Modified electrodes for sensors and supercapacitors as well as anti-corrosion are described. Sol-gel synthesis expands the capabilities of technologists to obtain highly porous, homogeneous, and hybrid thin-film materials for supercapacitor electrode application. The widespread materials are transition metal oxides, but due to their low conductivity, they greatly impede the rate capability of electrochemical supercapacitors. The way to optimize their properties is the production of complex oxides or different composites. Among the new materials, a special place is occupied by perovskites and materials with an olivine-type structure, which can be easily obtained by the sol-gel method. The sol-gel coating process has demonstrated excellent chemical stability to advance the corrosion resistance of the various metal alloy substrates.
- sol-gel
- corrosion protection
- coatings
- supercapacitors
- electrochemical sensors
1. Introduction
2. Sol-Gel Materials for Supercapacitors Application
A large specific surface area with high purity is required for the production of electrode materials for supercapacitors. Considering the advantages of the sol-gel method, it can be observed as a great potential process to prepare these materials. In general, tailor-made metal oxides and metal hydroxide-based electrodes follow a pseudocapacitance (redox) mechanism, whereas carbon-based electrodes store the charge via electric double-layer capacitance (EDLC). The materials to integrate both types of charge-storing mechanisms can be made using the universal process, which is a soft chemical approach. This is practiced to produce high porous metal oxides at adequate temperatures. The properties of supercapacitors depend on the electrochemical stability, conductivity, porosity, and structure features of the electrode materials for high specific capacitance at low resistance enables.3. Sol-Gel Technologies in Electrochemical Sensors
Electrochemical sensors are a class of chemical sensors in which an electrode is used as a transducer element in the presence of the analyte. Modern electrochemical sensors use several properties to determine various parameters in our daily life, whether physical, chemical, or biological parameters. In this regard, they are very widely applicable. Some examples are sensors for monitoring the environment, health, and appliances, as well as sensors related to machines such as cars, airplanes, mobile phones, and technological media. Currently, new materials, production methods, and strategies are actively used in the development of electrochemical sensors to increase selectivity and detection limits. Sol-gel matrices are useful in the production of electrochemical sensors due to their chemical inertia, mechanical stability, biocompatibility, and high specific surface area provided by porous grids with an open frame. All these features allow sol-gel nanocomposite sensors to demonstrate increased sensitivity and low detection limits about a wide range of target analytes [28]. The sol-gel method is most applicable in the formation of sensitive materials based on oxides, complex oxides, and their composites.3.1. Oxide Materials and Materials Based on Them
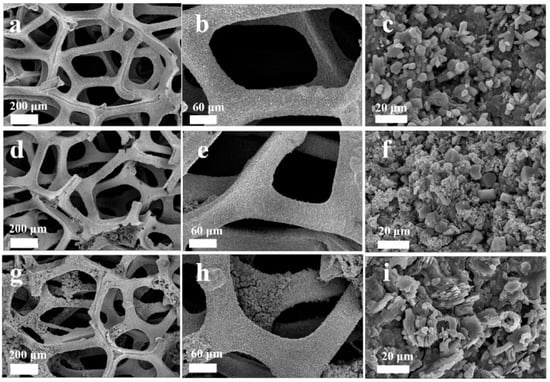
Materials based on oxides of various metals obtained by the sol-gel method are most actively used in electrochemical sensors. Zinc oxide-based materials are very popular. Golli, A.E et al. [29] reported the preparation of ZnO nanoparticles by the sol-gel method. Zinc acetate was dissolved in methanol at room temperature with constant stirring for 2 h, then copper chloride was added into the mixture, and the resultant product dried to get aerogel nanomaterial and the final product was annealed. The SEM image of fabricated Cu/ZnO showed spheroid-like spherical nanoparticles (Figure 1).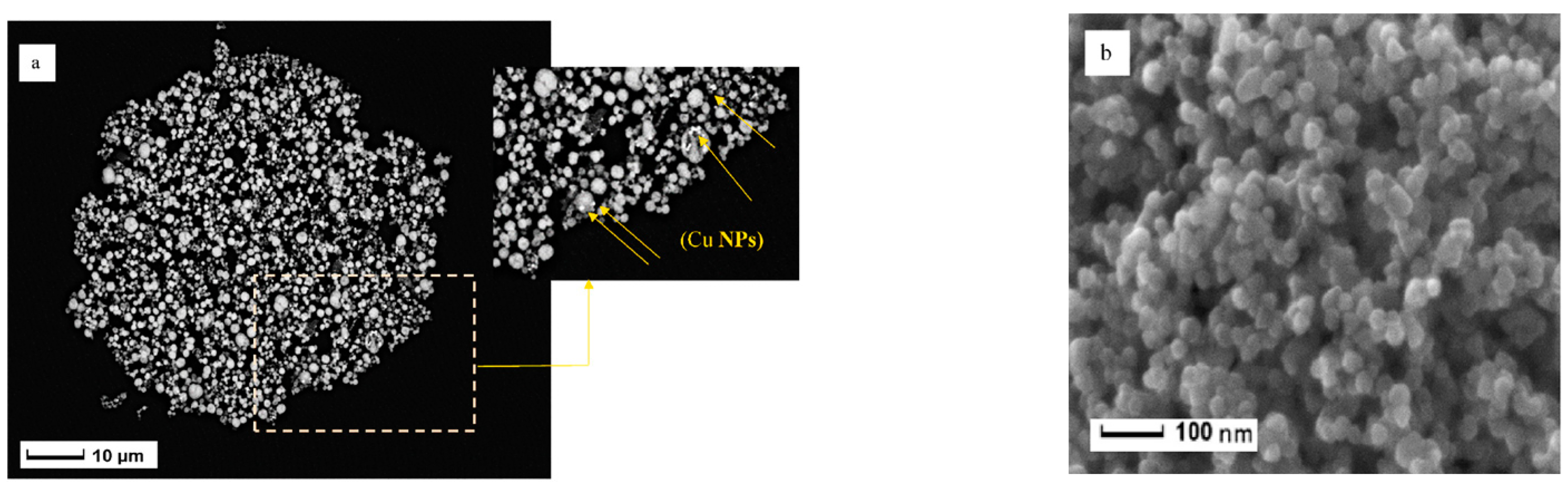
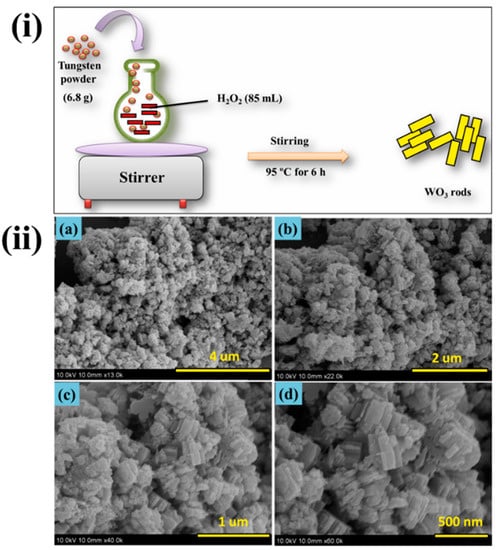
 Tin oxide films were used in the sensor platform to detect nitrites in milk, mineral water, and beer by impedance and conductometric methods. There is also research on the production of electrochemical sensors based on oxides and titanium and tungsten [38][39][40][41][42]. The preparation of tungsten trioxide [4039] and its morphology is shown in Figure 3. Using fabricated WO3 were studied for dopamine sensing vby cyclic voltammetry analysis, and the material showed better sensing of dopamine with excellent stability.
Tin oxide films were used in the sensor platform to detect nitrites in milk, mineral water, and beer by impedance and conductometric methods. There is also research on the production of electrochemical sensors based on oxides and titanium and tungsten [38][39][40][41][42]. The preparation of tungsten trioxide [4039] and its morphology is shown in Figure 3. Using fabricated WO3 were studied for dopamine sensing vby cyclic voltammetry analysis, and the material showed better sensing of dopamine with excellent stability.
3.2. Materials Based on Complex Oxides
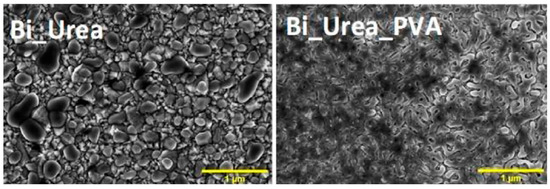
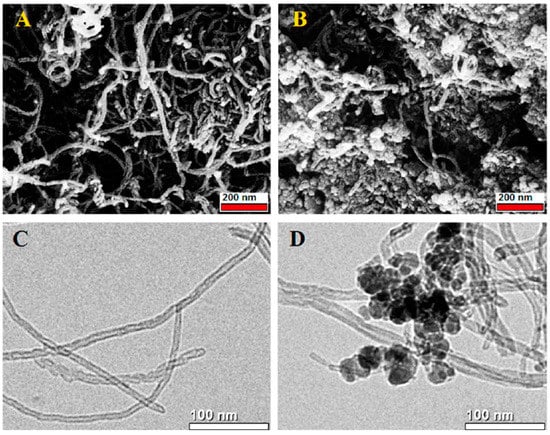
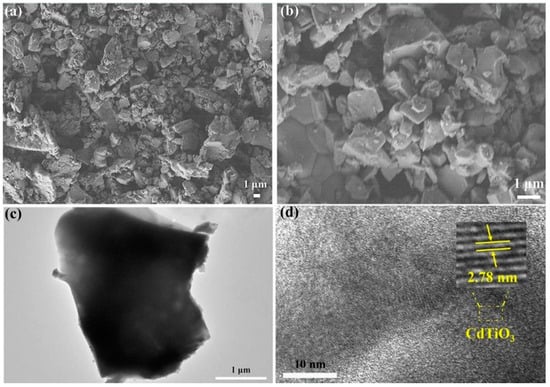
Double and triple oxides of semiconductor metals are a popular object of research in electrochemical sensors. This is due to their high conductivity and stability of chemical properties. Oftentimes, such materials are obtained using the sol-gel method. Peng S et al. [4847] prepared cadmium indium oxides using the sol-gel method in the aqueous medium and studied glucose biosensors. SEM images of nickel-coated CdIn2O4 showed multilayer stacked nanoplates, and it shows good selectivity of glucose. Similarly, Shu, H et al. [4948] prepared CdIn2O4 with iron modification by sol-gel method in the aqueous medium and examined glucose biosensors. SEM images of iron-modified CdIn2O4 showed aggregated nanoparticles (Figure 4) and it provides good electrochemical conductivity to the detection of glucose. Petruleviciene, M et al. [5049] prepared BiVO4 thin films on fluoride-doped tin oxide substrates by the sol-gel method in the aqueous medium. Fabrication of BiVO4 thin film, urea, and polyvinyl alcohol was used as a stabilizer and the layer deposition was annealed at 450 °C for 2 h. SEM images of BiVO4 thin films revealed that morphology, the influence of PVA additive on the urea-PVA treated coating, and nanoparticles are interconnected to BiVO4 thin films (Figure 15). PVA-modified coating BiVO4 thin films have a greater affinity to glucose adsorption. The MnFe2O4 nanoparticles and the MnFe2O4 nanoparticles modified carbon electrode paste (CPE) were prepared via the sol-gel process [5150]. The AuNPs were formed onto the MnFe2O4/CPE surface by electrodeposition of HAuCl4.3H2O. The SEM image indicates a nanostructure layer of the AuNPs onto the modified CPE/MnFe2O4NPs surface. This sensor showed good sensitivity to flunitrazepam. MWCNTs/CuFe2O4 (carbon nanotubes in combination with copper ferrite) nanocomposite was successfully fabricated by the sol-gel method [5251]. After the preparation of CuFe2O4, citric acid was added to the mixture and stirred at 80 °C for 3 h. Then, MWCNTs were added and ultra-sonicated at 80 °C for 1 h. The resultant material was calcined at 400 °C for 4 h. The SEM images of MWCNTs/CuFe2O4 nanoparticles showed tubular-like spherical morphology with a diameter of 32 nm (Figure 6). As a result, CuFe2O4 loading on MWCNTs reduces the particle size, which exposes more active centers in the nanocomposite. This feature of the morphology of the material, according to the authors of the work, contributes to a better sensitivity to bisphenol A. Zhang, Y et al. [5352] prepared La2NiFeO6 by the sol-gel method, and citric acid was used as a complexing agent. The resulting material was annealed at 400 °C for 4 h and the final materials were sintered at 1000 °C, 1100 °C, and 1200 °C respectively. The SEM images of La2NiFeO6 materials exhibit particle average size of 300 nm (sintered at 1000 °C) and 500 nm (sintered at 1100 °C), whereas materials sintered at 1000 °C show a slightly agglomerated average size of 1 micron (Figure 7).
| Sol-Gel Material | Detectable Substance |
Maximum Gas Sensitivity |
Linear Range | Ref. |
|---|---|---|---|---|
| Cu/ZnO nanocomposite | glucose | 36.641 μAmM−1cm−2 | 0.01–1, 1–7 mM | [29] |
| Cu-doped ZnO nanoparticles | myoglobin | 2.13–10.14 µAnM−1cm−2 | 3–15 nM | [30] |
| ZnO nanoparticles | clenbuterol | - | 0.3–1000 ng/mL | [31] |
| SiO2/Al2O3/C | nitrite | 410 μAμM−1 | 0.2–280 μM | [32] |
| SnO2 coatings | nitrite | 22.56 μAμM−1 | 10–400 μM | [33] |
| Pt-SnO2 nanoparticles | hydrogen | - | 0.08–500 ppm | [34] |
| Au–SnO2 nanoparticles | vitamin B12 | 110.843 μApM−1 | 0–1500 pM | [35] |
| Cu-doped SnO2 nanoparticles | ethyl acetate | 4.8 µA/ppb | 1–20 ppb | [36] |
| CuNPs on the C/SiO2 working electrode | glucose | - | 53–670 mg/L O2 | [37] |
| p-TiO2 nanoparticles | ethanol | ~50% | 1–100 ppm | [3938] |
| WO3 rods | dopamin | 3.66 μAμM−1cm−2 | 1–250 μM | [4039] |
| Nitrogen-doped carbon sheets wrapped in SnO2 nanoparticles | glucose | 215 nAμM−1cm−2 | 0.05–10 μM | [4140] |
| α-Fe2O3 doped CdSe | aqueous methanol | 0.2744 μAmM−1cm−2 | 0.2–48 mM | [4241] |
| MnO–CrN nanocomposite | hydrogen peroxide | 2156.25 μAmM−1cm−2 | 0.33–15 000 μM | [4443] |
| NiO nanoporous materials | glucose | 445 μAm−1cm−2 | - | [4544] |
| CuO-MgO composite | dopamine | 69 μAmM−1cm−2 | 10–100 μM | [4645] |
| WO3@SnO2-20 | ammonia | - | 5–50 ppm | [4746] |
| CdIn2O4 nanoparticles | glucose | 3292.5 μAmM−1cm−2 | 1.0 μM–1.0 mM | [4847] |
| Fe-CdIn2O4 nanoparticles | glucose | 8992 μAmM−1cm−2 | 0.01–1 mM | [4948] |
| BiVO4 | glucose | - | 1–35 mM | [5049] |
| MnFe2O4 | flunitrazepam | - | 0.1–100 μM | [5150] |
| Multiwalled carbon nanotubes CuFe2O4 | bisphenol | 0.355 μAμM−1 | 0.01–120 μM | [5251] |
| La2NiFeO6 | triethylamine | - | 0.5–200 ppm | [5352] |
| K2NiF4-type oxides La2NiO4 | hydrogen sulfide | −70 mV | 0.05–2 ppm | [5453] |
| CdTiO3 | acetylene | −91 mV | 5–100 ppm | [5554] |
| Ln2CuO4 nanocrystals | hydrogen peroxide | - | 0.50–15.87 μM | [5655] |
References
- Najafi, A.; Golestani-Fard, F.; Rezaie, H.R.; Saeb, S.P. Sol-Gel synthesis and characterization of SiC–B4C nanopowder. Ceram. Int. 2020, 47, 6376–6387.
- Fakhimi, O.; Najafi, A.; Khalaj, G. A facile route to obtain Al2O3 nanopowder via recycling aluminum cans by sol-gel method. Mater. Res. Express 2020, 7, 045008.
- Gaponenko, N.V. Sol-gel derived films in mesoporous matrices: Porous silicon, anodic alumina, and artificial opals. Synth. Met. 2001, 124, 125–130.
- Molchan, I.S.; Molchan, T.V.; Gaponenko, N.V.; Skeldon, P.; Thompson, G.E. Impurity-driven defect generation in porous anodic alumina. Electrochem. Commun. 2010, 12, 693–696.
- Han, F.; Qian, O.; Meng, G.; Lin, D.; Chen, G.; Zhang, S.; Pan, Q.; Zhang, X.; Zhu, X.; Wei, B. Structurally integrated 3D carbon tube grid-based high-performance filter capacitor. Mater. Sci. 2022, 377, 1004–1007.
- Yalovega, G.; Myasoedova, T.; Funik, A.; Plugotarenko, N.; Brzhezinskaya, M.; Bahmatskaya, A. Mechanism of the formation of copper-containing fractal-like crystallites in metal-organic thin films: Shape simulation and XANES analysis. Phys. Status Solidi B Basic Res. 2016, 253, 2217–2224.
- Yalovega, G.; Funik, A.; Myasoedova, T.; Brzhezinskaya, M. Copper-oxide metalorganic nanocomposite: Morphological and X-ray spectroscopy studies. J. Phys. Conf. Ser. 2016, 712, 012055.
- Shmatko, V.A.; Yalovega, G.E.; Myasoedova, T.N.; Shtekhin, I.E.; Petrov, V.V. Influence of the surface morphology and structure on the gas-sorption properties of SiO2CuOx nanocomposite materials: X-ray spectroscopy investigations. Phys. Solid State 2015, 57, 399–406.
- Plugotarenko, N.K.; Korolev, A.N.; Petrov, V.V.; Nazarova, T.N. Preparation of sols from water-alcohol solutions of tetraethyl orthosilicate and SnCl4 and the effect of sol composition on the surface morphology of sol-gel films. Inorg. Mater. 2007, 43, 1010–1014.
- Myasoedova, T.N.; Plugotarenko, N.K.; Moiseeva, T.A. Copper-containing films obtained by the simple citrate sol-gel route for NO2 detection: Adsorption and kinetic study. Chemosensors 2020, 8, 79.
- Myasoedova, T.N.; Mikhailova, T.S.; Yalovega, G.E.; Plugotarenko, N.K. Resistive low-temperature sensor based on the SiO2ZrO2 film for detection of high concentrations of NO2 gas. Chemosensors 2018, 6, 67.
- Myasoedova, T.N.; Mikhailova, T.S.; Plugotarenko, N.K. A Study on A NO2 Sensor Based on SiO2-ZrO2 Composite Film. In Proceedings of the 14th International Scientific-Technical Conference on Actual Problems of Electronic Instrument Engineering, APEIE 2018, Novosibirsk, Russia, 2–6 October 2018.
- Dhere, S. Electrode materials for supercapacitors synthesized by sol-gel process. Curr. Sci. 2018, 115, 436–449.
- Masalovich, M.; Zagrebelnyy, O.; Nikolaev, A.; Shilova, O.; Ivanova, A. Development of pseudocapacitive materials based on cobalt and iron oxide compounds for an asymmetric energy storage device. Electrochim. Acta 2022, 410, 139999.
- Lev, O.; Wu, Z.; Bharathi, S.; Glezer, V.; Modestov, A.; Gun, J.; Rabinovich, L.; Sampath, S. Sol-Gel Materials in Electrochemistry. Chem. Mater. 1997, 9, 2354–2375.
- Il’ina, E.A.; Lyalin, E.D.; Antonov, V.D.; Pankratov, A.A.; Vovkotrub, E.G. Sol-gel synthesis of Al- and Nb-co-doped Li7La3Zr2O12 solid electrolytes. Ionics 2020, 26, 3239–3247.
- Fernandes, M.; de Zea Bermudez, V. Chapter 13—Sol-gel materials for smart electrochromic devices. Chem. Solut. Synth. Mater. Des. Thin Film Device Appl. 2021, 2021, 439–475.
- Wang, D.; Bierwagen, G.P. Sol-gel coatings on metals for corrosion protection. Prog. Org. Coat. 2009, 64, 327–338.
- Schem, M.; Schmidt, T.; Gerwann, J.; Wittmar, M.; Veith, M.; Thompson, G.E.; Molchan, I.S.; Hashimoto, T.; Skeldon, P.; Phani, A.R.; et al. CeO2-filled sol-gel coatings for corrosion protection of AA2024-T3 aluminium alloy. Corros. Sci. 2009, 51, 2304–2315.
- Brinker, C.J.; Scherer, G.W. Sol-Gel Science: The Physics and Chemistry of Sol-Gel Processing; Academic Press: London, UK, 1990; p. 903.
- Rahman, I.A.; Padavettan, V. Synthesis of silica nanoparticles by sol-gel: Size-dependent properties, surface modification, and applications in silica-polymer nanocomposites—A review. J. Nanomater. 2012, 2012, 8.
- Catauro, M.; Tranquillo, E.; Poggetto, G.D.; Pasquali, M.; Dell’Era, A.; Vecchio, S.C. Influence of the heat treatment on the particles size and on the crystalline phase of TiO2 synthesized by the sol-gel method. Materials 2018, 11, 2364.
- Gupta, S.; Tripathi, M. A review on the synthesis of TiO2 nanoparticles by solution route. Open Chem. 2012, 10, 279–294.
- Lai, S.; Qu, X.; Zhao, H.; Hong, S.W.; Lee, K. Improved performance in asymmetric supercapacitors utilized by dual ion-buffering reservoirs based on honeycomb-structured NiCo2O4 and 3D rGO-PPy aerogels. Appl. Surf. Sci. 2022, 586, 152847.
- Vijayalakshmi, R.; Rajendran, V. Synthesis and characterization of nano-TiO2 via different methods. Arch. Appl. Sci. Res. 2012, 4, 1183–1190.
- Verma, R.; Mantri, B.; Srivastava, A.K. Shape control synthesis, characterizations, mechanisms and optical properties of large, scaled metal oxide nanostructures of ZnO and TiO2. Adv. Mater. Lett. 2015, 6, 324–333.
- Aparicio, M.; Jitianu, A.; Klein, L.C. Sol-Gel Processing for Conventional and Alternative Energy; Springer: New York, NY, USA, 2012; 397p.
- Luo, X.; Morrin, A.; Killard, A.J.; Smyth, M.R. Application of nanoparticles in electrochemical sensors and biosensors. Electroanalysis 2006, 18, 319–326.
- Golli, A.E.; Echabaane, M.; Dridi, C. Development of an electrochemical nanoplatform for non-enzymatic glucose sensing based on Cu/ZnO nanocomposite. Mater. Chem. Phys. 2022, 280, 125844.
- Haque, M.; Fouad, H.; Seo, H.-K.; Alothman, O.Y.; Ansari, Z.A. Cu-Doped ZnO Nanoparticles as an Electrochemical Sensing Electrode for Cardiac Biomarker Myoglobin Detection. IEEE Sens. J. 2020, 20, 8820–8832.
- Zhan, B.; Zhang, Y.; Zhao, X. High sensitive sol-gel based electrochemical immunosensor for Clenbuterol Determination. Int. J. Electrochem. Sci. 2021, 16, 211124.
- Alsaiari, M.; Saleem, A.; Alsaiari, R.; Muhammad, N.; Latif, U.; Tariq, M.; Almohana, A.; Rahim, A. SiO2/Al2O3/C grafted 3-n propylpyridinium silsesquioxane chloride-based non-enzymatic electrochemical sensor for determination of carcinogenic nitrite in food products. Food Chem. 2022, 369, 130970.
- Lete, C.; Chelu, M.; Marin, M.; Mihaiu, S.; Preda, S.; Anastasescu, M.; Calderón-Moreno, J.M.; Dinulescu, S.; Moldovan, C.; Gartner, M. Nitrite electrochemical sensing platform based on tin oxide films. Sens. Actuat. B Chem. 2020, 316, 128102.
- Yin, X.T.; Zhou, W.D.; Li, J.; Wang, Q.; Wu, F.Y.; Dastan, D.; Wang, D.; Garmestani, H.; Wang, X.M.; Ţălu, Ş. A highly sensitivity and selectivity Pt-SnO2 nanoparticles for sensing applications at extremely low level hydrogen gas detection. J. Alloy. Compd. 2019, 805, 229–236.
- Sharma, A.; Arya, S.; Chauhan, D.; Solanki, P.R.; Khajuria, S.; Khosla, A. Synthesis of Au-SnO2 nanoparticles for electrochemical determination of vitamin B12. J. Mater. Res. Tech. 2020, 9, 14321–14327.
- Khatoon, Z.; Fouad, H.; Seo, H.-K.; Alothman, O.Y.; Ansari, Z.A.; Ansari, S.G. Ethyl Acetate Chemical Sensor as Lung Cancer Biomarker Detection Based on Doped Nano-SnO2 Synthesized by Sol-Gel Process. IEEE Sens. J. 2020, 20, 12504–12511.
- Duan, W.; Gunes, M.; Baldi, A.; Gich, M.; Fernandez-Sanchez, C. Compact fluidic electrochemical sensor platform for on-line monitoring of chemical oxygen demand in urban wastewater. Chem. Eng. J. 2022, 449, 137837.
- Lete, C.; Chelu, M.; Marin, M.; Mihaiu, S.; Preda, S.; Anastasescu, M.; Calderón-Moreno, J.M.; Dinulescu, S.; Moldovan, C.; Gartner, M. Nitrite electrochemical sensing platform based on tin oxide films. Sens. Actuat. B Chem. 2020, 316, 128102.Bhowmik, B.; Dutta, K.; Bhattacharyya, P. An Efficient Room Temperature Ethanol Sensor Device Based on p-n Homojunction of TiO2 Nanostructures. IEEE Trans. Electron Devices 2019, 66, 1063–1068.
- Bhowmik, B.; Dutta, K.; Bhattacharyya, P. An Efficient Room Temperature Ethanol Sensor Device Based on p-n Homojunction of TiO2 Nanostructures. IEEE Trans. Electron Devices 2019, 66, 1063–1068. Ahmad, K.; Kim, H. Design and fabrication of WO3/SPE for dopamine sensing application. Mater. Chem. Phys. 2022, 287, 126298.
- Ahmad, K.; Kim, H. Design and fabrication of WO3/SPE for dopamine sensing application. Mater. Chem. Phys. 2022, 287, 126298. Atchudan, R.; Muthuchamy, N.; Edison, T.N.J.I.; Perumal, S.; Vinodh, R. An ultrasensitive photoelectrochemical biosensor for glucose based on bioderived nitrogen-doped carbon sheets wrapped titanium dioxide nanoparticles. Biosens. Bioelectron. 2019, 126, 160–169.
- Atchudan, R.; Muthuchamy, N.; Edison, T.N.J.I.; Perumal, S.; Vinodh, R. An ultrasensitive photoelectrochemical biosensor for glucose based on bioderived nitrogen-doped carbon sheets wrapped titanium dioxide nanoparticles. Biosens. Bioelectron. 2019, 126, 160–169. Ahmad, K.; Shinde, M.A.; Song, G.; Kim, H. Design and fabrication of MoSe2/WO3 thin films for the construction of electrochromic devices on indium tin oxide based glass and flexible substrates. Ceram. Int. 2021, 47, 34297–34306.
- Ahmad, K.; Shinde, M.A.; Song, G.; Kim, H. Design and fabrication of MoSe2/WO3 thin films for the construction of electrochromic devices on indium tin oxide based glass and flexible substrates. Ceram. Int. 2021, 47, 34297–34306. Abdullah, M.M.; Faisal, M.; Ahmed, J.; Harraz, F.A.; Jalalah, M.; Alsareii, S.A. Sensitive Detection of Aqueous Methanol by Electrochemical Route Using Mesoporous α-Fe2O3 Doped CdSe Nanostructures Modified. J. Electrochem. Soc. 2021, 168, 057525.
- Abdullah, M.M.; Faisal, M.; Ahmed, J.; Harraz, F.A.; Jalalah, M.; Alsareii, S.A. Sensitive Detection of Aqueous Methanol by Electrochemical Route Using Mesoporous α-Fe2O3 Doped CdSe Nanostructures Modified. J. Electrochem. Soc. 2021, 168, 057525. Tareen, A.K.; Khan, K.; Ahmad, W.; Khan, M.F.; Khan, Q.U.; Liu, X. A novel MnO–CrN nanocomposite based nonenzymatic hydrogen peroxide sensor. RSC Adv. 2021, 11, 19316–19322.
- Tareen, A.K.; Khan, K.; Ahmad, W.; Khan, M.F.; Khan, Q.U.; Liu, X. A novel MnO–CrN nanocomposite based nonenzymatic hydrogen peroxide sensor. RSC Adv. 2021, 11, 19316–19322. Naikoo, G.A.; Sheikh, M.U.D. Development of Highly Efficient NiO based Composite Materials for Ultra-Sensitive Glucose Sensors. In Proceedings of the 2019 Thirteenth International Conference on Sensing Trchnology (ICST), Nice, France, 27–31 October 2019.
- Naikoo, G.A.; Sheikh, M.U.D. Development of Highly Efficient NiO based Composite Materials for Ultra-Sensitive Glucose Sensors. In Proceedings of the 2019 Thirteenth International Conference on Sensing Trchnology (ICST), Nice, France, 27–31 October 2019. Paramparambath, S.; Shafath, S.; Maurya, M.R.; Cabibihan, J.-J.; Malik, R.A.; Sadasivuni, K.K. Nonenzymatic Electrochemical Sensor Based on CuO-MgO Composite for Dopamine Detection. IEEE Sens. J. 2021, 21, 25597–25606.
- Paramparambath, S.; Shafath, S.; Maurya, M.R.; Cabibihan, J.-J.; Malik, R.A.; Sadasivuni, K.K. Nonenzymatic Electrochemical Sensor Based on CuO-MgO Composite for Dopamine Detection. IEEE Sens. J. 2021, 21, 25597–25606. Lu, Q.; Huang, L.; Hao, X.; Li, W.; Wang, B.; Wang, T.; Liang, X.; Liu, F.; Wang, C.; Lu, G. Mixed potential type NH3 sensor based on YSZ solid electrolyte and metal oxides (NiO, SnO2, WO3) modified FeVO4 sensing electrodes. Sens. Actuat. B Chem. 2021, 343, 130043.
- Lu, Q.; Huang, L.; Hao, X.; Li, W.; Wang, B.; Wang, T.; Liang, X.; Liu, F.; Wang, C.; Lu, G. Mixed potential type NH3 sensor based on YSZ solid electrolyte and metal oxides (NiO, SnO2, WO3) modified FeVO4 sensing electrodes. Sens. Actuat. B Chem. 2021, 343, 130043. Peng, S.; Lai, T.; Kong, Y.; Ran, Y.; Su, L.; Ma, D.; Xiao, X.; Wang, Y. A novel non-enzymatic glucose electrochemical sensor with high sensitivity and selectivity based on CdIn2O4 nanoparticles on 3D Ni foam substrate. Nanotechnology 2021, 32, 405502.
- Peng, S.; Lai, T.; Kong, Y.; Ran, Y.; Su, L.; Ma, D.; Xiao, X.; Wang, Y. A novel non-enzymatic glucose electrochemical sensor with high sensitivity and selectivity based on CdIn2O4 nanoparticles on 3D Ni foam substrate. Nanotechnology 2021, 32, 405502. Shu, H.; Peng, S.; Lai, T.; Cui, X.; Ren, J.; Chen, T.; Xiao, X.; Wang, Y. Nickel foam electrode decorated with Fe-CdIn2O4 nanoparticles as an effective electrochemical sensor for non-enzymatic glucose detection. J. Electroanal. Chem. 2022, 919, 116524.
- Shu, H.; Peng, S.; Lai, T.; Cui, X.; Ren, J.; Chen, T.; Xiao, X.; Wang, Y. Nickel foam electrode decorated with Fe-CdIn2O4 nanoparticles as an effective electrochemical sensor for non-enzymatic glucose detection. J. Electroanal. Chem. 2022, 919, 116524. Petruleviciene, M.; Juodkazyte, J.; Savickaja, I.; Karpicz, R.; Morkvenaite-Vilkonciene, I.; Ramanavicius, A. BiVO4-based coatings for non-enzymatic photoelectrochemical glucose determination. J. Electroanalyt. Chem. 2022, 918, 16446.
- Petruleviciene, M.; Juodkazyte, J.; Savickaja, I.; Karpicz, R.; Morkvenaite-Vilkonciene, I.; Ramanavicius, A. BiVO4-based coatings for non-enzymatic photoelectrochemical glucose determination. J. Electroanalyt. Chem. 2022, 918, 16446. Asiabar, B.M.; Karimi, M.A.; Tavallali, H.; Nasrabadi, M.R. Application of MnFe2O4 and AuNPs modified CPE as a sensitive flunitrazepam electrochemical sensor. Microchem. J. 2021, 161, 105745.
- Asiabar, B.M.; Karimi, M.A.; Tavallali, H.; Nasrabadi, M.R. Application of MnFe2O4 and AuNPs modified CPE as a sensitive flunitrazepam electrochemical sensor. Microchem. J. 2021, 161, 105745. Baghayeri, M.; Amiri, A.; Fayazi, M.; Nodehi, M.; Esmaeelnia, A. Electrochemical detection of bisphenol a on a MWCNTs/CuFe2O4 nanocomposite modified glassy carbon electrode. Mater. Chem. Phys. 2021, 261, 124247.
- Baghayeri, M.; Amiri, A.; Fayazi, M.; Nodehi, M.; Esmaeelnia, A. Electrochemical detection of bisphenol a on a MWCNTs/CuFe2O4 nanocomposite modified glassy carbon electrode. Mater. Chem. Phys. 2021, 261, 124247. Zhang, Y.; Liu, T.; Wang, T.; Li, W.; Hao, X.; Lu, Q.; Yu, H.; Liang, X.; Liu, F.; Liu, F.; et al. Ce0.8Gd0.2O1.95-based mixed potential type triethylamine sensor utilizing La2NiFeO6 sensing electrode. Sens. Actuat. B Chem. 2021, 345, 130438.
- Zhang, Y.; Liu, T.; Wang, T.; Li, W.; Hao, X.; Lu, Q.; Yu, H.; Liang, X.; Liu, F.; Liu, F.; et al. Ce0.8Gd0.2O1.95-based mixed potential type triethylamine sensor utilizing La2NiFeO6 sensing electrode. Sens. Actuat. B Chem. 2021, 345, 130438. Hao, X.; Ma, C.; Yang, X.; Liu, T.; Wang, B.; Liu, F.; Liang, X.; Yang, C.; Zhu, H.; Lu, G. YSZ-based mixed potential H2S sensor using La2NiO4 sensing electrode. Sens. Actuat. B Chem. 2018, 255, 3033–3039.
- Hao, X.; Ma, C.; Yang, X.; Liu, T.; Wang, B.; Liu, F.; Liang, X.; Yang, C.; Zhu, H.; Lu, G. YSZ-based mixed potential H2S sensor using La2NiO4 sensing electrode. Sens. Actuat. B Chem. 2018, 255, 3033–3039. Wang, J.; Zhao, L.; Li, J.; Liu, F.; You, R.; Lv, S.; Yang, Z.; He, J.; Hao, Y.; Yan, X.; et al. Stabilized zirconia-based solid state electrochemical gas sensor coupled with CdTiO3 for acetylene detection. Sens. Actuat. B Chem. 2020, 316, 128199.
- Wang, J.; Zhao, L.; Li, J.; Liu, F.; You, R.; Lv, S.; Yang, Z.; He, J.; Hao, Y.; Yan, X.; et al. Stabilized zirconia-based solid state electrochemical gas sensor coupled with CdTiO3 for acetylene detection. Sens. Actuat. B Chem. 2020, 316, 128199. Wang, X.T.; Li, B.; Kong, D.R.; Zhang, Z.Y.; Zhang, X.F.; Deng, Z.P.; Huo, L.H.; Gao, S. T- and T-type layered perovskite Ln2CuO4 nanocrystals for enhanced sensing detection of hydrogen peroxide. J. Alloy. Compd. 2022, 911, 165037.
- Wang, X.T.; Li, B.; Kong, D.R.; Zhang, Z.Y.; Zhang, X.F.; Deng, Z.P.; Huo, L.H.; Gao, S. T- and T-type layered perovskite Ln2CuO4 nanocrystals for enhanced sensing detection of hydrogen peroxide. J. Alloy. Compd. 2022, 911, 165037.