Tomato cultivation is threatened by environmental stresses (e.g., heat, drought) and by viral infection (mainly viruses belonging to the tomato yellow leaf curl virus family—TYLCVs). Unlike many RNA viruses, TYLCV infection does not induce a hypersensitive response and cell death in tomato plants.
One of the major effects caused by TYLCV infection of tomato is a decrease in the activation of stress response proteins and metabolites to avoid an acute deleterious plant response, which may impair virus replication. Instead of severe, sometimes lethal response for most plant cells, TYLCV promotes the development of a protective homeostasis response in plants exposed to prolonged environmental stresses, favoring the survival of the plant. The down-regulation of stress proteins and metabolites coincides with the stabilization of their patterns, particularly in tomatoes resistant to TYLCV. These stable patterns are maintained not only in shoot, but also in roots of virus-infected tomatoes. Whether fruit production of pre-inoculation of R-TYLCV tomato seedlings in field and greenhouses located in hot and dry countries provides a solution, even partial, to global warming, remains to be analyzed on a large scale. This routine, adapted to tomato genotype, soil and climate, may help reduce drastically the amount of water used for irrigation by artificially alternating long periods of drought with short periods of irrigation.
1. Introduction
Environmental stresses affect crop production worldwide. Drought and heat are the most serious abiotic stresses especially in countries with hot climate. Moreover, they increase insect pressure (reviewed in
[1]), creating additional threats due to the viruses carried by insect vectors. For a long time, viruses were considered exclusively as pathogens that cause damage to plants, using host resources and compounds for their own reproduction. However, in recent years, new information described the beneficial roles of some viruses for their plant hosts. Viruses were defined as mutual interactors with plant cellular mechanisms, resulting in plant protection against some abiotic stresses
[2]. One of the pioneering studies in this direction described the ability of RNA viruses such as brome mosaic virus (BMV), tobacco mosaic virus (TMV), and tobacco rattle virus (TRV), to increase host plant tolerance to drought, while cucumber mosaic virus (CMV) induced plant resistance not only to drought, but also to cold
[3]. The virus-infected tissues were characterized by increased levels of osmoprotectants and antioxidants. A further advancement in the understanding of drought tolerance was achieved in
Nicotiana benthamiana and
Arabidopsis thaliana infected by potato virus X (PVX) and plum pox virus (PPV)
[4]. Even though a detailed description of PVX or PPV-induced improvement of plant survival under drought was not presented, increased levels of salicylic acid (SA), but not abscisic acid (ABA), were found in the infected plants. These results were unexpected, because ABA is the key hormone regulating plant responses to drought
[5], while SA is induced in response to infections by pathogens
[6][7][6,7]. However, the establishment of drought tolerance in an ABA-independent manner was also shown in transgenic Arabidopsis expressing the tomato yellow leaf curl C4 gene
[8] or the turnip mosaic virus (TuMV) 6K2 gene
[9]. TRV was shown to promote plant tolerance to low temperature
[10], and PVX to environmental oxidation
[11].
Begomoviruses are small circular ssDNA (cssDNA) viruses. Tomato yellow leaf curl virus (TYLCV) is a typical Begomovirus, which infects tomato plants (
Solanum lycopersicum). The TYLCV virion encapsidates a single cssDNA molecule (monopartite) of about 2800 nucleotides. Until very recently, it was thought that the monopartite TYLCV genome encodes six genes: the viral strand with two genes, V1 (coat protein CP) and V2, and the complementary strand with four genes, C1 to C4 (reviewed in
[12]). However, recent bioinformatic studies
[13] were instrumental in the discovery of six additional TYLCV open reading frames coding for proteins of less than 80 amino acids, some displaying specific subcellular localizations. For example, V3 expressed during viral infection, localizes in the Golgi apparatus, functions as an RNA silencing suppressor, and traffics along microfilaments to plasmodesmata to promote virus cell-to-cell movement
[14]. Another TYLCV protein, coined C5, is a pathogenicity determinant and RNA silencing suppressor
[15]. Another
recent study
[16] showed that by screening translation initiation sites of the bipartite tomato yellow leaf curl Thailand virus, potential viral proteins coded by the A and B genomes were discovered and found to be important for the translation of different protein isoforms localized in various cellular compartments.
TYLCV is spread between plants by its insect vector in a circulative persistent manner, the whitefly
Bemisia tabaci, which feeds on more than 600 plant species worldwide (reviewed in
[17]). In commercial tomatoes, yield losses may reach 100%
[18]. The threat of TYLCV pathogenicity has been reduced by the development of tomato lines and cultivars tolerant to TYLCV, first in Israel
[19] and later in many other countries
[20]. Upon infection, these tolerant tomatoes (coined here R-TYLCV) do not change drastically their morphological characteristics, show normal growth and yield even under massive inoculation by viruliferous whiteflies. After prolonged infection, R-TYLCV tomatoes contain virus amounts comparable to those present in TYLCV-susceptible tomatoes (S-TYLCV), while conserving they agronomic properties
[21].
2. Cell Death, Induced by Inactivation of HSP90, Is Suppressed in TYLCV-Infected Plants
Cell death can often be activated as a defense response against biotic and abiotic stresses
[22][23][23,24]. Silencing of the genes encoding the key cellular chaperone HSP90 and HSP90 co-chaperone, SGT1, caused necrosis in the tomato stem
[24][25]. The inactivation of the Hsp90 machinery resulted in massive accumulation of ubiquitinated proteins. A decrease in necrotic lesions was conspicuous in TYLCV-infected plants where the Hsp90/Sgt1 genes have been silenced. Host protein damage decreased as well.
The central role of HSP90 in protein damage was shown first in yeast
[25][26], then in plants
[26][27]. HSP90 develops complexes with 26S proteasome, activating the degradation of ubiquitinated proteins. Inactivation of the HSP90 machinery resulted in the dissociation of the 26S proteasome and in the loss of its peptidase activity. Likely, TYLCV mitigated the inactivation of 26S proteasome in Hsp90/Sgt1-silenced tomatoes, restoring the proper utilization of ubiquitinated proteins in cellular protein homeostasis, and leading to the suppression of cell death in virus-infected tomatoes. While uninfected tomatoes with silenced Hsp90/Sgt1 genes died after two weeks of growth, the infected tomatoes appeared healthy during prolonged growth
[24][25] (
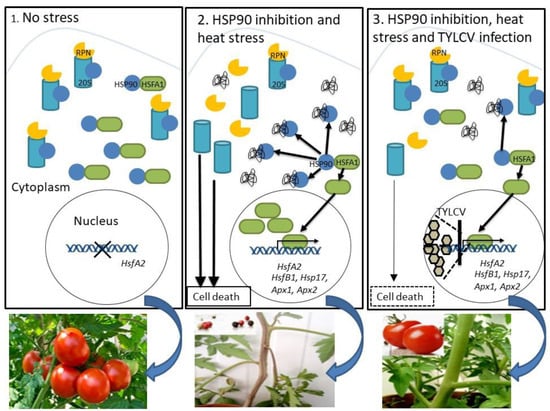
Figure 1).
Figure 1. Summary of the key processes of stress response regulation by HSP90 in tomato plants, and their down-regulation by TYLCV infection. Functional loss of the cellular chaperone HSP90 causes the dissociation of the 26S proteasome and a significant decrease in its peptidase activity, consequently, to an increase in the level of ubiquitinated proteins and signs of cell death. The inhibition of the 26S proteasome stimulated the expression of heat-inducible genes, including transcription factor HSFA2. In leaves of TYLCV-infected tomato, the levels of HSFA2 were lower than in leaves of uninfected plants. The amounts of HSFA2 greatly increased upon heat stress in uninfected tissues, and much less in TYLCV-infected leaves. The inhibition of HSP90 activity caused an additional increase in HSFA2 expression. Subsequent TYLCV infection reduced HSFA2 levels as well as expression levels of HsfB1, Hsp17, Apx1, and Apx2. TYLCV infection suppresses HSP90-dependent 26S proteasome inactivation, cell death and HSFA2 signal transduction pathways, resulting in normal tomato growth and fruit yielding
[24][25].