 represented in supplementary materials corresponds to various IONPs synthesis methods graphically presented which also includes key points for each corresponding method.
Table 1.
Merits and demerits of different IONP nanoparticle synthesis methods.
|
Type of Synthesis | |||||||||
Pros | |||||||||
Cons | |||||||||
Reference | |||||||||
|
Microwave | |||||||||
Short reaction time, higher yields, excellent reproducibility, easy handling | |||||||||
Expensive, unsuitable for scale-up and reaction monitoring | |||||||||
[28,29] | |||||||||
[ | ] | [29] |
|||||||
|
Spray pyrolysis | |||||||||
Finely dispersed particles of predictable size, shape and variable composition | |||||||||
Aggregated particles, expensive | |||||||||
[30–32] | |||||||||
[ | ] | ||||||||
|
Laser pyrolysis | |||||||||
Small particle size, narrow particle size distribution, near absence of aggregation | |||||||||
Complicated, very expensive | |||||||||
[21,31] | |||||||||
[ | ] | [31] |
|||||||
|
Pulsed wire discharge method | |||||||||
Fast process, higher purity of NPs | |||||||||
Batch process, limited production, high vacuum systems, costly process, contaminations in product | |||||||||
[33,34] | |||||||||
[ | ] | [34] |
|||||||
|
Chemical vapor condensation | |||||||||
Suitable for preparing small quantities to demonstrate desired properties in the laboratory | |||||||||
Low production, difficult to control size and particle size distribution | |||||||||
[ | ] | ||||||||
|
Co-precipitation | |||||||||
Convenient method, simple and rapid preparative method, easy control of particle size and composition | |||||||||
Extensive agglomeration, poor morphology and particle size distribution | |||||||||
[36–38] | |||||||||
[ | ] | ||||||||
|
Thermal decomposition | |||||||||
Producing highly monodispersed particles with a narrow size distribution | |||||||||
High cost, long-time synthesis reaction, high temperature | |||||||||
[39–41] | |||||||||
[ | ] | ||||||||
|
Microemulsion | |||||||||
Monodispersed nanoparticles with various morphology can be produced | |||||||||
Not very efficient and difficult to scale up | |||||||||
[39,42] | |||||||||
[ | ] | [42] |
|||||||
|
Polyol | |||||||||
Uniform size particles can be prepared, easy to scaleup | |||||||||
Needs high temperature, long time | |||||||||
[22,31] | |||||||||
[ | ] | [31] |
|||||||
|
Sol–Gel | |||||||||
Low processing cost, energy efficiency, high production rate, and rapid productivity | |||||||||
Limited efficiency, high cost | |||||||||
[43–45] | |||||||||
[ | ] | ||||||||
|
Sonochemical | |||||||||
Simple, low cost, safe, environment friendly, absence of many reactants | |||||||||
Very small concentration of prepared NPs, particle agglomeration is very narrow | |||||||||
[33,46] | |||||||||
[ | ] | [46] |
|||||||
Biological synthesis of nanoparticles using plants and bacteria | Selectivity and precision for nanoparticle formation, cost effective, eco friendly | Limited knowledge, difficulty in controlling size and properties |
[47,48] |
References
- Roca, A.G.; Gutiérrez, L.; Gavilán, H.; Fortes Brollo, M.E.; Veintemillas-Verdaguer, S.; del Puerto Morales, M. Design strategies for shape-controlled magnetic iron oxide nanoparticles. Adv. Drug Deliv. Rev. 2019, 138, 68–104, doi:10.1016/j.addr.2018.12.008.
- Noqta, O.A.; Aziz, A.A.; Usman, I.A.; Bououdina, M. Recent Advances in Iron Oxide Nanoparticles (IONPs): Synthesis and Surface Modification for Biomedical Applications. J. Supercond. Nov. Magn. 2019, 32, 779–795, doi:10.1007/s10948-018-4939-6.
- Iriarte-Mesa, C.; López, Y.C.; Matos-Peralta, Y.; de la Vega-Hernández, K.; Antuch, M. Gold, Silver and Iron Oxide Nanoparticles: Synthesis and Bionanoconjugation Strategies Aimed at Electrochemical Applications; Springer International Publishing: Berlin/Heidelberg, Germany, 2020; Volume 378; ISBN 0123456789.
- Trindade, T.; Thomas, P.J. Defining and Using Very Small Crystals; Elsevier Ltd.: Amsterdam, The Netherlands, 2013; Volume 4; ISBN 9780080965291, doi: 10.1016/B978-0-08-097774-4.00416-2.
- Tringides, M.C.; Jałochowski, M.; Bauer, E. Quantum size effects in metallic nanostructures. Phys. Today 2007, 60, 50–54, doi:10.1063/1.2731973.
- Li, Q.; Kartikowati, C.W.; Horie, S.; Ogi, T.; Iwaki, T.; Okuyama, K. Correlation between particle size/domain structure and magnetic properties of highly crystalline Fe3O4 nanoparticles. Sci. Rep. 2017, 7, 1–4, doi:10.1038/s41598-017-09897-5.
- Sun, Y.; Gray, S.K.; Peng, S. Surface chemistry: A non-negligible parameter in determining optical properties of small colloidal metal nanoparticles. Phys. Chem. Chem. Phys. 2011, 13, 11814–11826, doi:10.1039/c1cp20265k.
- Sudhakara, K.; Kumar, A.P.; Kumara, B.P.; Raghavendera, A.; Ravia, S.; Kenie, D.N.; Lee, Y.-I. Synthesis of γ-Fe2O3 Nanoparticles and Catalytic activity of Azide-Alkyne Cycloaddition Reactions. Asian J. Nanosci. Mater.2018, 1, 172–182, doi:10.26655/AJNANOMAT.2018.9.1.
- Cao, D.; Li, H.; Pan, L.; Li, J.; Wang, X.; Jing, P.; Cheng, X.; Wang, W.; Wang, J.; Liu, Q. High saturation magnetization of γ 3-Fe2 O3 nano-particles by a facile one-step synthesis approach. Sci. Rep. 2016, 6, 1–9, doi:10.1038/srep32360.
- Mohapatra, M.; Anand, S. Synthesis and applications of nano-structured iron oxides/hydroxides – a review. Int. J. Eng. Sci. Technol. 2011, 2, 127–146, doi:10.4314/ijest.v2i8.63846.
- Fang, S.; Bresser, D.; Passerini, S. Transition Metal Oxide Anodes for Electrochemical Energy Storage in Lithium- and Sodium-Ion Batteries. Adv. Energy Mater. 2020, 10, doi:10.1002/aenm.201902485.
- Magro, M.; Baratella, D.; Bonaiuto, E.; de A. Roger, J.; Vianello, F. New Perspectives on Biomedical Applications of Iron Oxide Nanoparticles. Curr. Med. Chem. 2018, 25, 540–555, doi:10.2174/0929867324666170616102922.
- Andrade, R.G.D.; Veloso, S.R.S.; Castanheira, E.M.S. Shape anisotropic iron oxide-based magnetic nanoparticles: Synthesis and biomedical applications. Int. J. Mol. Sci. 2020, 21, doi:10.3390/ijms21072455.
- Arakha, M.; Pal, S.; Samantarrai, D.; Panigrahi, T.K.; Mallick, B.C.; Pramanik, K.; Mallick, B.; Jha, S. Antimicrobial activity of iron oxide nanoparticle upon modulation of nanoparticle-bacteria interface. Sci. Rep. 2015, 5, 14813, doi:10.1038/srep14813.
- Kostyantyn, T.; Turek, Z.; Zanáška, M.; Kudrna, P.; Tichý, M. Iron Oxide and Iron Sulfide Films Prepared for Dye-Sensitized Solar Cells. Materials. 2020, 13,1797, doi:10.3390/ma13081797.
- Wang, Q.; Ma, Y.; Liu, L.; Yao, S.; Wu, W.; Wang, Z.; Lv, P.; Zheng, J.; Yu, K.; Wei, W. Plasma enabled Fe2O3/Fe3O4 nano-aggregates anchored on nitrogen-doped graphene as anode for sodium-ion batteries. Nanomaterials 2020, 10, 1–12, doi:10.3390/nano10040782.
- Cichello, S.A. Oxygen absorbers in food preservation: A review. J. Food Sci. Technol. 2015, 52, 1889–1895, doi:10.1007/s13197-014-1265-2.
- Rui, M.; Ma, C.; Hao, Y.; Guo, J.; Rui, Y.; Tang, X.; Zhao, Q.; Fan, X.; Zhang, Z.; Hou, T. Iron oxide nanoparticles as a potential iron fertilizer for peanut (Arachis hypogaea). Front. Plant Sci. 2016, 7,815, doi:10.3389/fpls.2016.00815.
- Durfey, C.L.; Swistek, S.E.; Liao, S.F.; Crenshaw, M.A.; Clemente, H.J.; Thirumalai, R.V.K.G.; Steadman, C.S.; Ryan, P.L.; Willard, S.T.; Feugang, J.M. Nanotechnology-based approach for safer enrichment of semen with best spermatozoa. J. Anim. Sci. Biotechnol. 2019, 10, 1–12, doi:10.1186/s40104-018-0307-4.
- Magdanz, V.; Gebauer, J.; Sharan, P.; Eltoukhy, S.; Voigt, D.; Simmchen, J. Sperm–Particle Interactions and Their Prospects for Charge Mapping. Adv. Biosyst. 2019, 3, 1–23, doi:10.1002/adbi.201900061.
- Arias, L.S.; Pessan, J.P.; Vieira, A.P.M.; De Lima, T.M.T.; Delbem, A.C.B.; Monteiro, D.R. Iron oxide nanoparticles for biomedical applications: A perspective on synthesis, drugs, antimicrobial activity, and toxicity. Antibiotics 2018, 7, 46, doi:10.3390/antibiotics7020046.
- Nene, A.G.; Takahashi, M.; Wakita, K.; Umeno, M. Size controlled synthesis of Fe3O4 nanoparticles by ascorbic acid mediated reduction of Fe(acac)3 without using capping agent. J. Nano Res. 2016, 40, 8–19, doi:10.4028/www.scientific.net/JNanoR.40.8.
- Huang, P.H.; Zhao, S.; Bachman, H.; Nama, N.; Li, Z.; Chen, C.; Yang, S.; Wu, M.; Zhang, S.P.; Huang, T.J. Acoustofluidic Synthesis of Particulate Nanomaterials. Adv. Sci. 2019, 6, doi:10.1002/advs.201900913.
- Jeevanandam, J.; Barhoum, A.; Chan, Y.S.; Dufresne, A.; Danquah, M.K. Review on nanoparticles and nanostructured materials: History, sources, toxicity and regulations. Beilstein J. Nanotechnol. 2018, 9, 1050–1074, doi:10.3762/bjnano.9.98.
- Nene, A.G.; Takahashi, M.; Somani, P.R.; Aryal, H.R.; Wakita, K.; Umeno, M. Synthesis and characterization of graphene-Fe3O4 nanocomposite. Carbon-Sci. Technol. 2016, 8, 13–24.
- Nene, A.G.; Somani, P.R.; Takahashi, M.; Umeno, M.; Technologies, G. Effect of experimental parameters on the synthesis of Fe3O4 nanoparticles by ascorbic acid mediated reduction of Fe(acac)3. Carbon Sci. Tech. 2019, 3, 6–26.
- Nene, A.G.; Takahashi, M.; Somani, P.R. Fe3O4 and Fe Nanoparticles by Chemical Reduction of Fe(acac) 3 by Ascorbic Acid: Role of Water Keywords Fe 3 O 4 Nanoparticles, Fe-Nanoparticles, Iron Oxide, Chemical Reduction Method. World J. Nano Sci. Eng. 2016, 6, 20–28, doi:10.4236/wjnse.2016.61002.
- Ambro, Ž.Č.G.; Orel, Z.C.; Žigon, M. Microwave-assisted non-aqueous synthesis of ZnO nanoparticles. Mater. Tehnol. 2011, 45, 173–177.
- Morán-Lázaro, J.P.; Guillen-López, E.S.; López-Urias, F.; Muñoz-Sandoval, E.; Blanco-Alonso, O.; Guillén-Bonilla, H.; Guillén-Bonilla, A.; Rodríguez-Betancourtt, V.M.; Sanchez-Tizapa, M.; Olvera-Amador, M.D.L.L. Synthesis of znmn2 o4 nanoparticles by a microwave-assisted colloidal method and their evaluation as a gas sensor of propane and carbon monoxide. Sensors (Switzerland) 2018, 18, doi:10.3390/s18030701.
- Jung, D.S.; Ko, Y.N.; Kang, Y.C.; Park, S. Bin. Recent progress in electrode materials produced by spray pyrolysis for next-generation lithium ion batteries. Adv. Powder Technol. 2014, 25, 18–31, doi:10.1016/j.apt.2014.01.012.
- Hasany, S.F.; Abdurahman, N.H.; Sunarti, A.R.; Jose, R. Magnetic iron oxide nanoparticles: Synthesis and applications. Curr. Nanosci. 2013, 9, 1–15.
- Eslamian, M.; Ahmed, M.; Ashgriz, N. Modelling of nanoparticle formation during spray pyrolysis. Nanotechnology 2006, 17, 1674–1685, doi:10.1088/0957-4484/17/6/023.
- Umer, A.; Naveed, S.; Ramzan, N.; Rafique, M.S. Selection of a suitable method for the synthesis of copper nanoparticles. Nano 2012, 7, 1230005, doi:10.1142/S1793292012300058.
- Gao, X.; Yokota, N.; Oda, H.; Tanaka, S.; Hokamoto, K.; Chen, P. One step preparation of Fe–FeO–graphene nanocomposite through pulsed wire discharge. Crystals 2018, 8, 104, doi:10.3390/cryst8020104.
- Dong, X.L.; Choi, C.J.; Kim, B.K. Structural and magnetic characterization of Fe nanoparticles synthesized by chemical vapor condensation process. J. Appl. Phys. 2002, 92, 5380–5385, doi:10.1063/1.1510168.
- Kumar, H.; Sangwan, P. Synthesis and Characterization of MnO 2 Nanoparticles using Co-precipitation Technique. Int. J. Chem. Chem. Eng. 2013, 3, 155–160.
- Wang, B.; Wei, Q.; Qu, S. Synthesis and characterization of uniform and crystalline magnetite nanoparticles via oxidation-precipitation and modified co-precipitation methods. Int. J. Electrochem. Sci. 2013, 8, 3786–3793.
- Hariani, P.L.; Faizal, M.; Ridwan, R.; Marsi, M.; Setiabudidaya, D. Synthesis and Properties of Fe3O4 Nanoparticles by Co-precipitation Method to Removal Procion Dye. Int. J. Environ. Sci. Dev. 2013, 4, 336–340, doi:10.7763/ijesd.2013.v4.366.
- Wu, W.; He, Q.; Jiang, C. Magnetic iron oxide nanoparticles: Synthesis and surface functionalization strategies. Nanoscale Res. Lett. 2008, 3, 397–415, doi:10.1007/s11671-008-9174-9.
- Singamaneni, S.; Bliznyuk, V.N.; Binek, C.; Tsymbal, E.Y. Magnetic nanoparticles: Recent advances in synthesis, self-assembly and applications. J. Mater. Chem. 2011, 21, 16819–16845, doi:10.1039/c1jm11845e.
- Campanini, M.; Ciprian, R.; Bedogni, E.; Mega, A.; Chiesi, V.; Casoli, F.; De Julián Fernández, C.; Rotunno, E.; Rossi, F.; Secchi, A.; et al. Lorentz microscopy sheds light on the role of dipolar interactions in magnetic hyperthermia. Nanoscale 2015, 7, 7717–7725, doi:10.1039/c5nr00273g.
- Ansari, S.A.M.K.; Ficiarà, E.; Ruffinatti, F.A.; Stura, I.; Argenziano, M.; Abollino, O.; Cavalli, R.; Guiot, C.; D’Agata, F. Magnetic iron oxide nanoparticles: Synthesis, characterization and functionalization for biomedical applications in the Central Nervous System. Materials 2019, 12, 465, doi:10.3390/ma12030465.
- Takai, Z.I.; Mustafa, M.K.; Asman, S.; Sekak, K.A. Preparation and characterization of magnetite (Fe3O4) nanoparticles by sol-gel method. Int. J. Nanoelectron. Mater. 2019, 12, 37–46.
- Rasheed, R.T.; Al-Algawi, S.D.; Kareem, H.H.; Mansoor, H.S. Preparation and Characterization of Hematite Iron Oxide (?-Fe2O3) by Sol-Gel Method. Chem. Sci. J. 2018, 09, 1000197, doi:10.4172/2150-3494.1000197.
- Thiagarajan, S.; Sanmugam, A.; Vikraman, D. Facile Methodology of Sol-Gel Synthesis for Metal Oxide Nanostructures. IntechOpen 2017, 38, doi:10.5772/intechopen.68708.
- Hassanjani-Roshan, A.; Vaezi, M.R.; Shokuhfar, A.; Rajabali, Z. Synthesis of iron oxide nanoparticles via sonochemical method and their characterization. Particuology 2011, 9, 95–99, doi:10.1016/j.partic.2010.05.013.
- Singh, P.; Kim, Y.J.; Zhang, D.; Yang, D.C. Biological Synthesis of Nanoparticles from Plants and Microorganisms. Trends Biotechnol. 2016, 34, 588–599, doi:10.1016/j.tibtech.2016.02.006.
- Varshney, R.; Bhadauria, S.; Gaur, M.S. A review: Biological synthesis of silver and copper nanoparticles. Nano Biomed. Eng. 2012, 4, 99–106, doi:10.5101/nbe.v4i2.p99-106.