Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 3 by Dean Liu and Version 2 by Dean Liu.
In periodontal surgery, HAhyaluronic acid exhibited better results in complete root coverage and mean root coverage when compared to the control group. Gingival recession reduction, clinical attachment level, and keratinized tissue gain were significantly increased compared to the control groups.
- gingival recession
- Miller class I and II
- hyaluronic acid
1. Introduction
Tooth root exposure due to the apical movement of the gingival margin, compared to the cement-enamel junction, is termed a gingival recession [1][2]. Persistent trauma or periodontal disease, anatomic factors of soft tissue (e.g., narrow band of keratinized mucosa), or areas of biofilm development (e.g., inadequately suited dental restorations/crowding) are causes to one or more teeth can be affected by the gingival recession, thus, resulting in tooth sensitivity, hygiene issues, root cavities, and other issues [2][3][4]. As a result, for patients with good dental hygiene, surgical treatment of gingival recession was necessary to reduce these problems. Sub-epithelial connective tissue grafts (SCTG), coronally advanced flaps (CAF), semilunar coronally advanced flaps, laterally positioned flaps, and free gingival grafts have all been used to address gingival recessions [5][6][7][8][9]. The primary downside of the SCTG procedure is the need for donor tissue. In cases with multiple recessions, it necessitates a substantial tissue volume, which causes post-operative pain [10]. Therefore, as a result, biomaterials and alternative grafts have been proposed, including acellular dermal matrix (ADM), enamel matrix derivatives (EMDs), and autologous plasma [11][12][13]. Recently, the role of hyaluronic acid (HA) as a chemotherapeutic agent has been a boon to the advancement in dentistry. HA is a major natural carbohydrate component of the extracellular matrix in many tissues, including the periodontium. The biological effects of HA depend heavily on molecular weight. Hyaluronic acid with molecular weights from 0.4 to 4.0 kDa acts as an inducer of inflammation and has a non-apoptotic property. HA, with a molecular weight of 20–200 kDa, takes part in biological processes such as embryonic development and wound healing. By contrast, high molecular weight hyaluronic acid (>500 kDa) has anti-angiogenic activity and can function as a space filler and a natural immunologic depressant [14]. It has unique physiochemical and biological properties such as viscoelastic, anti-inflammatory, hygroscopic, bacteriostatic, osteoinductive, and anti-edematous properties [15][16][17]. Moreover, numerous trials proved that HA enhances clot formation, induces angiogenesis, promotes osteogenesis, and plays important roles in cell differentiation, adhesion, and migration. HA-binding proteins mediate these to cell surface receptors. Thus, it has been proposed that HA might be a suitable material for both periodontal wound healing and regeneration in periodontal defects. With its application in intra-bony defects, HA has shown promising effects like clinical attachment level (CAL) gain, probing depth reduction, and complete root coverage (CRC) [15][16][18][19][20][21][22]. However, there is no qualitative or quantitative analysis of its efficacy with respect to gingival recession.
2. Concentration of HA Application
The method of applying HA and the time interval differed among the trials, but in all three, HA was at least applied once during the therapy procedure. Kumar et al. used Hyaluron gel (0.2% HA, Gengigel, Ricerfarma Pharmacheuticals, Milan, Italy). Nandanwar et al. chose Hyaloss matrix gel (HA, Hyaloss matrix, Meta, Italy), while a cross-linked high-concentrated HA gel (2 mg/mL HA, hyaDENT BG, Bioscience, Germany) was used by Pilloni et al. [17][21][22]. The follow-up period in two studies was 6 months, while the other presented 18 months [17][21][22].3. Complete and Mean Root Coverage
CRC and mean root coverage (MRC) have shown a significant difference in the included studies [17][22]. According to Kumar et al., when analyzed by Student’s t-test after six months of surgery, MRC was presented 68.3% in the study group and 61.7% in thecontrol group [21]. However, complete root coverage was not measured in this aforementioned study. By using Student’s unpaired t-test, Nandanwar et al. found a significant increase in CRC (77.7%) for the study group, six months post-operatively, compared to (65.25%) in the control group [22]. Mean root coverage in Nandanwar et al. study was measured 92.8% in the test group and 84% in the control group. Pilloni et al. also concluded that there is a significant increase in complete root coverage, with 80% in the test group and 33.3% in the control group 18 months postoperatively [17].4. Gingival Recession Reduction
Gingival recession reduction was found statistically significant in the included studies [17][22]. After six months, increased mean reduction was seen in the gingival recession for the test group (1.0 and 2.55 mm) than in the control group (1.1 and 2.11 mm) [21][22]. Similarly, Pilloni et al. discovered a significant difference between the groups, with the test group experiencing a 2.7 mm reduction in recession compared to 1.9 mm in the control group [17].5. Evaluation of CAL
CAL has shown significant improvement for all the trials included in this study. Pilloni et al. reported a statistically significant difference in CAL gain in the test group (p = 0.023) and (p = 0.011) for the control group [17]. By analyzing through Student’s unpaired t-test, Nandanwar et al. concluded that there is a significantly higher CAL gain in the test group (3.03 mm) compared to the control group (2.34 mm) [22]. Kumar et al. [21] observed significant gain in the CAL after 24 weeks post-operatively.6. Keratinized Tissue Gain
Heterogeneity was noted among the studies when comparing keratinized tissue gain. Pilloni et al. found no difference in keratinized tissue gain between the two groups (p = 0.116) [17]. Using Student’s unpaired t-test, Nandanwar et al. noted an increase in keratinized tissue gain of 2.53 mm in the test group compared to 1.96 mm for the control group [22].7. Synthesis of Result
Despite the fact that data were extracted in a systematic manner for this review, a meta-analysis was not possible owing to study heterogeneity and inconsistent data.8. Quality Assessment
Two studies were marked as “low risk,” while one study by Kumar et al. [21] was of “high risk”, the summary of which is described in Figure 1.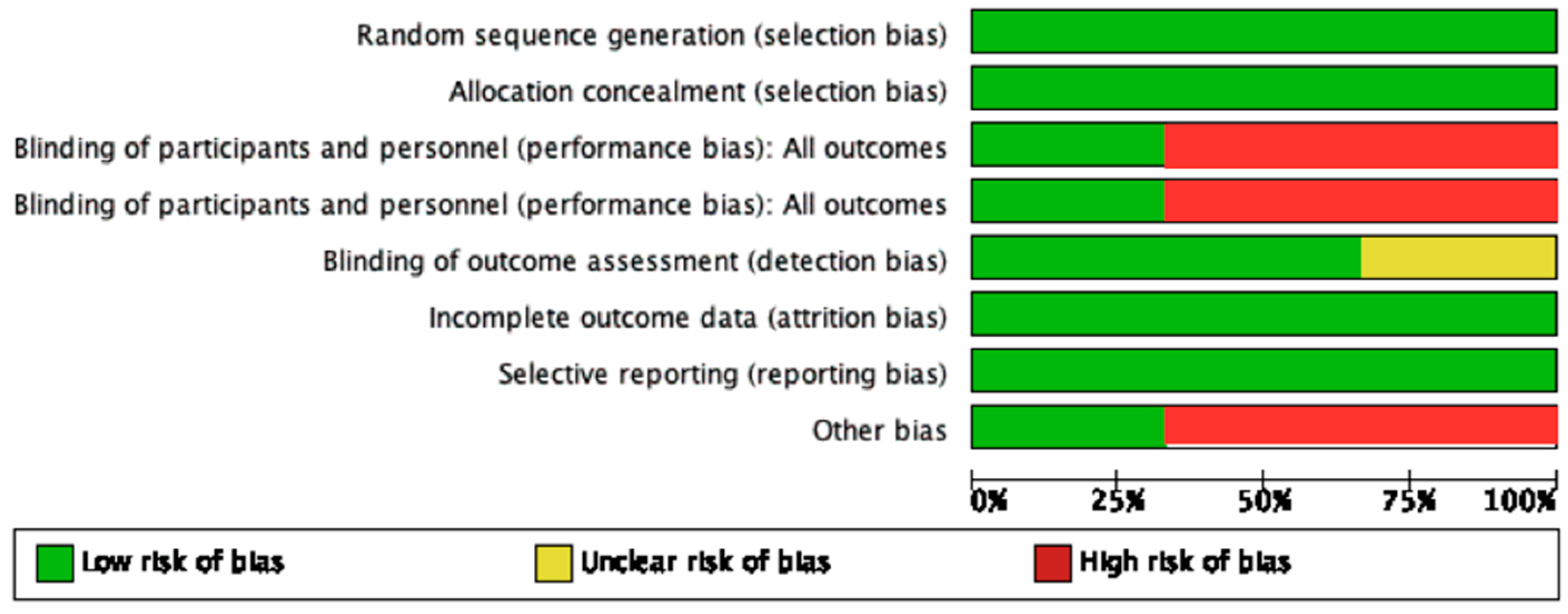
Figure 1. Summary of risk of bias.
References
- Loe, H.; Anerud, A.; Boysen, H. The natural history of periodontal disease in man: Prevalence, severity, and extent of gingival recession. J. Periodontol. 1992, 63, 489–495.
- Smith, R.G. Gingival recession. Reappraisal of an enigmatic condition and a new index for monitoring. J. Clin. Periodontol. 1997, 24, 201–205.
- Heasman, P.A.; Holliday, R.; Bryant, A.; Preshaw, P.M. Evidence for the occurrence of gingival recession and non-carious cervical lesions as a consequence of traumatic toothbrushing. J. Clin. Periodontol. 2015, 42, S237–S255.
- Daprile, G.; Gatto, M.R.; Checchi, L. The evolution of buccal gingival recessions in a student population: A 5-year follow-up. J. Periodontol. 2007, 78, 611–614.
- Carnio, J.; Neiva, R.F. Mineral trioxide aggregate and subepithelial connective tissue graft for treatment of iatrogenic gingival recession: Long-term results. Int. J. Periodontics Restor. Dent. 2014, 34, 71–77.
- Cairo, F.; Cortellini, P.; Pilloni, A.; Nieri, M.; Cincinelli, S.; Amunni, F.; Pagavino, G.; Tonetti, M.S. Clinical efficacy of coronally advanced flap with or without connective tissue graft for the treatment of multiple adjacent gingival recessions in the aesthetic area: A randomized controlled clinical trial. J. Clin. Periodontol. 2016, 43, 849–856.
- Santos, F.R.; Storrer, C.L.; Cunha, E.J.; Ulbrich, L.M.; Lopez, C.A.; Deliberador, T.M. Comparison of conventional and semilunar coronally positioned flap techniques for root coverage in teeth with cervical abrasion restored with pink resin. Clin. Cosmet. Investig. Dent. 2017, 9, 7–11.
- Yilmaz, E.; Ozcelik, O.; Comert, M.; Ozturan, S.; Seydaoglu, G.; Teughels, W.; Haytac, M.C. Laser-assisted laterally positioned flap operation: A randomized controlled clinical trial. Photomed. Laser Surg. 2014, 32, 67–74.
- Srinivas, B.V.; Rupa, N.; Kumari, H.K.V.; Rajender, A.; Reddy, M.N. Treatment of gingival recession using free gingival graft with fibrin fibronectin sealing system: A novel approach. J. Pharm. Bioallied Sci. 2015, 7, S734–S739.
- Zuhr, O.; Baumer, D.; Hurzeler, M. The addition of soft tissue replacement grafts in plastic periodontal and implant surgery: Critical elements in design and execution. J. Clin. Periodontol. 2014, 41, S123–S142.
- Aroca, S.; Keglevich, T.; Barbieri, B.; Gera, I.; Etienne, D. Clinical evaluation of a modified coronally advanced flap alone or in combination with a platelet-rich fibrin membrane for the treatment of adjacent multiple gingival recessions: A 6-month study. J. Periodontol. 2009, 80, 244–252.
- Aroca, S.; Keglevich, T.; Nikolidakis, D.; Gera, I.; Nagy, K.; Azzi, R.; Etienne, D. Treatment of class III multiple gingival recessions: A randomized-clinical trial. J. Clin. Periodontol. 2010, 37, 88–97.
- Modaressi, M.; Wang, H.L. Tunneling procedure for root coverage using acellular dermal matrix: A case series. Int. J. Periodontics Restor. Dent. 2009, 29, 395–403.
- Stern, R.; Asari, A.A.; Sugahara, K.N. Hyaluronan fragments: An information-rich system. Eur. J. Cell Biol. 2006, 85, 699–715.
- Sasaki, T.; Watanabe, C. Stimulation of osteoinduction in bone wound healing by high-molecular hyaluronic acid. Bone 1995, 16, 9–15.
- Dahiya, P.; Kamal, R. Hyaluronic Acid: A boon in periodontal therapy. N. Am. J. Med. Sci. 2013, 5, 309–315.
- Pilloni, A.; Schmidlin, P.R.; Sahrmann, P.; Sculean, A.; Rojas, M.A. Effectiveness of adjunctive hyaluronic acid application in coronally advanced flap in Miller class I single gingival recession sites: A randomized controlled clinical trial. Clin. Oral Investig. 2019, 23, 1133–1141.
- Asparuhova, M.B.; Chappuis, V.; Stahli, A.; Buser, D.; Sculean, A. Role of hyaluronan in regulating self-renewal and osteogenic differentiation of mesenchymal stromal cells and pre-osteoblasts. Clin. Oral Investig. 2020, 24, 3923–3937.
- Oksala, O.; Salo, T.; Tammi, R.; Hakkinen, L.; Jalkanen, M.; Inki, P.; Larjava, H. Expression of proteoglycans and hyaluronan during wound healing. J. Histochem. Cytochem. 1995, 43, 125–135.
- West, D.C.; Hampson, I.N.; Arnold, F.; Kumar, S. Angiogenesis induced by degradation products of hyaluronic acid. Science 1985, 228, 1324–1326.
- Kumar, R.; Srinivas, M.; Pai, J.; Suragimath, G.; Prasad, K.; Polepalle, T. Efficacy of hyaluronic acid (hyaluronan) in root coverage procedures as an adjunct to coronally advanced flap in Millers Class I recession: A clinical study. J. Indian Soc. Periodontol. 2014, 18, 746–750.
- Nandanwar, J.; Bhongade, M.L.; Puri, S.; Dhadse, P.; Datir, M.; Kasatwar, A. Comparison of effectiveness of hyaluronic acid in combination with polylactic acid/polyglycolic acid membrane and subepithelial connective tissue graft for the treatment of multiple gingival recession defects in human: A clinical study. J. Datta Meghe Inst. Med. Sci. Univ. 2018, 13, 48–53.
More
