You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 1 by Hao Liu and Version 2 by Catherine Yang.
Injury repair is a complex physiological process in which multiple cells and molecules are involved. Tenascin-C (TNC), an extracellular matrix (ECM) glycoprotein, is essential for angiogenesis during wound healing.
- injury repair
- tenascin-C
- angiogenesis
- extracellular matrix
1. The Construction and Expression Signature of TNC
The ECM is mainly composed of various macromolecular substances that constitute a complex network of cellular physiological activities [1][15]. Recent studies have shown that ECM plays an increasing role in tissue damage and regeneration, especially in angiogenesis, by providing important cues for cellular responses [2][3][16,17]. Tenascins are multifunctional glycoproteins found in the ECM of chordate animals. Studies have validated that tenascin-C, -R, -X, and -W are the four isoforms of the tenascin family, and have similar functions in regulating cell adhesion and cellular responses to injury repair [4][5][6][7][8][18,19,20,21,22].
Since it was first discovered in the 1980s, TNC has been the most studied and most representative member of the tenascin family [9][10][5,23]. The unique structure of TNC often exists in the form of hexamers, with a molecular weight of approximately 220–400 kDa [11][24]. TNC consists of four distinct domains, from N-terminal to C-terminal: the coil-like tenascin assembly (TA) domain; 14.5 epidermal growth factor-like (EGF-L) domain; 17 fibronectin type III (FN III)-like domains; and a fibrinogen-like globule (FBG) domain (Figure 1A). This multi-modular structure enables tenascin to interact with a large number of highly diverse ligands, such as FNIII-like domains that bind different proteins, such as annexin II, syndecan-4, and integrins αvβ3 and α7β1 through alternative splicing [12][13][14][25,26,27]. In addition, FNIII-like domains bind to a variety of growth factors, including the vascular endothelial growth factor (VEGF), epidermal growth factor receptor (EGFR), and transforming growth factor-β (TGF-β). The interactions allow TNC to promote cell migration, cell adhesion, matrix aggregation, protease, and proinflammatory factor synthesis. Therefore, given the structural, expression, and functional characteristics of TNC, the alternative spliceosome of TNC plays different roles in various injuries (Figure 1B) [15][16][17][18][19][20][28,29,30,31,32,33].
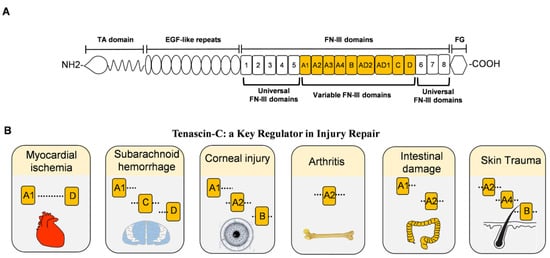
Figure 1. The structure of TNC and damage-related variable FNIII domains. (A). Full-length domain of human TNC protein. From the N-terminal to the C-terminal includes the tenascin assembly domain (TA), epidermal growth factor-like (EGF-L) repeat, fibronectin type III (FN III) domain, and fibrinogen globulin (FG) domain. The FNIII domains include eight fixed FNIII domains and nine variable FNIII domains. (B). The role of the variable FNIII domains in different injuries. All variable FNIII domains play a role in myocardial infarction. A1, C, and D domains aggravate the symptoms of subarachnoid hemorrhage. Corneal injury exhibits A1, A2, and B domains abnormally, while arthritis expresses the A2 domain leading to synovial injury. In intestinal damage, A1 and A2 domains aggravate intestinal bleeding. For skin trauma, domains A2, A4, and B are associated with angiogenesis.
In adults, the expression of TNC is strictly regulated by cell type and the tissue microenvironment. TNC is nearly undetectable in normal adults, with the exception of embryonic development and a few connective tissues. TNC mainly exists around motor cells, especially in the central nervous system and bone joint system [21][34]. TNC plays a role in regulating cell adhesion, motility, and tissue remodeling and is closely related to inflammatory signaling, through which angiogenesis can be achieved. Changes in TNC expression caused by external stimuli cause angiogenesis and are involved in the processes of trauma, tumor, and degeneration. Whereas in the pathological state, TNC exists around the injury, the tumor matrix, and the site of regeneration [22][23][35,36].
2. TNC in Healing Wound
Injury defined by damage to normal tissues is caused by multiple factors [24][37]. The integrity of the tissue is destroyed, along with the loss of a certain amount of normal tissue [25][38]. In the wound healing process, there are four relatively distinct phases that include hemostasis, inflammation, proliferation, and remodeling. The first step is the hemostasis phase of wound healing, which entails clotting of blood mediated by platelets [26][39]. Angiogenesis is a complex and dynamic process that plays an important role at each stage of wound healing by transforming vascular precursor cells into new blood vessels [27][40]. The generation of new capillary beds through angiogenesis is essential for normal healing [28][41]. Tissue vascular networks after injury usually involve angiogenesis, vasculogenesis, and arteriogenesis. Vasculogenesis is the process of growing new blood vessels from existing vasculature. Arteriogenesis is the process by which arteries remodel [29][30][42,43].
The ECM, including collagen, TNC, matrix metalloproteinases (MMPs), etc., are major players in tissue injury and regeneration, providing important regulatory clues for the formation of new capillaries [31][32][44,45]. Usually, angiogenesis increases greatly in the early stage of injury and will increase to 2–3 or even 10 times after injury [33][34][46,47]. In the later stages of repair, cell death and vascular degeneration return to normal levels to maintain homeostasis [35][48]. TNC is an important component of the ECM and is mainly expressed during tissue repair and tumor growth. The expression level of TNC is dynamically consistent with the changes in angiogenesis after injury in both time and space [35][48] (Figure 2). Studies suggested that the physiological repair of tissue injury can be mediated by regulating the dynamic expression of TNC.
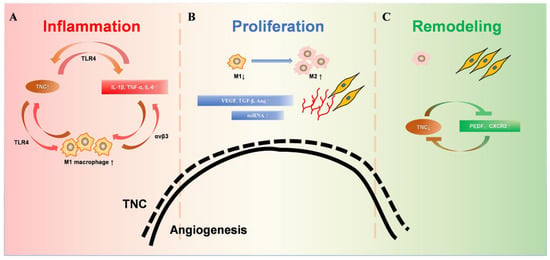
Figure 2. Dynamic changes of TNC during wound healing. (A). During the inflammatory phase, TNC induces the synthesis of proinflammatory cytokines through TLR4. TNC enhanced the M1 macrophage polarization of bone-marrow-derived macrophages. Furthermore, proinflammatory cytokines up-regulated the expression of TNC significantly, which amplifies inflammatory responses by creating a positive feedback loop. (B). The proliferative phase is mainly granulation tissue, including a large number of new capillaries, fibroblasts, and M1 and M2 macrophages. The substantial increase in TNC promotes angiogenesis, accompanied by promotion of macrophage polarization into the M2 phenotype. Increased M2 phenotype macrophages promote collagen formation and extracellular matrix proliferation. (C). During the remodeling stage, the decreased TNC expression leads to decreased M2 phenotype macrophages. Increased fibroblasts and collagen fiber formation are involved in tissue remodeling. Capillaries atrophy and decrease, and TNC decreases to the pre-injury state.
3. TNC in the Remodeling Phase
With the resolution of inflammation, TNC expression gradually decreases in the late stages of injury. The dynamic reduction in TNC, which decreases the angiogenic-related response, is an important marker of entering the remodeling phase. Over time, most neovascularization degenerates in the late stages of injury until the final vascular density returns to the state of normal uninjured skin.
Moreover, molecules related to TNC play essential roles in tissue remodeling during the late stage of injury repair, which are further discussed below.
3.1. TNC and PEDF
Pigment epithelium derived factor (PEDF) is a glycoprotein with a molecular weight of 50 kDa [36][99]. It is the most effective neoangiogenesis inhibitor [37][100]. The anti-angiogenic efficacy of PEDF is selective because it targets neovascularization and is almost ineffective for existing vessels [38][101]. Thus, PEDF is of great importance for accelerating the wound healing process.
Loss of PEDF can promote angiogenesis in retinal endothelial cells [39][102]. Flow cytometry, MTT assay, and transwell migration assays have shown that PEDF deletion is mainly associated with increased cell proliferation, decreased apoptosis, and increased angiogenesis. Molecular studies show that the mechanism of PEDF deficiency promoting angiogenesis is primarily related to increased levels of TNC, cohesin, thrombospondin-1, collagen IV, and decreased osteopontin content [40][103]. Therefore, in the process of vascular degeneration in the later stage of trauma, the increase in anti-inflammatory levels reduces TNC expression and promotes an increase in PEDF levels, thus, inhibiting the process of angiogenesis.
3.2. TNC and CXCR3
The chemokine receptor CXCR3 is a member of the G-protein coupled receptor family and is mainly expressed in the parenchymal cells of damaged organs and inflammatory cells [41][104]. Since CXCR3 can bind to its ligands CXCL9, CXCL10, and CXCL11 to inhibit angiogenesis, CXCR3 may play an important role in wound healing and tumor growth [42][105].
A recent study has shown that transplantation of mesenchymal stem cells (MSCs) can promote wound healing by regulating inflammatory responses, inducing angiogenesis, and enhancing epithelial growth and extracellular matrix formation [43][106]. The pro-angiogenic ability of MSCs can be accelerated by the use of type I collagen and TNC hydrogels. MSCs mainly inhibit the expression of CXCR3 in fibroblasts, thereby reducing the formation of hypertrophic scar tissue and regulating angiogenesis. The removal of TNC-containing hydrogels significantly inhibited the effects of MCCs. During the post-injury scarring phase, TNC may modulate the post-injury repair ability by increasing the level of CXCR3 in fibroblasts and reducing capillary formation.
