Cisplatin (cis-diamminedichloroplatinum (II), DDP) is an antineoplastic agent widely used in the treatment of solid tumors because of its extensive cytotoxic activity. However, the main limiting side effect of DDP use is nephrotoxicity, a rapid deterioration in kidney function due to toxic chemicals. Noncoding RNAs (ncRNAs), a class of epigenetic processes, are molecules that regulate gene expression under physiological and pathological conditions. MicroRNAs (miRNAs) are the most characterized class of ncRNAs and are engaged in many cellular processes. DDP-induced nephrotoxicity can present in a several ways, but the most common and serious presentation is acute kidney injury (AKI), which occurs in 20–30% of patients.
- apoptosis
- miRNAs
- noncoding RNAs
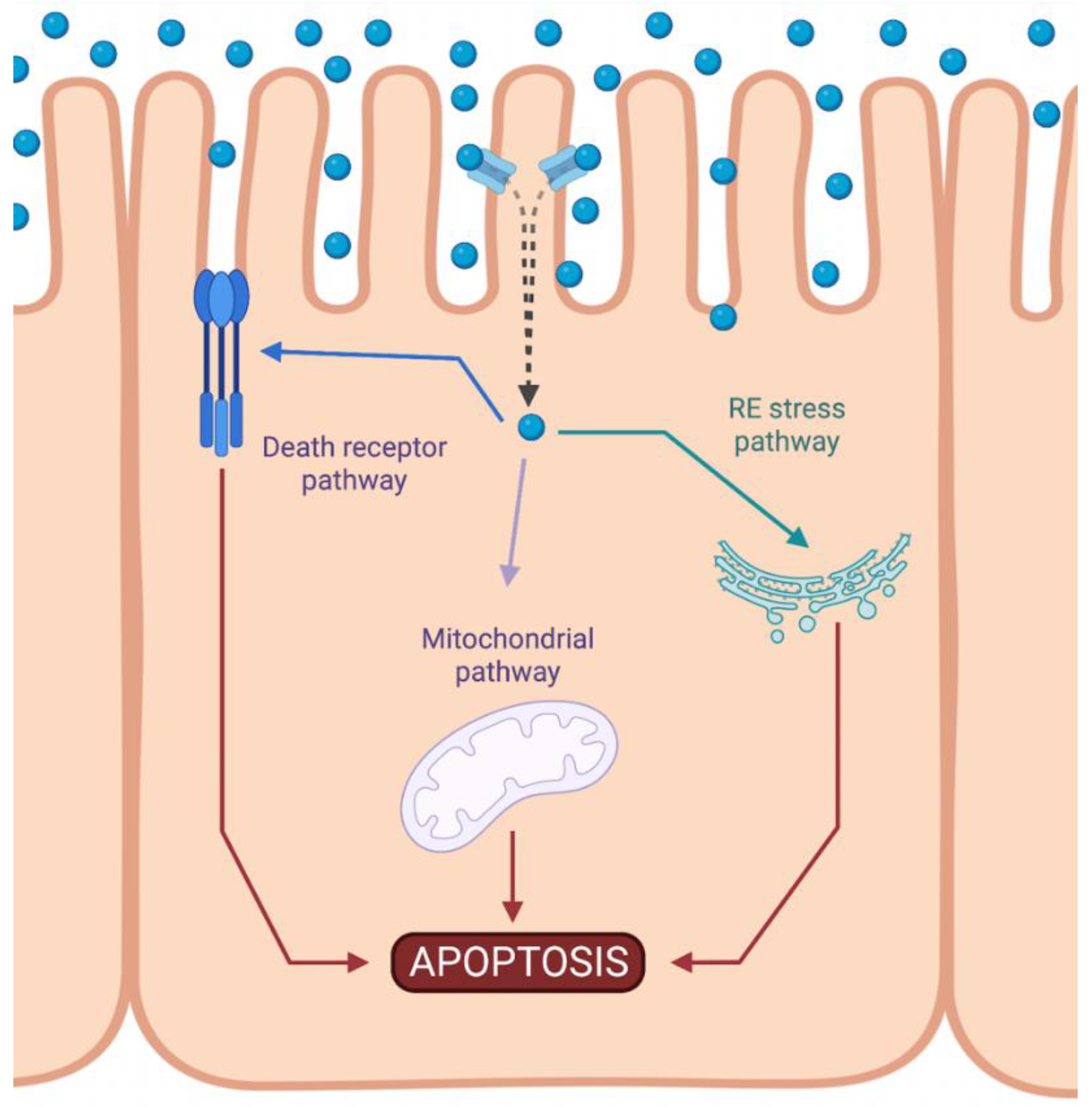
1. Intrinsic Apoptotic Pathway during DDP-Induced Nephrotoxicity
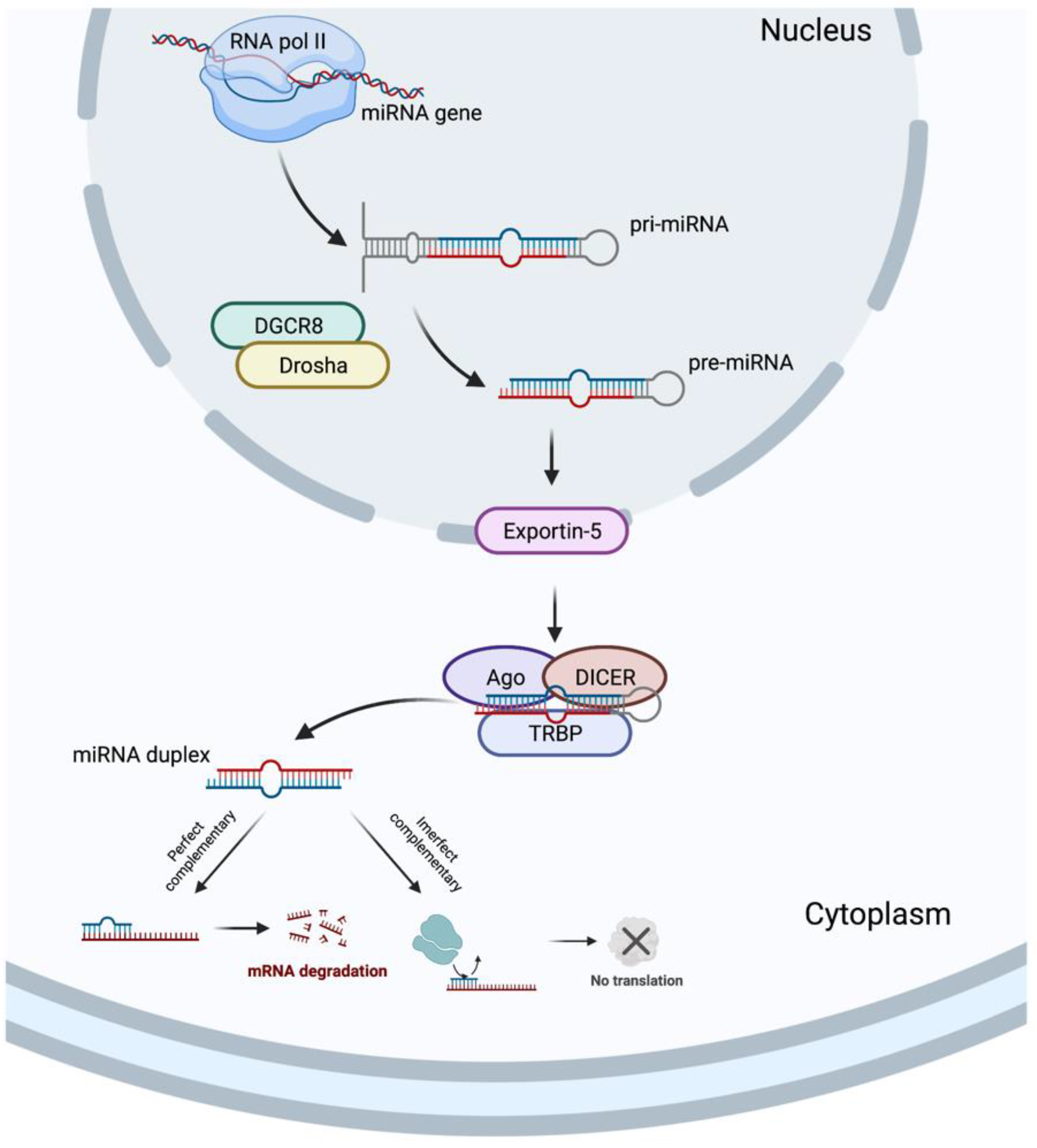
2. Involvement of microRNAs upon Intrinsic Apoptosis Pathway during DDP-Induced Nephrotoxicity
3. Potential Utility of Natural Products in DDP-Induced Apoptosis
References
- Liu, Z.; Li, Z.; Chen, Z.; Li, C.; Lei, L.; Wu, X.; Li, Y. Numb ameliorates necrosis and inflammation in acute kidney injury induced by cisplatin. Chem Biol. Interact. 2020, 330, 109251.
- Ramesh, G.; Reeves, W.B. TNFR2-mediated apoptosis and necrosis in cisplatin-induced acute renal failure. Am. J. Physiol. Renal. Physiol. 2003, 285, F610–F618.
- Landau, S.I.; Guo, X.; Velazquez, H.; Torres, R.; Olson, E.; Garcia-Milian, R.; Moeckel, G.W.; Desir, G.V.; Safirstein, R. Regulated necrosis and failed repair in cisplatin-induced chronic kidney disease. Kidney Int. 2019, 95, 797–814.
- Bhatt, K.; Zhou, L.; Mi, Q.S.; Huang, S.; She, J.X.; Dong, Z. MicroRNA-34a is induced via p53 during cisplatin nephrotoxicity and contributes to cell survival. Mol. Med. 2010, 16, 409–416.
- Du, B.; Dai, X.M.; Li, S.; Qi, G.L.; Cao, G.X.; Zhong, Y.; Yin, P.D.; Yang, X.S. MiR-30c regulates cisplatin-induced apoptosis of renal tubular epithelial cells by targeting Bnip3L and Hspa5. Cell Death Dis. 2017, 8, e2987.
- Guo, Y.; Ni, J.; Chen, S.; Bai, M.; Lin, J.; Ding, G.; Zhang, Y.; Sun, P.; Jia, Z.; Huang, S.; et al. MicroRNA-709 Mediates Acute Tubular Injury through Effects on Mitochondrial Function. J. Am. Soc. Nephrol. 2018, 29, 449–461.
- Zou, D.; Ganugula, R.; Arora, M.; Nabity, M.B.; Sheikh-Hamad, D.; Kumar, M. Oral delivery of nanoparticle urolithin A normalizes cellular stress and improves survival in mouse model of cisplatin-induced AKI. Am. J. Physiol. Renal Physiol. 2019, 317, F1255–F1264.
- Yang, Y.; Li, Z.L.; Wang, F.M.; Tang, R.N.; Tu, Y.; Liu, H. MicroRNA26a inhibits cisplatin-induced renal tubular epithelial cells apoptosis through suppressing the expression of transient receptor potential channel 6 mediated dynamin-related protein 1. Cell Biochem. Funct. 2020, 38, 384–391.
- Harrill, A.H.; Lin, H.; Tobacyk, J.; Seely, J.C. Mouse population-based evaluation of urinary protein and miRNA biomarker performance associated with cisplatin renal injury. Exp. Biol. Med. 2018, 243, 237–247.
- Zhang, S.; Sun, H.; Kong, W.; Zhang, B. Functional role of microRNA-500a-3P-loaded liposomes in the treatment of cisplatin-induced AKI. IET Nanobiotechnol. 2020, 14, 465–469.
- Jiang, L.; Liu, X.Q.; Ma, Q.; Yang, Q.; Gao, L.; Li, H.D.; Wang, J.N.; Wei, B.; Wen, J.; Li, J.; et al. hsa-miR-500a-3P alleviates kidney injury by targeting MLKL-mediated necroptosis in renal epithelial cells. FASEB J. 2019, 33, 3523–3535.
- Wei, Q.; Dong, G.; Franklin, J.; Dong, Z. The pathological role of Bax in cisplatin nephrotoxicity. Kidney Int. 2007, 72, 53–62.
- Qin, W.; Xie, W.; Yang, X.; Xia, N.; Yang, K. Inhibiting microRNA-449 Attenuates Cisplatin-Induced Injury in NRK-52E Cells Possibly via Regulating the SIRT1/P53/BAX Pathway. Med. Sci. Monit. 2016, 22, 818–823.
- Zhu, H.Y.; Liu, M.Y.; Hong, Q.; Zhang, D.; Geng, W.J.; Xie, Y.S.; Chen, X.M. Role of microRNA-181a in the apoptosis of tubular epithelial cell induced by cisplatin. Chin. Med. J. 2012, 125, 523–526.
- Candé, C.; Cohen, I.; Daugas, E.; Ravagnan, L.; Larochette, N.; Zamzami, N.; Kroemer, G. Apoptosis-inducing factor (AIF): A novel caspase-independent death effector released from mitochondria. Biochimie 2002, 84, 215–222.
- Hwa Lee, R.; Mi Song, J.; Young Park, M.; Kyung Kang, S.; Keun Kim, Y.; Sup Jung, J. Cisplatin-induced apoptosis by translocation of endogenous Bax in mouse collecting duct cells11Abbreviations: ROS, reactive oxygen species; SAPK/JNK, stress-activated protein kinase/c-Jun NH2-terminal kinase; RT-PCR, reverse transcription-polymerase chain reaction; ECL, enhanced chemiluminescence; LDH, lactic dehydrogenase; DPPD, diphenyl-p-phenylene-diamine; DFO, deferoxamine; DMTU, dimethylthiourea; and BHA, butylated hydroxyanisole. Biochem. Pharmacol. 2001, 62, 1013–1023.
- Li, L.Y.; Luo, X.; Wang, X. Endonuclease G is an apoptotic DNase when released from mitochondria. Nature 2001, 412, 95–99.
- Hegde, R.; Srinivasula, S.M.; Zhang, Z.; Wassell, R.; Mukattash, R.; Cilenti, L.; DuBois, G.; Lazebnik, Y.; Zervos, A.S.; Fernandes-Alnemri, T.; et al. Identification of Omi/HtrA2 as a mitochondrial apoptotic serine protease that disrupts inhibitor of apoptosis protein-caspase interaction. J. Biol. Chem. 2002, 277, 432–438.
- Adrain, C.; Creagh, E.M.; Martin, S.J. Apoptosis-associated release of Smac/DIABLO from mitochondria requires active caspases and is blocked by Bcl-2. EMBO J. 2001, 20, 6627–6636.
- Chipuk, J.E.; Kuwana, T.; Bouchier-Hayes, L.; Droin, N.M.; Newmeyer, D.D.; Schuler, M.; Green, D.R. Direct activation of Bax by p53 mediates mitochondrial membrane permeabilization and apoptosis. Science 2004, 303, 1010–1014.
- Zou, H.; Li, Y.; Liu, X.; Wang, X. An APAF-1.cytochrome c multimeric complex is a functional apoptosome that activates procaspase-9. J. Biol. Chem. 1999, 274, 11549–11556.
- Saleh, A.; Srinivasula, S.M.; Acharya, S.; Fishel, R.; Alnemri, E.S. Cytochrome c and dATP-mediated oligomerization of Apaf-1 is a prerequisite for procaspase-9 activation. J. Biol. Chem. 1999, 274, 17941–17945.
- Du, C.; Fang, M.; Li, Y.; Li, L.; Wang, X. Smac, a Mitochondrial Protein that Promotes Cytochrome c–Dependent Caspase Activation by Eliminating IAP Inhibition. Cell 2000, 102, 33–42.
- Deveraux, Q.L.; Reed, J.C. IAP family proteins--suppressors of apoptosis. Genes Dev. 1999, 13, 239–252.
- Ma, M.; Luo, Q.; Fan, L.; Li, W.; Li, Q.; Meng, Y.; Yun, C.; Wu, H.; Lu, Y.; Cui, S.; et al. The urinary exosomes derived from premature infants attenuate cisplatin-induced acute kidney injury in mice via microRNA-30a-5p/mitogen-activated protein kinase 8 (MAPK8). Bioengineered 2022, 13, 1650–1665.
- Park, M.S.; De Leon, M.; Devarajan, P. Cisplatin induces apoptosis in LLC-PK1 cells via activation of mitochondrial pathways. J. Am. Soc.Nephrol. 2002, 13, 858–865.
- Chen, C.L.; Liao, J.W.; Hu, O.Y.; Pao, L.H. Blockade of KCa3.1 potassium channels protects against cisplatin-induced acute kidney injury. Arch. Toxicol. 2016, 90, 2249–2260.
- de Almeida, D.C.; Bassi, E.J.; Azevedo, H.; Anderson, L.; Origassa, C.S.; Cenedeze, M.A.; de Andrade-Oliveira, V.; Felizardo, R.J.; da Silva, R.C.; Hiyane, M.I.; et al. A Regulatory miRNA-mRNA Network Is Associated with Tissue Repair Induced by Mesenchymal Stromal Cells in Acute Kidney Injury. Front. Immunol. 2016, 7, 645.
- Hussein, R.M.; Al-Dalain, S.M. Betaine downregulates microRNA 34a expression via a p53-dependent manner in cisplatin-induced nephrotoxicity in rats. J. Biochem. Mol. Toxicol. 2021, 35, e22856.
- Yin, X.; Apostolov, E.O.; Shah, S.V.; Wang, X.; Bogdanov, K.V.; Buzder, T.; Stewart, A.G.; Basnakian, A.G. Induction of renal endonuclease G by cisplatin is reduced in DNase I-deficient mice. J. Am. Soc. Nephrol. 2007, 18, 2544–2553.
- Cilenti, L.; Kyriazis, G.A.; Soundarapandian, M.M.; Stratico, V.; Yerkes, A.; Park, K.M.; Sheridan, A.M.; Alnemri, E.S.; Bonventre, J.V.; Zervos, A.S. Omi/HtrA2 protease mediates cisplatin-induced cell death in renal cells. Am. J. Physiol. Renal. Physiol. 2005, 288, F371–F379.
- Orzaez, M.; Sancho, M.; Marchan, S.; Mondragon, L.; Montava, R.; Valero, J.G.; Landeta, O.; Basanez, G.; Carbajo, R.J.; Pineda-Lucena, A.; et al. Apaf-1 inhibitors protect from unwanted cell death in in vivo models of kidney ischemia and chemotherapy induced ototoxicity. PLoS ONE 2014, 9, e110979.
- Qi, Z.; Li, Z.; Li, W.; Liu, Y.; Wang, C.; Lin, H.; Liu, J.; Li, P. Pseudoginsengenin DQ Exhibits Therapeutic Effects in Cisplatin-Induced Acute Kidney Injury via Sirt1/NF-kappaB and Caspase Signaling Pathway without Compromising Its Antitumor Activity in Mice. Molecules 2018, 23, 3038.
- Lee, R.C.; Feinbaum, R.L.; Ambros, V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 1993, 75, 843–854.
- Wightman, B.; Ha, I.; Ruvkun, G. Posttranscriptional regulation of the heterochronic gene lin-14 by lin-4 mediates temporal pattern formation in C. elegans. Cell 1993, 75, 855–862.
- Shivdasani, R.A. MicroRNAs: Regulators of gene expression and cell differentiation. Blood 2006, 108, 3646–3653.
- Denli, A.M.; Tops, B.B.; Plasterk, R.H.; Ketting, R.F.; Hannon, G.J. Processing of primary microRNAs by the Microprocessor complex. Nature 2004, 432, 231–235.
- Chendrimada, T.P.; Gregory, R.I.; Kumaraswamy, E.; Norman, J.; Cooch, N.; Nishikura, K.; Shiekhattar, R. TRBP recruits the Dicer complex to Ago2 for microRNA processing and gene silencing. Nature 2005, 436, 740–744.
- Guo, C.; Dong, G.; Liang, X.; Dong, Z. Epigenetic regulation in AKI and kidney repair: Mechanisms and therapeutic implications. Nat. Rev. Nephrol. 2019, 15, 220–239.
- Bragado, P.; Armesilla, A.; Silva, A.; Porras, A. Apoptosis by cisplatin requires p53 mediated p38alpha MAPK activation through ROS generation. Apoptosis 2007, 12, 1733–1742.
- Wu, J.; Li, D.D.; Li, J.Y.; Yin, Y.C.; Li, P.C.; Qiu, L.; Chen, L.M. Identification of microRNA-mRNA networks involved in cisplatin-induced renal tubular epithelial cells injury. Eur. J. Pharmacol. 2019, 851, 1–12.
- Cummings, B.S.; Schnellmann, R.G. Cisplatin-induced renal cell apoptosis: Caspase 3-dependent and -independent pathways. J. Pharmacol. Exp. Ther. 2002, 302, 8–17.
- Li, Z.; Hou, G. LincRNA-p21 Inhibits Cisplatin-Induced Apoptosis of Human Renal Proximal Tubular Epithelial Cells by Sponging miR-449a. Kidney Blood Press Res. 2021, 46, 495–501.
- Zhang, M.; Bi, H.; Wang, S.; Sun, X.; Li, Y. Long non-coding RNA GAS5 aggravate renal epithelial cell apoptosis in cisplatin-induced AKI by regulating miR-205-5p. Res. Sq. 2021.
- Chang, S.; Chang, M.; Liu, G.; Xu, D.; Wang, H.; Sun, R.; Feng, M. LncRNA OIP5-AS1 reduces renal epithelial cell apoptosis in cisplatin-induced AKI by regulating the miR-144-5p/PKM2 axis. Biomed. J. 2021.
- Zhang, W.; Chen, C.; Jing, R.; Liu, T.; Liu, B. Remote Ischemic Preconditioning Protects Cisplatin-Induced Acute Kidney Injury through the PTEN/AKT Signaling Pathway. Oxid. Med. Cell Longev. 2019, 2019, 7629396.
- Li, J.; Fan, X.; Wang, Q.; Gong, Y.; Guo, L. Long Noncoding RNA PRNCR1 Reduces Renal Epithelial Cell Apoptosis in Cisplatin-Induced AKI by Regulating miR-182-5p/EZH1. Kidney Blood Press Res. 2021, 46, 162–172.
- Zhang, H.; Zhang, X.; Yuan, X.; Wang, L.; Xiao, Y. MicroRNA-205 inhibits renal cells apoptosis via targeting CMTM4. Iran, J. Basic Med. Sci. 2015, 18, 1020–1026.
- Zhao, Y.; Lang, Y.; Zhang, M.; Liang, S.; Zhu, X.; Liu, Z. miR-125b Disrupts Mitochondrial Dynamics via Targeting Mitofusin 1 in Cisplatin-Induced Acute Kidney Injury. Kidney Dis. 2022, 8, 137–147.
- Wu, Z.; Li, C.; Li, Q.; Li, J.; Lu, X. Puerarin alleviates cisplatin-induced acute renal damage and upregulates microRNA-31-related signaling. Exp. Ther. Med. 2020, 20, 3122–3129.
- Sun, C.-Y.; Zhang, Q.-Y.; Zheng, G.-J.; Feng, B. Phytochemicals: Current strategy to sensitize cancer cells to cisplatin. Biomed. Pharmacother. 2019, 110, 518–527.
- Verma, V.K.; Malik, S.; Mutneja, E.; Sahu, A.K.; Rupashi, K.; Dinda, A.K.; Arya, D.S.; Bhatia, J. Mechanism involved in fortification by berberine in cddp-induced nephrotoxicity. Curr. Mol. Pharmacol. 2020, 13, 342–352.
- Domitrović, R.; Cvijanović, O.; Pernjak-Pugel, E.; Škoda, M.; Mikelić, L.; Crnčević-Orlić, T. Berberine exerts nephroprotective effect against cisplatin-induced kidney damage through inhibition of oxidative/nitrosative stress, inflammation, autophagy and apoptosis. Food Chem. Toxicol. 2013, 62, 397–406.
- Hagar, H.; El Medany, A.E.; Salam, R.; El Medany, G.E.; Nayal, O.A. Betaine supplementation mitigates cisplatin-induced nephrotoxicity by abrogation of oxidative/nitrosative stress and suppression of inflammation and apoptosis in rats. Exp. Toxicol. Pathol. 2015, 67, 133–141.
- Turgut, N.H.; Gungor, H.; Ekici, M.; Erdogan, M.A.; Karayigit, M.O.; Kara, H. Boldine provides protective effect against nephrotoxicity induced by cisplatin in Wistar rats: Role of oxidative stress, inflammation and caspase-3. Biocell 2022, 46, 2111–2122.
- Liu, X.H.; Li, J.; Li, Q.X.; Ai, Y.X.; Zhang, L. Protective effects of ligustrazine on cisplatin-induced oxidative stress, apoptosis and nephrotoxicity in rats. Environ. Toxicol. Pharmacol. 2008, 26, 49–55.
- Liu, X.H.; Li, Q.X. Effects of ligustrazine on renal cell apoptosis and expression of apoptosis-related proteins in rats with cisplatin-induced renal injury. Chin. J. Pharmacol. Toxicol. 2005, 19, 352–356.
- Wang, S.-W.; Xu, Y.; Weng, Y.-Y.; Fan, X.-Y.; Bai, Y.-F.; Zheng, X.-Y.; Lou, L.-J.; Zhang, F. Astilbin ameliorates cisplatin-induced nephrotoxicity through reducing oxidative stress and inflammation. Food Chem. Toxicol. 2018, 114, 227–236.
- Gao, S.; Chen, T.; Choi, M.Y.; Liang, Y.; Xue, J.; Wong, Y.S. Cyanidin reverses cisplatin-induced apoptosis in HK-2 proximal tubular cells through inhibition of ROS-mediated DNA damage and modulation of the ERK and AKT pathways. Cancer Lett. 2013, 333, 36–46.
- Malik, S.; Suchal, K.; Bhatia, J.; Gamad, N.; Dinda, A.K.; Gupta, Y.K.; Arya, D.S. Molecular mechanisms underlying attenuation of cisplatin-induced acute kidney injury by epicatechin gallate. Lab. Investig. 2016, 96, 853–861.
- Ma, N.; Wei, W.; Fan, X.; Ci, X. Farrerol Attenuates Cisplatin-Induced Nephrotoxicity by Inhibiting the Reactive Oxygen Species-Mediated Oxidation, Inflammation, and Apoptotic Signaling Pathways. Front. Physiol. 2019, 10, 1419.
- Tomar, A.; Vasisth, S.; Khan, S.I.; Malik, S.; Nag, T.C.; Arya, D.S.; Bhatia, J. Galangin ameliorates cisplatin induced nephrotoxicity in vivo by modulation of oxidative stress, apoptosis and inflammation through interplay of MAPK signaling cascade. Phytomedicine 2017, 34, 154–161.
- Huang, Y.C.; Tsai, M.S.; Hsieh, P.C.; Shih, J.H.; Wang, T.S.; Wang, Y.C.; Lin, T.H.; Wang, S.H. Galangin ameliorates cisplatin-induced nephrotoxicity by attenuating oxidative stress, inflammation and cell death in mice through inhibition of ERK and NF-kappaB signaling. Toxicol. Appl. Pharmacol. 2017, 329, 128–139.
- Chen, X.; Wei, W.; Li, Y.; Huang, J.; Ci, X. Hesperetin relieves cisplatin-induced acute kidney injury by mitigating oxidative stress, inflammation and apoptosis. Chem. Biol. Interact. 2019, 308, 269–278.
- Ma, P.; Zhang, S.; Su, X.; Qiu, G.; Wu, Z. Protective effects of icariin on cisplatin-induced acute renal injury in mice. Am. J. Transl. Res. 2015, 7, 2105–2114.
- Zhou, Y.D.; Hou, J.G.; Yang, G.; Jiang, S.; Chen, C.; Wang, Z.; Liu, Y.Y.; Ren, S.; Li, W. Icariin ameliorates cisplatin-induced cytotoxicity in human embryonic kidney 293 cells by suppressing ROS-mediated PI3K/Akt pathway. Biomed. Pharmacother. 2019, 109, 2309–2317.
- Pei, Z.; Wu, M.; Yu, H.; Long, G.; Gui, Z.; Li, X.; Chen, H.; Jia, Z.; Xia, W. Isoliquiritin Ameliorates Cisplatin-Induced Renal Proximal Tubular Cell Injury by Antagonizing Apoptosis, Oxidative Stress and Inflammation. Front. Med. 2022, 9, 873739.
- Fan, X.; Wei, W.; Huang, J.; Liu, X.; Ci, X. Isoorientin Attenuates Cisplatin-Induced Nephrotoxicity Through the Inhibition of Oxidative Stress and Apoptosis via Activating the SIRT1/SIRT6/Nrf-2 Pathway. Front. Pharmacol. 2020, 11, 264.
- Wang, H.; Xia, W.; Long, G.; Pei, Z.; Li, Y.; Wu, M.; Wang, Q.; Zhang, Y.; Jia, Z.; Chen, H. Isoquercitrin Ameliorates Cisplatin-Induced Nephrotoxicity Via the Inhibition of Apoptosis, In fl ammation, and Oxidative Stress. Front. Pharmacol. 2020, 11, 599416.
- Domitrovic, R.; Cvijanovic, O.; Pugel, E.P.; Zagorac, G.B.; Mahmutefendic, H.; Skoda, M. Luteolin ameliorates cisplatin-induced nephrotoxicity in mice through inhibition of platinum accumulation, inflammation and apoptosis in the kidney. Toxicology 2013, 310, 115–123.
- Kang, K.P.; Park, S.K.; Kim, D.H.; Sung, M.J.; Jung, Y.J.; Lee, A.S.; Lee, J.E.; Ramkumar, K.M.; Lee, S.; Park, M.H.; et al. Luteolin ameliorates cisplatin-induced acute kidney injury in mice by regulation of p53-dependent renal tubular apoptosis. Nephrol. Dial. Transplant. 2011, 26, 814–822.
- Bello-Alvarez, C.; Moreno-Londoño, Á.P.; Pedraza-Chaverri, J. Effect of Pretreatment of Morin on the Cisplatin-Induced Toxicity on LLC-PK1 and T24 Cells. Nat. Prod. Commun. 2018, 13, 1934578X1801300416.
- Chtourou, Y.; Aouey, B.; Aroui, S.; Kebieche, M.; Fetoui, H. Anti-apoptotic and anti-inflammatory effects of naringin on cisplatin-induced renal injury in the rat. Chem. Biol. Interact. 2016, 243, 1–9.
- Casanova, A.G.; Hernandez-Sanchez, M.T.; Martinez-Salgado, C.; Morales, A.I.; Vicente-Vicente, L.; Lopez-Hernandez, F.J. A meta-analysis of preclinical studies using antioxidants for the prevention of cisplatin nephrotoxicity: Implications for clinical application. Crit. Rev. Toxicol. 2020, 50, 780–800.
- Casanova, A.G.; Prieto, M.; Colino, C.I.; Gutierrez-Millan, C.; Ruszkowska-Ciastek, B.; de Paz, E.; Martin, A.; Morales, A.I.; Lopez-Hernandez, F.J. A Micellar Formulation of Quercetin Prevents Cisplatin Nephrotoxicity. Int. J. Mol. Sci. 2021, 22, 729.
- Radwan, R.R.; Abdel Fattah, S.M. Mechanisms involved in the possible nephroprotective effect of rutin and low dose gamma irradiation against cisplatin-induced nephropathy in rats. J. Photochem. Photobiol. B 2017, 169, 56–62.
- Sun, C.Y.; Nie, J.; Zheng, Z.L.; Zhao, J.; Wu, L.M.; Zhu, Y.; Su, Z.Q.; Zheng, G.J.; Feng, B. Renoprotective effect of scutellarin on cisplatin-induced renal injury in mice: Impact on inflammation, apoptosis, and autophagy. Biomed. Pharmacother. 2019, 112, 108647.
- Li, Y.; Ye, Z.; Lai, W.; Rao, J.; Huang, W.; Zhang, X.; Yao, Z.; Lou, T. Activation of Sirtuin 3 by Silybin Attenuates Mitochondrial Dysfunction in Cisplatin-induced Acute Kidney Injury. Front. Pharmacol. 2017, 8, 178.
- Ninsontia, C.; Pongjit, K.; Chaotham, C.; Chanvorachote, P. Silymarin selectively protects human renal cells from cisplatin-induced cell death. Pharm. Biol. 2011, 49, 1082–1090.
- Tantituvanont, A.; Jiamchaisri, P.; Chunhacha, P.; Pongjit, K.; Ninsontia, C.; Meksawan, K.; Sritularak, B.; Chanvorachote, P. Silymarin inhibits cisplatin-mediated apoptosis via inhibition of hydrogen peroxide and hydroxyl radical generation. Songklanakarin J. Sci. Technol. 2015, 37, 155–162.
- Badawy, A.M.; El-Naga, R.N.; Gad, A.M.; Tadros, M.G.; Fawzy, H.M. Wogonin pre-treatment attenuates cisplatin-induced nephrotoxicity in rats: Impact on PPAR-gamma, inflammation, apoptosis and Wnt/beta-catenin pathway. Chem. Biol. Interact. 2019, 308, 137–146.
- Meng, X.M.; Li, H.D.; Wu, W.F.; Ming-Kuen Tang, P.; Ren, G.L.; Gao, L.; Li, X.F.; Yang, Y.; Xu, T.; Ma, T.T.; et al. Wogonin protects against cisplatin-induced acute kidney injury by targeting RIPK1-mediated necroptosis. Lab. Investig. 2018, 98, 79–94.
- Ali, B.H.; Abdelrahman, A.; Al Suleimani, Y.; Manoj, P.; Ali, H.; Nemmar, A.; Al Za’abi, M. Effect of concomitant treatment of curcumin and melatonin on cisplatin-induced nephrotoxicity in rats. Biomed. Pharmacother. 2020, 131, 110761.
- Al Fayi, M.; Otifi, H.; Alshyarba, M.; Dera, A.A.; Rajagopalan, P. Thymoquinone and curcumin combination protects cisplatin-induced kidney injury, nephrotoxicity by attenuating NFκB, KIM-1 and ameliorating Nrf2/HO-1 signalling. J. Drug Target. 2020, 28, 913–922.
- Abd El-Kader, M.; Taha, R.I. Comparative nephroprotective effects of curcumin and etoricoxib against cisplatin-induced acute kidney injury in rats. Acta Histochem. 2020, 122, 151534.
- Topcu-Tarladacalisir, Y.; Sapmaz-Metin, M.; Karaca, T. Curcumin counteracts cisplatin-induced nephrotoxicity by preventing renal tubular cell apoptosis. Ren. Fail. 2016, 38, 1741–1748.
- Bami, E.; Ozakpınar, O.B.; Ozdemir-Kumral, Z.N.; Köroglu, K.; Ercan, F.; Cirakli, Z.; Sekerler, T.; Izzettin, F.V.; Sancar, M.; Okuyan, B. Protective effect of ferulic acid on cisplatin induced nephrotoxicity in rats. Environ. Toxicol. Pharmacol. 2017, 54, 105–111.
- Guada, M.; Ganugula, R.; Vadhanam, M.; Ravi Kumar, M.N.V. Urolithin A Mitigates Cisplatin-Induced Nephrotoxicity by Inhibiting Renal Inflammation and Apoptosis in an Experimental Rat Model. J. Pharmacol. Exp. Ther. 2017, 363, 58–65.
- Fatima, S.; Al-Mohaimeed, N.; Al-Shaikh, Y.; Tyagi, P.; Banu, N.; Hasan, S.; Arjumand, S. Combined treatment of epigallocatechin gallate and Coenzyme Q10 attenuates cisplatin-induced nephrotoxicity via suppression of oxidative/nitrosative stress, inflammation and cellular damage. Food Chem. Toxicol. 2016, 94, 213–220.
- Zou, P.; Song, J.; Jiang, B.; Pei, F.; Chen, B.; Yang, X.; Liu, G.; Hu, Z. Epigallocatechin-3-gallate protects against cisplatin nephrotoxicity by inhibiting the apoptosis in mouse. Int. J. Clin. Exp. Pathol. 2014, 7, 4607–4616.
- Mao, R.W.; He, S.P.; Lan, J.G.; Zhu, W.Z. Honokiol ameliorates cisplatin-induced acute kidney injury via inhibition of mitochondrial fission. Br. J. Pharmacol. 2022, 179, 3886–3904.
- Chen, C.; Ai, Q.; Wei, Y. Hydroxytyrosol protects against cisplatin-induced nephrotoxicity via attenuating CKLF1 mediated inflammation, and inhibiting oxidative stress and apoptosis. Int. Immunopharmacol. 2021, 96, 107805.
- Potočnjak, I.; Škoda, M.; Pernjak-Pugel, E.; Peršić, M.P.; Domitrović, R. Oral administration of oleuropein attenuates cisplatin-induced acute renal injury in mice through inhibition of ERK signaling. Mol. Nutr. Food Res. 2016, 60, 530–541.
- Aladaileh, S.H.; Al-Swailmi, F.K.; Abukhalil, M.H.; Ahmeda, A.F.; Mahmoud, A.M. Punicalagin prevents cisplatin-induced nephrotoxicity by attenuating oxidative stress, inflammatory response, and apoptosis in rats. Life Sci. 2021, 286, 120071.
- Domitrović, R.; Potočnjak, I.; Crnčević-Orlić, T.; Škoda, M. Nephroprotective activities of rosmarinic acid against cisplatin-induced kidney injury in mice. Food Chem. Toxicol. 2014, 66, 321–328.
- Ansari, M.A. Sinapic acid modulates Nrf2/HO-1 signaling pathway in cisplatin-induced nephrotoxicity in rats. Biomed. Pharmacother. 2017, 93, 646–653.
- Kandemir, F.M.; Yildirim, S.; Caglayan, C.; Kucukler, S.; Eser, G. Protective effects of zingerone on cisplatin-induced nephrotoxicity in female rats. Environ. Sci. Pollut. Res. 2019, 26, 22562–22574.
- He, L.; Zhang, Y.; Kang, N.; Wang, Y.; Zhang, Z.; Zha, Z.; Yang, S.; Xu, Q.; Liu, Y. Anemoside B4 attenuates nephrotoxicity of cisplatin without reducing anti-tumor activity of cisplatin. Phytomedicine 2019, 56, 136–146.
- Sahu, B.D.; Rentam, K.K.R.; Putcha, U.K.; Kuncha, M.; Vegi, G.M.N.; Sistla, R. Carnosic acid attenuates renal injury in an experimental model of rat cisplatin-induced nephrotoxicity. Food Chem. Toxicol. 2011, 49, 3090–3097.
- El-sayed, E.S.M.; Ar, A.A.; Am, M.; Aa, E.A. Thymol and carvacrol prevent cisplatin-induced nephrotoxicity by abrogation of oxidative stress, inflammation, and apoptosis in rats. J. Biochem. Mol. Toxicol. 2015, 29, 165–172.
- Potočnjak, I.; Domitrović, R. Carvacrol attenuates acute kidney injury induced by cisplatin through suppression of ERK and PI3K/Akt activation. Food Chem. Toxicol. 2016, 98, 251–261.
- Zhang, Y.; Tao, X.; Yin, L.; Xu, L.; Xu, Y.; Qi, Y.; Han, X.; Song, S.; Zhao, Y.; Lin, Y.; et al. Protective effects of dioscin against cisplatin-induced nephrotoxicity via the microRNA-34a/sirtuin 1 signalling pathway. Br. J. Pharmacol. 2017, 174, 2512–2527.
- Soodvilai, S.; Meetam, P.; Siangjong, L.; Chokchaisiri, R.; Suksamrarn, A.; Soodvilai, S. Germacrone reduces cisplatin-induced toxicity of renal proximal tubular cells via inhibition of organic cation transporter. Biol. Pharm. Bull. 2020, 43, 1693–1698.
- Hu, J.N.; Xu, X.Y.; Jiang, S.; Liu, Y.; Liu, Z.; Wang, Y.P.; Gong, X.J.; Li, K.K.; Ren, S.; Li, W. Protective effect of ginsenoside Rk1, a major rare saponin from black ginseng, on cisplatin-induced nephrotoxicity in HEK-293 cells. Kaohsiung J. Med. Sci. 2020, 36, 732–740.
- Qi, Z.; Li, W.; Tan, J.; Wang, C.; Lin, H.; Zhou, B.; Liu, J.; Li, P. Effect of ginsenoside Rh2 on renal apoptosis in cisplatin-induced nephrotoxicity in vivo. Phytomedicine 2019, 61, 152862.
- Han, M.-S.; Han, I.-H.; Lee, D.; An, J.M.; Kim, S.-N.; Shin, M.-S.; Yamabe, N.; Hwang, G.S.; Yoo, H.H.; Choi, S.-J.; et al. Beneficial effects of fermented black ginseng and its ginsenoside 20(S)-Rg3 against cisplatin-induced nephrotoxicity in LLC-PK1 cells. J. Ginseng Res. 2016, 40, 135–140.
- Li, W.; Yan, M.H.; Liu, Y.; Liu, Z.; Wang, Z.; Chen, C.; Zhang, J.; Sun, Y.S. Ginsenoside Rg5 Ameliorates Cisplatin-Induced Nephrotoxicity in Mice through Inhibition of Inflammation, Oxidative Stress, and Apoptosis. Nutrients 2016, 8, 566.
- Wang, Z.; Li, Y.F.; Han, X.Y.; Sun, Y.S.; Zhang, L.X.; Liu, W.; Liu, X.X.; Li, W.; Liu, Y.Y. Kidney Protection Effect of Ginsenoside Re and Its Underlying Mechanisms on Cisplatin-Induced Kidney Injury. Cell Physiol. Biochem. 2018, 48, 2219–2229.
- Mohamed, M.E.; Abduldaium, Y.S.; Younis, N.S. Ameliorative effect of linalool in cisplatin-induced nephrotoxicity: The role of HMGB1/TLR4/NF-κB and NRF2/HO1 pathways. Biomolecules 2020, 10, 1488.
- Ma, Z.N.; Li, Y.Z.; Li, W.; Yan, X.T.; Yang, G.; Zhang, J.; Zhao, L.C.; Yang, L.M. Nephroprotective effects of saponins from leaves of panax quinquefolius against cisplatin-induced acute kidney injury. Int. J. Mol. Sci. 2017, 18, 1407.
- Liu, X.; Huang, Z.; Zou, X.; Yang, Y.; Qiu, Y.; Wen, Y. Panax notoginseng saponins attenuates cisplatin-induced nephrotoxicity via inhibiting the mitochondrial pathway of apoptosis. Int. J. Clin. Exp. Pathol. 2014, 7, 8391–8400.
- Fei, B.; Liu, Z.; Xie, L.; Lv, L.; Zhu, W.; Liu, J.; Dai, Y.; She, W. Panax notoginseng Saponins protect auditory cells against cisplatin-induced ototoxicity by inducing the AKT/Nrf2 signaling-mediated redox pathway. Mol. Med. Rep. 2020, 22, 3533–3540.
- Hu, J.N.; Leng, J.; Shen, Q.; Liu, Y.; Li, X.D.; Wang, S.H.; Li, H.P.; Wang, Z.; Wang, Y.P.; Li, W. Platycodin D suppresses cisplatin-induced cytotoxicity by suppressing ROS-mediated oxidative damage, apoptosis, and inflammation in HEK-293 cells. J. Biochem. Mol. Toxicol. 2021, 35, e22624.
- Kim, T.W.; Song, I.B.; Lee, H.K.; Lim, J.H.; Cho, E.S.; Son, H.Y.; Park, S.J.; Kim, J.W.; Yun, H.I. Platycodin D, a triterpenoid sapoinin from Platycodon grandiflorum, ameliorates cisplatin-induced nephrotoxicity in mice. Food Chem. Toxicol. 2012, 50, 4254–4259.
- Kim, Y.J.; Lee, M.Y.; Son, H.Y.; Park, B.K.; Ryu, S.Y.; Jung, J.Y. Red ginseng ameliorates acute cisplatin-induced nephropathy. Planta Med. 2014, 80, 645–654.
- Ma, X.; Dang, C.; Kang, H.; Dai, Z.; Lin, S.; Guan, H.; Liu, X.; Wang, X.; Hui, W. Saikosaponin-D reduces cisplatin-induced nephrotoxicity by repressing ROS-mediated activation of MAPK and NF-kappaB signalling pathways. Int. Immunopharmacol. 2015, 28, 399–408.
- Sherif, I.O. Amelioration of cisplatin-induced nephrotoxicity in rats by triterpenoid saponin of Terminalia arjuna. Clin. Exp. Nephrol. 2015, 19, 591–597.
- El Shaffei, I.; Abdel-Latif, G.A.; Farag, D.B.; Schaalan, M.; Salama, R.M. Ameliorative effect of betanin on experimental cisplatin-induced liver injury; the novel impact of miRNA-34a on the SIRT1/PGC-1alpha signaling pathway. J. Biochem. Mol. Toxicol. 2021, 35, 1–14.
