1. Intrinsic Apoptotic Pathway during DDP-Induced Nephrotoxicity
Cisplatin-induced nephrotoxicity is an adverse side effect of this antineoplastic drug therapy and involves several types of cellular death, such as the necrosis (also called uncontrolled cell death)
[1][2][3][21,22,23], apoptosis (programmed cell death)
[4][5][6][7][8][9][24,25,26,27,28,29], and necroptosis (regulated inflammatory cell death)
[10][11][30,31] of renal cells. Concerning programmed apoptosis cell death, the three pathways that have been described are called intrinsic, extrinsic, and endoplasmic reticulum stress (
Figure 12).
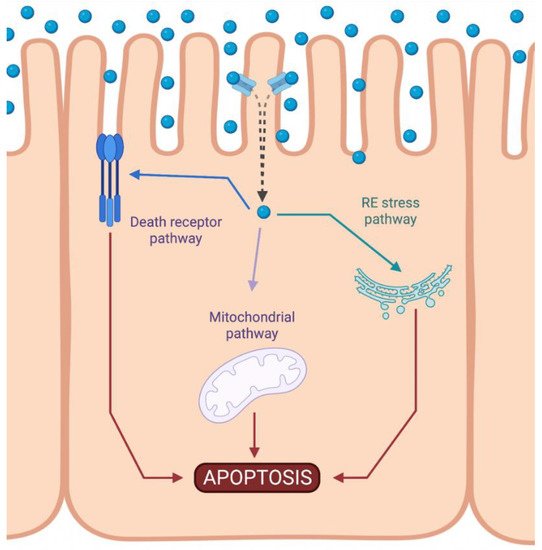
Figure 12. Activation of apoptotic pathways during cisplatin-induced nephrotoxicity. Cisplatin (blue circle) can activate both mitochondrial (purple) and death receptor (blue) pathways of apoptosis. Likewise, endoplasmic reticulum (ER) stress may also be induced (green). Created with BioRender.com.
The intrinsic pathway refers to a primarily mitochondrial-mediated apoptotic pathway that results in cell damage. The intrinsic mitochondrial pathway is the main apoptotic pathway prompted during DDP-induced nephrotoxicity. In this pathway, cellular stress leads to the activation of the proapoptotic B-cell lymphoma 2 (Bcl-2) proteins, Bcl-2-associated X protein (Bax), and Bcl-2 homologous antagonist/killer (Bak)
[12][32], and the reduction of antiapoptotic proteins such as Bcl-2, Bcl-extra-large (Bcl-XL), and myeloid cell leukemia 1 (Mcl-1)
[13][14][33,34]. This triggers mitochondrial outer-membrane permeabilization (MOMP), releasing apoptotic factors, such as apoptosis-inducing factor (AIF)
[15][35], cytochrome c
[16][36], endonuclease G
[17][37], HtrA2/Omi
[18][38], Smac/DIABLO
[19][39], and others. Chipuk et al. also showed that p53 directly activated Bax and triggered apoptosis
[20][40].
Cytochrome c is a crucial mediator of the mitochondrial pathway. Once in the cytosol, cytochrome c induces a dATP-mediate oligomerization of apoptotic protease-activating factor-1 (Apaf-1) in a 2:1 ratio. This complex then recruits the initiator caspase of this pathway, procaspase-9, and induces its autoactivation
[21][41]. Finally, caspase-9, in turn, activates downstream caspases, such as caspase-3, and initiates the process of caspase-dependent apoptosis
[22][42]. In normal conditions, the second mitochondria-derived activator of caspase (Smac), a protein located in the mitochondria, however, is released into the cytosol when cells undergo apoptosis
[23][43]. Some studies have shown that inhibitors of apoptosis proteins (IAPs), such as XIAP, cIAP1, and cIAP2, are group proteins that negatively regulate both caspases and cell death
[24][44]. Thus, Smac is able to promote caspase-9 activation by binding to IAPs and removing their inhibitory activity
[23][43].
The involvement of the intrinsic pathway of apoptosis in DDP-induced nephrotoxicity has been reported. On in vitro cultured cells, DDP treatment led to a reduction of proapoptotic Bcl-2 protein expression, which was accompanied with an increase of antiapoptotic protein Bax and Bak
[13][14][16][25][26][27][28][33,34,36,45,46,47,48], leading to apoptosis. In the same way, in the rodent model, a reduced Bcl-2 protein expression was also observed after a single dose of DDP
[25][45]. After DDP treatment, the translocation of endogenous Bax from the cytosolic to the membrane fractions was observed and, subsequently, the release of cytochrome c. In addition, using adult Wistar rats, a single dose of DDP triggered severe kidney tissue damage, accompanied by an increase in cytochrome c activity
[29][49].
Endonuclease G has also been observed to be induced during DDP injury in mice
[30][50]. Furthermore, using primary mouse proximal tubule cells, Cilenti et al. showed that the level of Omi protein was also upregulated after DDP treatment, and this upregulation was followed by the release of Omi from mitochondria to the cytoplasm, and the subsequent degradation of XIAP
[31][51]. Cisplatin also increases the expression of Apaf-1
[32][52] and then activates caspase-9/-3
[33][53], leading to cell death.
2. Involvement of microRNAs upon Intrinsic Apoptosis Pathway during DDP-Induced Nephrotoxicity
In 1993, two different studies, by Lee et al.
[34][54] and Wightman et al.
[35][55], discovered the miRNAs, which revolutionized the field of molecular biology. MicroRNAs are a class of ncRNAs of approximately 22 bp in length that recognize target sites, most commonly found in the 3′-untranslated regions (3′-UTRs) of mRNAs, through imperfect base-pairing, with one or more mismatches in sequence complementarity
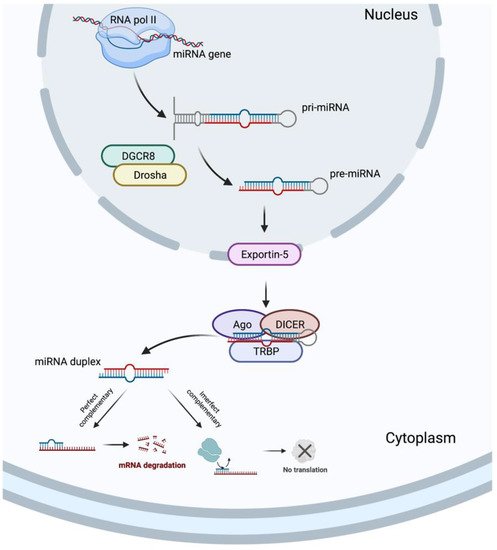
[36][56]. Briefly, the miRNAs biogenesis is started when a pri-miRNA is transcribed from its miRNA gene and then recognized and cleaved into pre-miRNA by RNA binding protein DiGeorge Syndrome Critical Region 8 (DGCR8) and a ribonuclease III enzyme, Drosha
[37][57], resulting in the formation of a pre-miRNA, which is exported to the cytoplasm by an Exportin-5 and then processed by the RNase III endonuclease, DICER, in collaboration with the transactivation response RNA binding protein (TRBP) and Argonaute (Ago), which remove the terminal loop, resulting in a mature miRNA duplex
[38][58]. Both 5p or 3p strands originated from the 5′ or 3′ end of the pre-miRNA harping, respectively, are derived from the mature miRNA duplex. Thus, the final result is the generation of a miRNA, which can negatively regulate gene targets at the post-transcriptional level by perfect complementarity of their “seed” region to the 3′-UTR of its target mRNA, inducing their degradation, or by an imperfect complementarity, resulting in translational repression
[39][59] (
Figure 23).
Figure 23.
MicroRNA biogenesis. Created with BioRender.com.
The ability of DDP to induce cell death requires the sequential activation of the p53/ROS/p38a MAPK cascade
[40][77]. On this basis, a microarray analysis identified 47 differentially expressed miRNAs during DDP-cytotoxicity of HK-2 cells. Moreover, a pathway analysis indicated that the top upregulated pathways included the MAPK and p53 signaling pathways. A further network analysis showed that the MAPK signaling pathway and apoptosis were identified as core pathways and miR-9-3p and miR-371b-5p as the most critical miRNAs during DDP-induced cytotoxicity
[41][65].
p53 mediates cisplatin-induced apoptosis in renal proximal tubular cells, and p53 can activate caspase-3
[42][78]. Related to this, Li et al.
[43][76] described that HRPTEp cells treated with DDP for 48 h showed that DDP led to significantly upregulated miR-449a. In the same way, the overexpression of miR-449a led to an increased apoptotic rate of HRPTEpCs after DDP insult, while antagomir-449a reversed it. Another study demonstrated that HK-2 cells stimulated with DDP showed that the downregulation of miR-205-5p promoted cell apoptosis, observed by an enhanced caspase-3 activity and apoptosis rate of in vitro cultured cells. However, enhancing miR-205-5p expression suppressed apoptotic rate
[44][72]. The levels of miR-144-5p were also downregulated in DDP-stimulated HK-2 cells, and the expression levels of apoptosis-related proteins showed that enhancing miR-144-5p expression was able to increase the expression levels of caspase-3/-9, and Bax, and to decrease the expression levels of Bcl-2, by regulating PKM2 expression
[45][71]. Opposite results were observed by Zhang et al.
[46][70], since they demonstrated that remote ischemic preconditioning, a strategy to induce resistance in a target organ, exerted a protective effect on DDP-induced AKI in mice by reversing the downregulation of miR-144 and the dysregulation of caspase-3, Bax, and Bcl-2 expression in renal tissues of DDP-induced AKI in mice and NRK-52 cells. A similar study also showed that an enhanced miR-182-5p expression could reduce renal epithelial Bcl-2 levels and promote Bax and cleaved caspase-3 after in vitro DDP insult, and inhibiting its expression attenuated the damage of DDP in HK-2 cells
[47][74]. Zhu et al.
[14][34] found that miR-181a expression downregulated after DDP-induced apoptosis. They also found that Bcl-2 was upregulated and Bax was downregulated after the transient transfection of the miR-181a inhibitor in HK-2 cells, suggesting that miR-181a was directly involved in the apoptotic process. Using qRT-PCR analysis, Zhang et al.
[48][73] observed a downregulation of miR-205 in HK-2 cells treated with DDP. The transient overexpression of miR-205 using mimics demonstrated that HK-2 cells were more resistant to DDP-induced apoptosis, by modulating CMTM4 protein expression. In another study, qPCR was performed to evaluate the miR-125b expression in in vitro and in vivo DDP-induced damage, and the results showed the upregulation of miR-125b after DDP injection and in cultured tubular epithelial cells treated with DDP. Moreover, DDP-induced apoptosis was decreased by using a miR-125b inhibitor, as shown by the TUNEL assay and the reduced Bax expression
[49][68]. Another study demonstrated that DDP notably increased miR-31 expression and apoptosis-associated proteins (caspase-3 and Bax), while decreasing the antiapoptotic factor, Bcl-2, in kidney samples
[50][66].
Thus, it is evident that many miRNAs exert a fundamental role in activating or inhibiting critical molecules in the progression of the mitochondrial pathway of apoptosis.
3. Potential Utility of Natural Products in DDP-Induced Apoptosis
Cisplatin is an effective chemotherapeutic drug whose clinical use and efficacy are limited by its nephrotoxicity, which affects mainly the renal tubular cells. It accumulates in proximal and distal epithelial tubule cells and causes cell death. Consequently, various classes of natural products have been tested for their capacity to prevent DDP-induced nephrotoxicity. Furthermore, natural products also overcome resistance, sensitizing cancer cells to DDP
[51][79].
Alkaloids are a class of natural compound. Alkaloids represent a vast group of naturally occurring compounds which contain at least one nitrogen atom (amino or amido in some cases). Some alkaloids shown to prevent DDP-induced apoptosis include the berberine
[52][53][80,81], betaine
[29][54][49,82], boldine
[55][83], and ligustrazine
[56][57][84,85].
Flavonoids are a class of polyphenolic secondary metabolites found in plants. Several studies have shown that the use of certain flavonoids prevent DDP-induced apoptosis, most notably the use of astilbin
[58][86], cyanidin
[59][87], epicatechin gallate
[60][88], farrerol
[61][89], galangin
[62][63][90,91], hespertin
[64][92], icariin
[65][66][93,94], isoliquiritin
[67][95], isoorientin
[68][96], isoquercitrin
[69][97], luteolin
[70][71][98,99], morin
[72][100], naringin
[73][101], puerarin
[50][66], quercetin
[74][75][102,103], rutin
[76][104], scutellarin
[77][105], silybin
[78][106], silymarin
[79][80][107,108], and wogonin
[81][82][109,110].
Another class of natural product consists of phenolic compounds. They are secondary metabolites produced in the shikimic acid of plants, which contain benzene rings, with one or more hydroxyl substituents. Phenolic compounds with research related to this side effect include curcumin
[83][84][85][86][87][111,112,113,114,115], ellagic acid
[88][116], epigallocatechin gallate
[89][90][117,118], ferulic acid
[87][115], honokiol
[91][119], hydroxytyrosol
[92][120], oleuropein
[93][121], punicalagin
[94][122], rosmarinic acid
[95][123], sinapic acid
[96][124], and zingerone
[97][125].
Likewise, terpenoids are a large and diverse group of lipids resulting from five-carbon isoprene units assembled in thousands of combinations and are isolated from plants and microbial sources. The following terpenoids have shown potential in some studies: anemoside B4
[98][126], carnosic acid
[99][127], carvacrol
[100][101][128,129], dioscin
[102][130], germacrone
[103][131], ginsenoside 20 (S)-RG3/Re/RG5/Rk1/Rh2
[104][105][106][107][108][132,133,134,135,136], linalool
[109][137],
Panax quinquefolius saponins
[110][138],
Panax notoginseng saponins
[111][112][139,140], platycodin D
[113][114][141,142], pseudoginsengenin DQ
[33][53], red ginseng
[115][143], saikosaponin D
[116][144], and
Terminalia arjuna triterpenoid saponins
[117][145].
However, only a few studies have shown the use of antioxidants in epigenetic regulation, specifically on miRNAs, during DDP-induced apoptosis. For example, betanin, a natural red glycoside food dye obtained from beets, has been shown to reduce organ damage induced by DDP by reducing miRNA-34a expression and enhancing the SIRT1/PGC-α pathway
[118][146]. Moreover, puerarin, another natural flavonoid extracted from the Chinese medical herb
Radix puerariae, alleviated DDP-induced AKI by suppressing miR-31 expression, enhancing Numb activation, thereby inhibiting the Notch signaling pathway
[50][66]. Finally, dioscin, a steroid saponin commonly found in various herbs, protected against DDP-induced injury to NRK-52E and HK-2 cells by decreasing miR-34a expression, which was accompanied by increased levels of SIRT1, and thus, cell damage
[102][130].
Therefore, natural products could be developed as a new candidate to alleviate DDP-induced cell injury. In addition, they could be used as an ncRNA-based therapy to counteract apoptosis and other pathways induced during nephrotoxicity.