Gut-derived metabolites such as indolepropionic acid (IPA) are known to paly an important contribution in the development of metabolic diseases. The source of IPA depends on the dietary tryptophan intake, especially fiber, and presence of specific bacterial species in the human gut. In the present narrative review we elaborate on the role of IPA in type 2 diabetes (T2D) as well as non-alcoholic fatty liver disease (NAFLD). WResearchers further explored the association of IPA with well known T2D-genetic variants and IPA-linked mechanisms affecting glucose metabolism and liver fibrosis.
- indolepropionic acid
- type 2 diabetes
- insulin
- NAFLD
1. Introduction
2. Diet, Gut Microbiota, and IPA
Abundance and diversity of gut microbiota are linked in complex ways to obesity, T2D, NAFLD, metabolic syndrome, inflammatory bowel disease, cardiovascular disease, and neurological disorders (6)[6]. In addition to endogenous metabolism, it has been shown that certain gut bacteria can synthesize IPA from tryptophan via the indole pathway (7)[7]. Clostridium (Cl.) sporogenes and Cl. botulinum but also Peptostreptococcus anaerobius and three strains of Cl. calaveris may also synthesize IPA (8)[8].
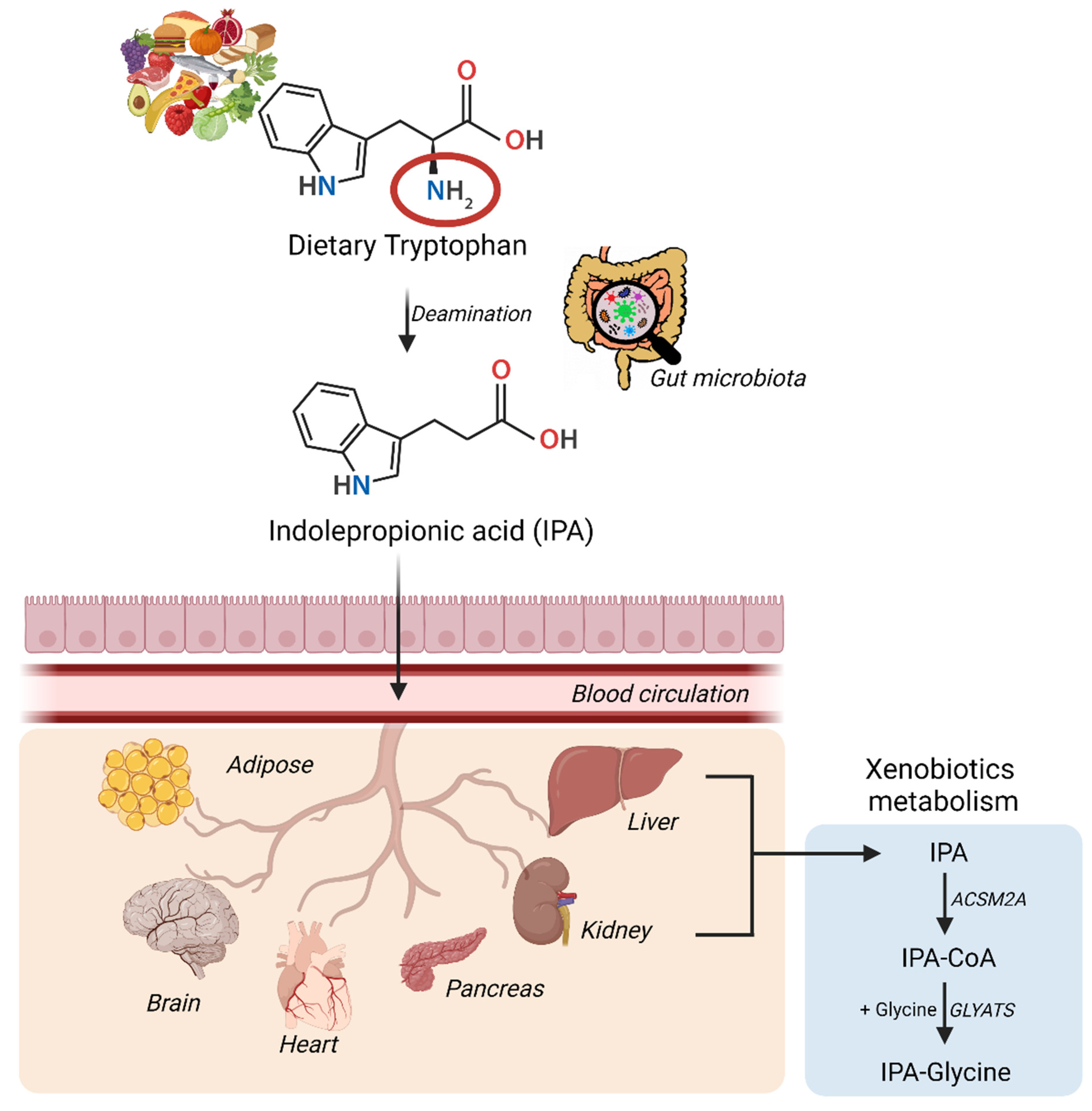
The synthesis and conversion of IPA in humans have been summarized in the figure above. IPA via the blood circulation can gain excess to multiple organs either via passive diffusion or with the help of amino acid transporters and can induce multiple beneficial effects.
3. IPA, T2D, & NAFLD
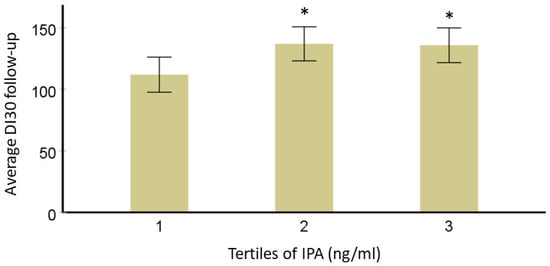
The mechanistic effect of IPA on glucose metabolism has not yet been studied in detail and the studies that have identified IPA and the risk of development of T2D have been combined. in the present review. In this narrative review, we Researchers further explored if T2D-specific genetic variants (TCF7L2 rs7903146 and rs12255372; FTO rs9939609 and PPARG rs1801282) from the same DPS population modified this association. A total of 522 individuals with IGT were originally randomly allocated into either a lifestyle intervention or control group for a mean four-year intervention (active study) period. DI30 is the estimate for beta cell function or peripheral insulin sensitivity, as validated before. Mathematically, DI30 = Matsuda insulin sensitivity index × ratio of total insulin area under the curve (AUC) and total glucose AUC during 0–30 min of the oral glucose tolerance test. After data normalization, analysis of variance models adjusted for study group, age, sex, and BMI were used to study the associations of IPA with long-term follow-up insulin secretion (DI30), fasting, and postprandial glucose or insulin additionally considering the effect of TCF7L2, FTO or PPARG genotypes (categorical variable; dominant model). For testing correlations, wresearchers applied Pearson’s correlation test and the value of p-value < 0.05 was considered significant. In models adjusted for age, sex, BMI, and study group, persons in the lowest third for serum IPA concentration have lower averaged DI during the 7-year FU compared with those in the second and third categories. This association did not change by TCF7L2 variants rs7903146 and rs12255372, which are known to be associated with insulin secretion, which was also the case in the DPS population. Another variant associated with the development of T2D in the DPS, PPARG rs1801282 may be associated with improvements in insulin sensitivity. When this variant was included in the model, the association of IPA with DI30 was not lost. Also, the interaction of variant rs1801282 with IPA was not significant.
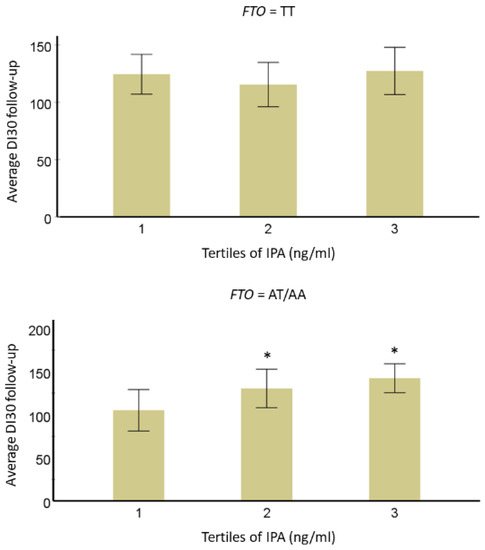
Researchers observed a significant trend for the interaction of IPA with
FTOvariant rs9939609 (
p = 0.05). In individuals with the minor allele A, we found that those in the lowest IPA tertile had lower averaged IPA concentrations as compared with the second (= 0.05). In individuals with the minor allele A, researchers found that those in the lowest IPA tertile had lower averaged IPA concentrations as compared with the second (
p-value = 0.003) and third (
p-value = 0.018) tertiles for DI
30.
Gut-liver axis is the passage for metabolites to gain direct access to liver and lead to liver-related diseases (9)[9]. Existing evidence links gut-derived indole and indole derivatives to liver health and integrity (10)[10]. IPA and other tryptophan derivatives have also been found to be associated with cardiovascular events, a common co-morbidity in NAFLD, NASH, and T2D (11)[11]. IPA is well known for its hydroxyl radical and lipid peroxide scavenging activity and has been found to regulate low-grade inflammation and affecting mechanisms linked to liver health and functioning (12)[12]. In various studies involving obese adolescents and adults, IPA levels have been identified to be altered (13,14)[13][14]. These results demand future research projects focusing on a deep understanding of the mechanisms of action of IPA specifically in metabolic disorders, yet not forgetting the important role of diet in the prevention of these diseases.
4. Conclusions
WResearchers conclude that the discovery of the connection between the microbiota and gut-derived metabolites, particularly IPA, has opened up new options for T2D prevention and treatment. In addition to providing new therapeutic options, the biological effects of IPA have increased ourthe understanding of the intricate relationship between diet, gut microbiota, and the risk of cardio-metabolic disorders. New information on IPA's beneficial effects on intestinal integrity, neuronal function, oxidative stress, lipid metabolism, glucose metabolism, including insulin secretion, inflammation, liver injury, and cellular damage has already been provided by its numerous interactions with various biological processes. To be more specific, the fact that there is a strong connection between IPA and T2D and NAFLD opens the door to developing strategies for using IPA as potential treatments. These findings call for subsequent research projects that place a strong emphasis on gaining a comprehensive understanding of the mechanisms of action of IPA, particularly in cardio-metabolic disorders, without neglecting the significant role that diet plays in the prevention of these conditions.References
1. Konopelski, P.; Mogilnicka, I. Biological Effects of Indole-3-Propionic Acid, a Gut Microbiota-Derived Metabolite, and Its Precursor Tryptophan in Mammals’ Health and Disease. Int. J. Mol. Sci. 2022, 23, 1222.
2. Massey, W.; Brown, J.M. The Gut Microbial Endocrine Organ in Type 2 Diabetes. Endocrinology 2021, 162, bqaa235.
3. De Mello, V.D.; Paananen, J.; Lindström, J.; Lankinen, M.A.; Shi, L.; Kuusisto, J.; Pihlajamäki, J.; Auriola, S.; Lehtonen, M.; Rolandsson, O.; et al. Indolepropionic Acid and Novel Lipid Metabolites Are Associated with a Lower Risk of Type 2 Diabetes in the Finnish Diabetes Prevention Study. Sci. Rep. 2017, 7, 46337.
4. O’Keefe, S.J. The Association between Dietary Fibre Deficiency and High-Income Lifestyle-Associated Diseases: Burkitt’s Hypothesis Revisited. Lancet Gastroenterol. Hepatol. 2019, 4, 984–996.
5. Sehgal, R.; Ilha, M.; Vaittinen, M.; Kaminska, D.; Männistö, V.; Kärjä, V.; Tuomainen, M.; Hanhineva, K.; Romeo, S.; Pajukanta, P.; et al. Indole-3-Propionic Acid, a Gut-Derived Tryptophan Metabolite, Associates with Hepatic Fibrosis. Nutrients 2021, 13, 3509.
6. Zhu, T.; Goodarzi, M.O. Metabolites Linking the Gut Microbiome with Risk for Type 2 Diabetes. Curr. Nutr. Rep. 2020, 9, 83–93.
7. Roager, H.M.; Licht, T.R. Microbial Tryptophan Catabolites in Health and Disease. Nat. Commun. 2018, 9, 3210–3294.
8. Dodd, D.; Spitzer, M.H.; Van Treuren, W.; Merrill, B.D.; Hryckowian, A.J.; Higginbottom, S.K.; Le, A.; Cowan, T.M.; Nolan, G.P.; Fischbach, M.A.; et al. A Gut Bacterial Pathway Metabolizes Aromatic Amino Acids into Nine Circulating Metabolites. Nature 2017, 551, 648–652.
9. Pan, X.; Wen, S.W.; Kaminga, A.C.; Liu, A. Gut Metabolites and Inflammation Factors in Non-Alcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. Sci. Rep. 2020, 10, 8848.
10. Hendrikx, T.; Schnabl, B. Indoles: Metabolites Produced by Intestinal Bacteria Capable of Controlling Liver Disease Manifestation. J. Intern. Med. 2019, 286, 32–40.
11. Zhang, B.; Jiang, M.; Zhao, J.; Song, Y.; Du, W.; Shi, J. The Mechanism Underlying the Influence of Indole-3-Propionic Acid: A Relevance to Metabolic Disorders. Front. Endocrinol. 2022, 13, 841703.
12. Negatu, D.A.; Gengenbacher, M.; Dartois, V.; Dick, T. Indole Propionic Acid, an Unusual Antibiotic Produced by the Gut Microbiota, With Anti-Inflammatory and Antioxidant Properties. Front. Microbiol. 2020, 11, 575586.
13. Farook, V.S.; Reddivari, L.; Chittoor, G.; Puppala, S.; Arya, R.; Fowler, S.P.; Hunt, K.J.; Curran, J.E.; Comuzzie, A.G.; Lehman, D.M.; et al. Metabolites as Novel Biomarkers for Childhood Obesity-Related Traits in Mexican-American Children. Pediatr. Obes. 2015, 10, 320–327.
14. Jennis, M.; Cavanaugh, C.R.; Leo, G.C.; Mabus, J.R.; Lenhard, J.; Hornby, P.J. Microbiota-Derived Tryptophan Indoles Increase after Gastric Bypass Surgery and Reduce Intestinal Permeability in Vitro and in Vivo. Neurogastroenterol. Motil. 2018, 30, e13178.
References
- Konopelski, P.; Mogilnicka, I. Biological Effects of Indole-3-Propionic Acid, a Gut Microbiota-Derived Metabolite, and Its Precursor Tryptophan in Mammals’ Health and Disease. Int. J. Mol. Sci. 2022, 23, 1222.
- Massey, W.; Brown, J.M. The Gut Microbial Endocrine Organ in Type 2 Diabetes. Endocrinology 2021, 162, bqaa235.
- De Mello, V.D.; Paananen, J.; Lindström, J.; Lankinen, M.A.; Shi, L.; Kuusisto, J.; Pihlajamäki, J.; Auriola, S.; Lehtonen, M.; Rolandsson, O.; et al. Indolepropionic Acid and Novel Lipid Metabolites Are Associated with a Lower Risk of Type 2 Diabetes in the Finnish Diabetes Prevention Study. Sci. Rep. 2017, 7, 46337.
- O’Keefe, S.J. The Association between Dietary Fibre Deficiency and High-Income Lifestyle-Associated Diseases: Burkitt’s Hypothesis Revisited. Lancet Gastroenterol. Hepatol. 2019, 4, 984–996.
- Sehgal, R.; Ilha, M.; Vaittinen, M.; Kaminska, D.; Männistö, V.; Kärjä, V.; Tuomainen, M.; Hanhineva, K.; Romeo, S.; Pajukanta, P.; et al. Indole-3-Propionic Acid, a Gut-Derived Tryptophan Metabolite, Associates with Hepatic Fibrosis. Nutrients 2021, 13, 3509.
- Zhu, T.; Goodarzi, M.O. Metabolites Linking the Gut Microbiome with Risk for Type 2 Diabetes. Curr. Nutr. Rep. 2020, 9, 83–93.
- Roager, H.M.; Licht, T.R. Microbial Tryptophan Catabolites in Health and Disease. Nat. Commun. 2018, 9, 3210–3294.
- Dodd, D.; Spitzer, M.H.; Van Treuren, W.; Merrill, B.D.; Hryckowian, A.J.; Higginbottom, S.K.; Le, A.; Cowan, T.M.; Nolan, G.P.; Fischbach, M.A.; et al. A Gut Bacterial Pathway Metabolizes Aromatic Amino Acids into Nine Circulating Metabolites. Nature 2017, 551, 648–652.
- Pan, X.; Wen, S.W.; Kaminga, A.C.; Liu, A. Gut Metabolites and Inflammation Factors in Non-Alcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. Sci. Rep. 2020, 10, 8848.
- Hendrikx, T.; Schnabl, B. Indoles: Metabolites Produced by Intestinal Bacteria Capable of Controlling Liver Disease Manifestation. J. Intern. Med. 2019, 286, 32–40.
- Zhang, B.; Jiang, M.; Zhao, J.; Song, Y.; Du, W.; Shi, J. The Mechanism Underlying the Influence of Indole-3-Propionic Acid: A Relevance to Metabolic Disorders. Front. Endocrinol. 2022, 13, 841703.
- Negatu, D.A.; Gengenbacher, M.; Dartois, V.; Dick, T. Indole Propionic Acid, an Unusual Antibiotic Produced by the Gut Microbiota, With Anti-Inflammatory and Antioxidant Properties. Front. Microbiol. 2020, 11, 575586.
- Farook, V.S.; Reddivari, L.; Chittoor, G.; Puppala, S.; Arya, R.; Fowler, S.P.; Hunt, K.J.; Curran, J.E.; Comuzzie, A.G.; Lehman, D.M.; et al. Metabolites as Novel Biomarkers for Childhood Obesity-Related Traits in Mexican-American Children. Pediatr. Obes. 2015, 10, 320–327.
- Jennis, M.; Cavanaugh, C.R.; Leo, G.C.; Mabus, J.R.; Lenhard, J.; Hornby, P.J. Microbiota-Derived Tryptophan Indoles Increase after Gastric Bypass Surgery and Reduce Intestinal Permeability in Vitro and in Vivo. Neurogastroenterol. Motil. 2018, 30, e13178.
